Abstract
Purpose
Tumor motion is a major challenge in stereotactic ablative body radiotherapy (SABR) for non-small cell lung cancer (NSCLC), causing excessive irradiation to compensate for this motion. Real-time tumor tracking with a magnetic resonance imaging-guided linear accelerator (MR-Linac) could address this problem. This study aimed to assess the effects and advantages of MR-Linac in SABR for the treatment of lung tumors.
Methods
Overall, 41 patients with NSCLC treated with SABR using MR-Linac between March 2019 and December 2021 were included. For comparison, 40 patients treated with SABR using computed tomography-based modalities were also enrolled. The SABR dose ranged from 48 to 60 Gy in 3–5 fractions. The primary endpoint was a lower radiation volume compared to CT-based SABR. The secondary endpoint was the local control rate of SABR using the MR-Linac.
Results
The median follow-up time was 19 months (range: 3–105 months). There was no significant difference in the gross tumor volume between the MR and CT groups (7.1 ± 9.3 cm3 vs 8.0 ± 6.8 cm3, p = 0.643), but the planning target volume was significantly smaller in the MR group (20.8 ± 18.8 cm3 vs 34.1 ± 22.9 cm3, p = 0.005). The 1-year local control rates for the MR and CT groups were 92.1 and 75.4%, respectively (p = 0.07), and the 1-year overall survival rates were 87.4 and 87.0%, respectively (p = 0.30).
Conclusion
Lung SABR with MR-Linac can reduce the radiation field without compromising the local control rate. Further follow-up is needed to assess the long-term effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection with lobectomy and mediastinal lymph node sampling remains the first-line treatment for early-stage non-small cell lung cancer (NSCLC) (Park et al. 2020). However, not all patients are eligible for surgical resection, particularly those with concomitant comorbidities or weakened cardiopulmonary function, the elderly, and other high-risk patients (Shinde et al. 2018). Stereotactic ablative body radiotherapy (SABR) is a non-invasive treatment using radiotherapy for a small-sized tumor mass, with accurate targeting delivered in high doses per fraction within five sessions (Timmerman et al. 2018). Various dose fractionation schedules have been reported, and a biologically effective dose (BED10) of ≥ 100 Gy is considered sufficient to achieve local control (LC) and has been recommended for primary lung tumors (Onishi et al. 2007). Accordingly, SABR is recommended as the treatment of choice for inoperable patients with early-stage NSCLC owing to its convenience and acceptable outcomes (Howington et al. 2013; Shibamoto et al. 2015). Many prospective studies on SABR in patients with early-stage NSCLC have been reported. The 3-year LC rates range from 70 to 94%, with limited acute and late toxicities (Nagata et al. 2005; Hof et al. 2007; Koto et al. 2007; Baumann et al. 2009). Further, the 3-year overall survival (OS) rate is reported to be 60–70% (Nagata et al. 2005; Koto et al. 2007; Baumann et al. 2009). As a treatment option for oligometastasis in NSCLC, recent studies on SABR have reported improved LC and survival in patients with oligometastatic NSCLC (Shultz et al. 2014; Palma et al. 2019).
However, a challenge in lung SABR is the breathing-related movement of the tumor and the adjacent organs. To compensate for these movements, a four-dimensional computed tomography (4-D CT) scan is often used to generate an internal target volume (ITV). However, the ITV generation technique enlarges the planning target volume (PTV) size, which causes a larger irradiated volume than the true tumor mass and can underestimate true respiratory tumor movement (Wojcieszynski et al. 2017). One potential solution to this problem is to directly monitor the target movement during treatment, and this is possible with a magnetic resonance imaging-guided linear accelerator (MR-Linac). The MR-Linac is a hybrid device that combines magnetic resonance imaging (MRI) with linear accelerators. The operator can observe the exact movement of a tumor throughout the treatment period and control the beam can be turned on and off, depending on the tumor position, using cine MRI and structural tracking. Consequently, SABR using MR-Linac may potentially prevent geometric tumor misses and allow for a smaller PTV. Nevertheless, practical studies on this hypothesis have not yet been carried out, and thus, the feasibility of lung SABR with MR-Linac is still unclear. Thus, this study aimed to evaluate the effects and advantages of MR-Linac in comparison with those of conventional CT-based modalities in SABR for the treatment of lung tumors.
