Abstract
Purpose
Radiotherapy is the mainstay for treating brain metastasis (BM). The objective of this study is to evaluate the overall survival (OS) of patients with BM of lung cancer treated with different radiotherapy modalities.
Methods
Patients with BM of lung cancer who underwent radiotherapy between July 2007 and November 2017 were collected, and their baseline demographics, clinicopathological characteristics and treatments were recorded. Survival was estimated by the Kaplan–Meier method and compared by using the log-rank test. Univariate and multivariate analysis of the prognostic factors were performed using the Cox proportional hazard regression model.
Results
A total of 144 patients were enrolled, of whom 77 underwent whole-brain radiotherapy (WBRT), 39 underwent whole brain radiotherapy with consecutive boost (WBRT + boost), and 28 underwent integrated simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT). The OS in SIB-IMRT group was significantly longer than that in WBRT group (median OS 14 (95% confidence interval [CI] 8.8–19.1) vs.7 (95% CI 5.5–8.5) months, log-rank p < 0.001) and WBRT + boost group (median OS: 14 (95% CI 8.8–19.1) vs.11 (95% CI 8.3–13.7) months, log-rank p = 0.037). Multivariable analysis showed that mortality risk of patients treated with SIB-IMRT decrease by 56, 59, 64 and 64% in unadjusted model (hazard ratio [HR] = 0.44; 95% CI 0.28–0.70, p < 0.001), model 1 (HR = 0.41; 95% CI 0.26–0.65, p < 0.001), model 2 (HR = 0.36; 95% CI 0.21–0.61, p < 0.001), and model 3 (HR = 0.36; 95% CI 0.21–0.61, p < 0.001).
Conclusions
For patients with BM of lung cancer, SIB-IMRT seems to be associated with a more favorable prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, lung cancer is one of the malignant tumors with the highest morbidity and mortality worldwide (Siegel et al. 2018). Brain metastasis (BM) is particularly common in lung cancer, occurring in approximately 30–50% of patients, even over 50% in small cell lung cancer (SCLC) (Brown et al. 2016; Preusser et al. 2018). BM is a vital lethal in lung cancer patients, it could result in a dismal prognosis which is only 2.3–7.1 months of median survival time depending on subtype (Gaspar et al. 1997). But due to the restriction of most drugs to cross the blood brain barrier and the invasion of surgical resection, radiotherapy had always been a more appropriate treatment to BM. Whole brain radiotherapy (WBRT) had been established as the most fundamental modality for most patients with BM, especially in patients who have numerous BMs, it could prolong the survival time of 4–7 months (Andrews et al. 2004). Some studies had revealed that whole brain radiotherapy with consecutive boost (WBRT + boost) could reduce local recurrence rate, improve the quality of life (QoL), and further prolong the survival time (Dobi et al. 2020; Sperduto et al. 2014). In recent years, simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT) had been applied to treat BM increasingly, but its real prognostic value, especially in the setting of lung cancer, remained unclear. And it deserves to be investigated that whether SIB-IMRT would bring further survival benefit compared with WBRT and WBRT + boost. Therefore, to provide evidence for the option of radiotherapy modalities for patients with BM and potentially guide future research in clinical practice, we carried out this study to directly evaluate the survival differences among these three modalities, and analyzed other potential prognostic factors associated with overall survival (OS).
Patients and methods
Study patients
We retrospectively reviewed 168 patients with brain metastases of lung cancer underwent radiotherapy at our hospital between July 2007 and November 2017. All patients were native Chinese (Asians). Patients were included who were diagnosed with histopathological primary lung cancer and developed subsequent or simultaneous BM confirmed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) treated with either WBRT, WBRT + boost and SIB-WBRT. While the exclusion criteria were as follows: (1) patients who underwent either stereotactic radiosurgery (SRS), or stereotactic radiation therapy (SRT), or Gamma Knife and cyberknife; (2) patients who underwent any form of intracranial radiotherapy like prophylactic cranial irradiation (PCI) before; (3) patients with metastatic lung tumor or other primary malignant tumor; (4) patients with leptomeningeal metastases; (5) the number of BMs was more than ten. Lastly, 144 patients were enrolled in our study.
We collected baseline demographic, clinicopathologic characteristics and treatment-related variables including age at first BM diagnosis, gender, smoking status, lung cancer histopathology, region (urban or rural), symptoms at first BM diagnosis, number of BMs, Karnofsky Performance Status (KPS), radiotherapy modalities, total dose and dose-fractionation regimens, extracranial metastasis status, treatment to primary lung tumor, date of lung cancer diagnosis and date of first BM diagnosis. We obtained recursive partitioning analysis (RPA) class, diagnosis-specific graded prognostic assessment (DS-GPA) score and brain metastasis-free interval (BMFI) by using foregoing data. Furthermore, we stratified patients into three DS-GPA classes, which were class 1 (0–1.0 points), class 2 (1.5–2.5 points) and class 3 (3.0–4.0 points).
Follow-up
The follow-up data were collected by the hospital official contacting with patients or their relatives by telephone or obtained from inpatient medical records. Each enrolled patient had complete inpatient medical records. OS was calculated as the time interval from the date of radiotherapy to BM to the date of either cancer-related death or the last follow-up (May 2019). BMFI was calculated as the time interval from the date of lung cancer diagnosis to the date of first BM diagnosis. The median follow-up was 10 (range 1–77) months.
Radiotherapy treatment
Patients were positioned supine in a custom-made thermoplastic mask for reproducibility and received a non-enhanced CT simulation scan including the entire head with 3 mm slice thickness. And the CT images were then fused with contrast-enhanced T1-weighted MRI sequence if any. Gross Tumor Volume (GTV) was defined as BMs, Clinical Target Volume (CTV) was defined as whole brain tissue, Planning Target Volume (PTV) was defined as CTV plus 3 mm margin, Planning Gross Target Volume (PGTV) was defined as GTV plus 3 mm margin. Routine organs at risk (OARs) including brainstem, eyes, lens, optic chiasma, and optic nerves were delineated. All treatment planning was delivered by IMRT. The total biological effective dose (BED) was calculated as equivalent dose in 2 Gy fractions (EQD2) (α/β = 2 Gy for normal brain and 10 Gy for BM). Detailed dose are as follows: PTV: 40.0 Gy/20 f (EQD2 = 40.0 Gy) (5 fractions/week) in WBRT group; PTV: 40.0 Gy/20 f (EQD2 = 40.0 Gy) (5 fractions/week), PGTV: 16.0–26.0 Gy/6–13 f (EQD2 = 19.6–26.0 Gy) (5 fractions/week) in WBRT + boost group; and PTV: 36.0–41.4 Gy/ 20.0–24.0 f (EQD2 = 35.40–40.45 Gy) (5 fractions/week), PGTV: 56.0–62.4 Gy/20–24 f (EQD2 = 59.73–65.52 Gy) (5 fractions/week) in SIB-IMRT group.
Statistical analysis
Descriptive characteristics were calculated using 2-tailed \(\chi\)2 test (or Fisher exact test), and continuous variables were calculated using t test. Survival were plotted by means of the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate survival analyses were performed for identifying the radiotherapy modality and other potential prognostic factors using the Cox proportional hazards models. There were four models we constructed: unadjusted model, no covariates were adjusted; model 1, adjusted for age and gender; model 2, covariates were included as potential confounders in the final models if they changed the estimates of radiotherapy modality on mortality risk by more than 10%; model 3, covariates which were significantly associated with mortality risk in univariable Cox regression analyses were included. We would like to use these models with different covariates and potential confounders to verify whether the results of them are identical. All statistical analyses and visualizations were performed