Materials and methods
Study design and patients
This retrospective study evaluated patients treated with SABR using MR-Linac (ViewRay Linac MRIdian system, ViewRay, Cleveland, Ohio, USA) between March 2019 and December 2021 at Incheon St. Mary’s Hospital. For comparison, patients treated with SABR using computed tomography (CT)-based modalities (Tomotherapy and Novalis Tx, BrainLAB, Heimstetten, Germany) at the same institution between March 2013 and August 2021 were also enrolled. The inclusion criteria were: (1) non-small cell lung carcinoma; (2) tumors less than 4.5 cm in the longest diameter; and (3) treatment with SABR on a lung lesion with a BED10 > 100 Gy. The exclusion criteria were: (1) previous treatment on the same site with radiotherapy; (2) treatment with mediastinal lymph nodes, bronchial tree, or pleura; and (3) Eastern Cooperative Oncology Group (ECOG) performance status score of ≥ 3. Among the 103 treated patients, 22 patients who did not complete the treatment for any reason or who were lost to follow-up (n = 15) and patients who were simultaneously treated for multiple lung lesions (n = 7) were excluded. Finally, 81 patients were included in the study.
Simulation
The patients were divided into two groups. The MR group included patients who received magnetic resonance imaging (MRI)-guided real-time tumor tracking technique by MR-Linac for SABR. Meanwhile, the CT group included patients who received CT-based modalities for SABR. Patients in the MR group underwent MRI scan simulation using the ViewRay Linac MRIdian system with 0.35 Tesla. The patients were placed in the supine position with both arms elevated. Chest Vac-Lok™ (CIVCO Medical Solutions, Coralville, Iowa, USA) was used for immobilization. An MRI coil was placed above the patient’s chest, and MRI scans were performed with a slice thickness of 3 mm. Then, a real-time cine MRI scan was performed for 10 s using the end-exhale breath-hold method. Patients were instructed to take shallow and steady breathes while cine images were taken. In one selected sagittal plane image, the lung lesion was contoured by a physician, and the movements of the lesion in all directions were verified while tracking the target. After MRI simulation, CT simulation was performed using the same immobilization device with a contrast-enhanced CT scanner (LightSpeed RT 16 (GE, Waukesha, WI, USA)). The enhanced CT simulation images were fused with the MRI simulation images to obtain the electron density data for radiotherapy planning.
Meanwhile, patients in the CT group underwent CT only simulation. The position was the same as that of the MR group, with both arms raised together in the supine position. Whole-body Vac-Lok™ was used for immobilization. To manage respiratory motion, abdominal compression with forced shallow breathing was performed. Four-dimensional CT scans with a 2.5-mm slice thickness were routinely performed for accurate delineation of target volumes. Tumor motion related to respiration was quantified using 4-D CT scans.
Planning
For the MR group, the gross tumor volume (GTV) for lung lesions was contoured by a radiation oncologist on the MR simulation image, referring to the CT simulation image and previously acquired diagnostic images, such as enhanced chest CT or positron emission tomography CT. The PTV was created by adding a 5–7 mm margin to the GTV, depending on the movements that were confirmed during real-time tracking performed at the time of simulation. Step-and-shoot intensity-modulated radiation therapy (IMRT) planning was performed using 13–15 beams with an MRIdian treatment planning system (ViewRay Inc., Mountain View, CA, USA) (Fig. 1).
Sample of MR-Linac plan (GTV = red line; PTV = cyan line; orange line = 95% isodose line; yellow line = 90% isodose line; green line = 70% isodose line; blue line = 50% isodose line). Prescription dose was planned to cover more than 95% of the PTV volume. GTV = gross tumor volume; PTV planning target volume
For the CT group, the GTV was delineated in every phase (five inspiratory, five expiratory, and one resting phase) acquired by 4-D CT scans on the pulmonary CT windows. The 11 GTVs acquired at each respiratory phase were integrated to generate the internal target volume (ITV). To compensate for the intra- and inter-fractional variations in patient position and beam geometries, 5 mm margins were added to the ITV to define the PTV. Sliding-window IMRT planning with an iplan (BrainLab (Heimstetten, Germany)) was used for cases treated with Novalis. Meanwhile, helical IMRT planning with a Tomo planning station (Accuray) was used for tomotherapy cases.
Treatment protocol
In the MR tracking group, real-time MR tumor tracking was performed using deformable image-registration-based beam control during radiotherapy. At each session of RT, the patients underwent MRI scanning for 25 s with breath holding. Adjustments were made for the target lesion according to the simulation image. Subsequently, cine MRI was performed for 10 s. One sagittal plane was chosen to observe the target during the treatment delivery. The GTV and planning PTV were set to track the target and boundary, respectively. During radiation delivery, the beam automatically turned off if the target was out of the boundary by more than 3% (Fig. 2). In the CT group, the patients were immobilized and placed in the same position as in the simulation. During each treatment, image guidance was used to confirm the location of the target.
Sample of real-time MR-guided tumor tracking and gating radiotherapy through CINE MRI (Red line = Target (gross tumor volume); blue line = Boundary (5 mm margin on target)). During radiotherapy, the beam was automatically turned off if the target was out of the boundary by more than a threshold of 3%
Follow-up protocol
For most cases, the first follow-up was conducted 1 month after SABR. Chest physical examination and chest CT also were performed in addition to physical examination and laboratory workups. Treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1. The patients were followed-up every 3 months for the first 2 years then every 6 months for up to 5 years thereafter. Treatment-related toxicity was investigated according to the Common Terminology Criteria for Adverse Events version 5.0.
Endpoints and variable definitions
The primary endpoint was a lower radiation volume in MR-based SABR than in CT-based SABR. The dimensions of the GTV and PTV were calculated to assess whether MR-Linac reduced the radiation volume. The potential risk of reducing the radiotherapy volume is a geometrical miss. Therefore, we set the secondary endpoint as the LC rate of SABR using MR-Linac to ensure its feasibility. LC was defined as the absence of progressive soft-tissue CT density and size increments at the treated site. LC was determined as the duration from the end date of SABR to the date of disease relapse in the initial involved site, death from any cause, or last follow-up visit if a patient was free of disease. Progression-free survival (PFS) was defined as the duration from the end date of SABR to the date of disease relapse at any site, death from any cause, or last follow-up visit if a patient was free of disease. OS was defined as the time from the end date of SABR to death from any cause or the last follow-up visit.
Statistical analyses
Between-group comparisons were performed using a t test. Survival curves for LC, PFS, and OS were generated using the Kaplan–Meier method. All statistical analyses were performed using R version 4.0.0 (R Development Core Team, Vienna, Austria). p values less than 0.05 were considered statistically significant.
Results
Patient and tumor characteristics
In total, 41 and 40 patients were included in the MR and CT groups, respectively. The patient characteristics are summarized in Table 1. Intrapulmonary metastasis (56.1%) was the main disease extent in the MR group, whereas it was solitary pulmonary nodules (62.5%) in the CT group (p = 0.004). There was no significant between-group difference in the lobar location of the tumor (p = 1.000). The number of fractionations prescribed for SABR was different between the two groups (p < 0.001); most of the patients in the CT group were treated with four fractions, while almost half of the patients in the MR group were treated with five fractions. However, all the patients in this study received a minimum BED10 of 100 Gy. There was no significant difference in the BED10 on PTV between the two groups (116.7 ± 20.1 Gy for the MR group vs. 116.2 ± 16.3 Gy for the CT group, p = 0.915). No other patient characteristics differed significantly between the MR and CT groups.
Tumor volumes
The tumor sizes in both the groups are shown in Table 1. There was no significant difference in GTV between the two groups (7.1 ± 9.3 cm3 for the MR group vs. 8.0 ± 6.8 cm3 for the CT group, p = 0.643). However, the PTV was smaller in the MR group by almost 40%, with a significant difference (20.8 ± 18.8 cm3 for the MR group vs 34.1 ± 22.9 cm3 for the CT group, p = 0.005). The dosimetric parameters of the ipsilateral lungs are presented in Table 2. However, the reduction of PTV in the MR group did not result in lower low-to-moderate dose and mean dose to the ipsilateral lung compared to that in the CT group (V5 (percentage of volume receiving more than 5 Gy: 36.8 ± 10.8% vs. 32.4 ± 11.7%; V10: 28.4 ± 8.6% vs. 28.1 ± 11.1%; V20: 19.5 ± 6.5% vs. 19.0 ± 10.1%; mean dose: 644.6 ± 203.9 Gy vs. 641.5 ± 298.8 Gy for the MR group vs. CT group).
Treatment outcome
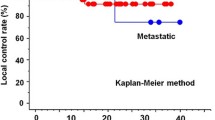
The median follow-up time was 19 months (range, 3–105 months) in the total population, 11 months (range, 3–32 months) in the MR group, and 34 months (range, 3–105 months) in the CT group. In total, 17 and 22 patients in the MR and CT groups, respectively, experienced disease recurrence during follow-up. There were 4 and 17 cases of local recurrence and 13 and 5 cases of distant recurrence in the MR and CT groups, respectively. The LC rate in the MR group was superior to that in the CT group, with borderline significance (p = 0.07). The 1-year LC rate in the MR and CT groups were 92.1 and 75.4%, respectively (Fig. 3A). There were no significant between-group differences in the 1-year PFS rate (80.2% for the MR group vs. 80.4% for the CT group, p = 0.50; Fig. 3B) and the 1-year OS rate (87.4% for the MR group and vs 87.0% for the CT group, p = 0.30; Fig. 3C).
Toxicity
The toxicities associated with SABR are summarized in Table 3. Most of the patients were tolerable to the treatment. There was no significant difference in the frequency of radiation-related toxicities between the MR and CT groups (p = 0.188). Overall, eight patients (19.5%) in the MR group experienced grade 2 toxicity. There were seven cases of radiation pneumonitis and one case of dyspnea. In the CT group, 14 patients (35%) developed grade 2 toxicity, including 10 (25.0%) patients with radiation pneumonitis, 3 (7.5%) patients with dyspnea, and 1 (2.5%) patient with rib fracture without displacement. No other toxicities of grade ≥ 3 was observed in either group. The incidence of radiation-induced toxicity is not proportional to the radiation dose to the ipsilateral lung.
Discussion
In our study, compared with the ITV generation technique, use of the MR-Linac could significantly reduce the volume of the irradiated field without compromising the LC rate during SABR for patients with NSCLC.
SABR uses tight margins to preserve normal tissue and deliver a high dose to the tumor, leading to inhomogeneous dose distributions. Respiratory motion is one of the major challenges in SABR for lung tumors (Shirato et al. 2012). Lung movement can be as high as 40 mm during free breathing (Hanley et al. 1999). Therefore, controlling lung movement is important to improve target coverage and preserve normal tissues near the target in patients with lung SABR. Respiratory motion management strategies, such as conventional margins on free-breathing CT, respiratory gating, non-invasive tumor tracking, and ITV generation using 4-D CT, are used for the management of physiologic lung movement (Brandner et al. 2017; Molitoris et al. 2018).
The conventional free-breathing CT technique uses large margins that cover the entire range of motion of the tumor. Although it is the simplest method, it leads to unnecessary radiation exposure to adjacent normal organs. The respiratory gating technique turns the beam on and off at specific breathing amplitudes or phases using a breathing monitor to predict the phase of the breathing cycle (Giraud and Houle 2013). The tumor tracking technique uses a dynamic multi-leaf collimator to continuously reshape the treatment machine apertures to follow real-time target movement (Brandner et al. 2017). An external monitoring system is also required. Although respiratory gating, tumor tracking, and ITV generation allow for lower PTV margins than in conventional margins, they do not directly monitor the internal target movement at the time of treatment (Ehrbar et al. 2017; Prunaretty et al. 2019). These strategies are assumed to involve a constant and reproducible respiratory cycle. However, variations in respiratory motion may occur cycle to cycle, and geometric and dosimetric variations may exist in the direction in which the center and boundary of the tumor move according to each respiratory cycle. Moreover, in some cases, tumor movement does not correlate with the entire lung or diaphragmatic motion (Sawant et al. 2014). Therefore, respiratory gating, tumor tracking, and ITV generation techniques can potentially underestimate true tumor movement and cause tumor excursion during treatment. This is especially true for tumors with a wide range of motion, such as those at the base of the lung (Kim et al. 2016). The most accurate way of monitoring target movement is to directly observe the tumor itself in real-time.
The real-time MR tracking technique had the advantage of increased confidence achieved by directly monitoring real-time tumor movement during treatment. During RT delivery with the MR-Linac, real-time MRI tumor tracking and gating were performed using deformable image registration-based beam control. A preview cine MRI scan was performed before the start of each treatment session. The most appropriate sagittal plane was selected for deformable image registration to preview the corresponding plane of the simulated image. The GTV and PTV contoured during radiation planning were set as the target and the boundary, respectively. During treatment, the live cine MRI scan was in turn registered as deformable, and based on that deformation, the gating target was deformed. The beam was automatically turned off if the target was outside the boundary by more than 3%. This MR tracking technique reduced the PTV because higher accuracy allowed small margins to the tumor. Only a 5–7 mm margin from the GTV was required for PTV generation in this study.
There was no significant difference in GTV between the two groups. However, PTV using the MR tracking technique was approximately 40% smaller than that using the ITV generation technique, and the difference was statistically significant. Similar results have been reported by Wojcieszynski et al. (Wojcieszynski et al. 2017). They reported a dosimetric comparison of SABR for 10 patients with lung cancer using the MR tracking technique and ITV generation technique. The PTV obtained using the ITV generation technique was twice as large as that obtained using the MR tracking technique. In another study, enlarged PTV size and lung V20 were significant risk factors that increased the incidence of pneumonitis in patients undergoing lung SABR (Matsuo et al. 2012). However, in our study, despite a smaller PTV in the MR group, the dosimetric parameters for the ipsilateral lung were not significantly different between the groups. This result can be largely attributed to the underestimation of the entire lung volume in patients in the MR group. Because the MRI scans were performed in the end-exhale breathing hold position, the dosimetric parameters of the lung in the MR group may have been slightly overestimated.
Differences in the total lung volume according to the respiratory cycle have been reported by Yamada et al. (Yamada et al. 2020). They found that the total lung volume was 50% higher during inspiration than that during expiration. Numerous studies have observed that compared with the free breathing, the deep inspiration breath-hold has better dosimetric benefit of the mean dose and V20 of the ipsilateral lung in for breast cancer (Remouchamps et al. 2003; Nissen and Appelt 2013; Oechsner et al. 2019). However, a deep inspiration breath-hold is not commonly used in SABR using traditional CT-based treatment because of concerns regarding reproducibility and the potential for geometric misses (Wojcieszynski et al. 2017). Target movement can directly be observed during treatment in SABR using an MR-Linac. Thus, it is possible to directly assess whether the deep inspiration breath-hold is repeatedly reproduced, and the target is placed in an appropriate position. The use of MR-Linac equipment with a deep inspiration breath-hold could be expected to increase lung volume and decrease the mean lung dose and lung V20.
The current study found no significant difference in the treatment outcomes of the NSCLC patients treated with SABR using the MR tracking technique and those of patients treated with the ITV generation technique using 4-D CT. The 1-year LC and OS rates for the MR group were 92.1 and 87.4%, respectively, while those for the CT group were 75.4 and 87.0%, respectively. The MR group showed superior LC outcomes, although the difference showed borderline significance. We assume that accurate targeting with real-time MR tracking contributed to this result. The difference between the two groups became more profound with longer follow-up. Given the difference in disease entities between the two groups, distant metastasis was not considered as an endpoint. However, the distant metastasis outcomes were also consistent with those in previously published reports (Temming et al. 2018; Videtic et al. 2019; Lee et al. 2021): only 17% of patients had distant metastasis despite the relatively high proportion of patients with advanced disease. Treatment toxicity in the current study was limited to grades 1 and 2, and most cases were radiation pneumonitis that rapidly improved with steroid treatment. None of the patients had grade ≥ 3 toxicity. The overall grade 2 toxicity rate in the MR group was 19.5% (8/42 patients), and there was no significant between-group difference. Considering previous studies on SABR for NSCLC (Zhao et al. 2016; Murray et al. 2017), this is considered an acceptable toxicity rate.
This study has some limitations. The dataset included a heterogeneous population of patients. The disease extent varied, and the patients received differing treatments prior to SABR. This might have affected treatment outcomes (e.g., PFS and OS) in this study. In addition, this study included a relatively small number of patients and a short follow-up duration. These limitations were inevitable because our institution adopted MR-Linac equipment only 3 years ago. Despite these limitations, this study suggested the possibility of real-time tracking using the MR-Linac for SABR in patients with NSCLC.
In conclusion, MR-Linac in SABR for lung cancer reduces the volume of the irradiated field without compromising the local control rate. With further follow-up, recruitment of additional patients, and improvements in our planning ability with more experience, we anticipate to report better results in the future.
Availability of data and material
All data generated or analyzed during this study are included in this article.
References
Baumann P, Nyman J, Hoyer M et al (2009) Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 27:3290–3296. https://doi.org/10.1200/JCO.2008.21.5681
Brandner ED, Chetty IJ, Giaddui TG, Xiao Y, Huq MS (2017) Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: a review from NRG oncology. Med Phys 44:2595–2612. https://doi.org/10.1002/mp.12227
Ehrbar S, Johl A, Tartas A et al (2017) ITV, mid-ventilation, gating or couch tracking - a comparison of respiratory motion-management techniques based on 4D dose calculations. Radiother Oncol 124:80–88. https://doi.org/10.1016/j.radonc.2017.05.016
Giraud P, Houle A (2013) Respiratory gating for radiotherapy: main technical aspects and clinical benefits. ISRN Pulmonology 2013:519602. https://doi.org/10.1155/2013/519602
Hanley J, Debois MM, Mah D et al (1999) Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys 45:603–611. https://doi.org/10.1016/s0360-3016(99)00154-6
Hof H, Muenter M, Oetzel D, Hoess A, Debus J, Herfarth K (2007) Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC). Cancer 110:148–155. https://doi.org/10.1002/cncr.22763
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC (2013) Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 143:e278S-e313S. https://doi.org/10.1378/chest.12-2359
Kim J, Wu Q, Zhao B, Wen N, Ajlouni M, Movsas B, Chetty IJ (2016) To gate or not to gate - dosimetric evaluation comparing Gated vs. ITV-based methodologies in stereotactic ablative body radiotherapy (SABR) treatment of lung cancer. Radiat Oncol 11:125. https://doi.org/10.1186/s13014-016-0699-2
Koto M, Takai Y, Ogawa Y et al (2007) A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 85:429–434. https://doi.org/10.1016/j.radonc.2007.10.017
Lee P, Loo BW Jr, Biswas T et al (2021) Local control after stereotactic body radiation therapy for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 110:160–171. https://doi.org/10.1016/j.ijrobp.2019.03.045
Matsuo Y, Shibuya K, Nakamura M et al (2012) Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 83:e545-549. https://doi.org/10.1016/j.ijrobp.2012.01.018
Molitoris JK, Diwanji T, Snider JW 3rd et al (2018) Advances in the use of motion management and image guidance in radiation therapy treatment for lung cancer. J Thorac Dis 10:S2437–S2450. https://doi.org/10.21037/jtd.2018.01.155
Murray P, Franks K, Hanna GG (2017) A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. Br J Radiol 90:20160732. https://doi.org/10.1259/bjr.20160732
Nagata Y, Takayama K, Matsuo Y et al (2005) Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 63:1427–1431. https://doi.org/10.1016/j.ijrobp.2005.05.034
Nissen HD, Appelt AL (2013) Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol 106:28–32. https://doi.org/10.1016/j.radonc.2012.10.016
Oechsner M, Dusberg M, Borm KJ, Combs SE, Wilkens JJ, Duma MN (2019) Deep inspiration breath-hold for left-sided breast irradiation: analysis of dose-mass histograms and the impact of lung expansion. Radiat Oncol 14:109. https://doi.org/10.1186/s13014-019-1293-1
Onishi H, Shirato H, Nagata Y et al (2007) Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2:S94-100. https://doi.org/10.1097/JTO.0b013e318074de34
Palma DA, Olson R, Harrow S et al (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393:2051–2058. https://doi.org/10.1016/s0140-6736(18)32487-5
Park K, Vansteenkiste J, Lee KH et al (2020) Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol 31:191–201. https://doi.org/10.1016/j.annonc.2019.10.026
Prunaretty J, Boisselier P, Ailleres N et al (2019) Tracking, gating, free-breathing, which technique to use for lung stereotactic treatments? A dosimetric comparison. Rep Pract Oncol Radiother 24:97–104. https://doi.org/10.1016/j.rpor.2018.11.003
Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW (2003) Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 55:392–406. https://doi.org/10.1016/s0360-3016(02)04143-3
Sawant A, Keall P, Pauly KB et al (2014) Investigating the feasibility of rapid MRI for image-guided motion management in lung cancer radiotherapy. Biomed Res Int 2014:485067. https://doi.org/10.1155/2014/485067
Shibamoto Y, Hashizume C, Baba F et al (2015) Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 10:960–964. https://doi.org/10.1097/jto.0000000000000525
Shinde A, Li R, Kim J, Salgia R, Hurria A, Amini A (2018) Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin Oncol 45:210–219. https://doi.org/10.1053/j.seminoncol.2018.06.002
Shirato H, Onimaru R, Ishikawa M et al (2012) Real-time 4-D radiotherapy for lung cancer. Cancer Sci 103:1–6. https://doi.org/10.1111/j.1349-7006.2011.02114.x
Shultz DB, Filippi AR, Thariat J, Mornex F, Loo BW Jr, Ricardi U (2014) Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. J Thorac Oncol 9:1426–1433. https://doi.org/10.1097/jto.0000000000000317
Temming S, Kocher M, Stoelben E et al (2018) Risk-adapted robotic stereotactic body radiation therapy for inoperable early-stage non-small-cell lung cancer. Strahlenther Onkol 194:91–97. https://doi.org/10.1007/s00066-017-1194-x
Timmerman RD, Paulus R, Pass HI et al (2018) Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG oncology RTOG 0618 trial. JAMA Oncol 4:1263–1266. https://doi.org/10.1001/jamaoncol.2018.1251
Videtic GM, Paulus R, Singh AK et al (2019) Long-term follow-up on NRG oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys 103:1077–1084. https://doi.org/10.1016/j.ijrobp.2018.11.051
Wojcieszynski AP, Hill PM, Rosenberg SA et al (2017) Dosimetric comparison of real-time MRI-guided tri-cobalt-60 versus linear accelerator-based stereotactic body radiation therapy lung cancer plans. Technol Cancer Res Treat 16:366–372. https://doi.org/10.1177/1533034617691407
Yamada Y, Yamada M, Chubachi S et al (2020) Comparison of inspiratory and expiratory lung and lobe volumes among supine, standing, and sitting positions using conventional and upright CT. Sci Rep 10:16203. https://doi.org/10.1038/s41598-020-73240-8
Zhao J, Yorke ED, Li L et al (2016) Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys 95:1357–1366. https://doi.org/10.1016/j.ijrobp.2016.03.024
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Y-KK conceptualized and designed the work. Y-KK and HJK collected data on patients and treatments. HJK wrote the main manuscript text. HJK and SJL prepared figure. All authors reviewed the manuscript. Y-KK made final approvement.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
This study was approved by the institutional review board of the Catholic Medical Center ethics committee (IRB No. OC22RISI0014).
Consent to participate
As a retrospective study using medical records that do not contain patient's personal information, the consent process was exempted.
Consent for publication
As a retrospective study using medical records that do not contain patient's personal information, the consent process was exempted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, H.J., Kwak, YK., Kim, M. et al. Application of real-time MRI-guided linear accelerator in stereotactic ablative body radiotherapy for non-small cell lung cancer: one step forward to precise targeting. J Cancer Res Clin Oncol 148, 3215–3223 (2022). https://doi.org/10.1007/s00432-022-04264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04264-y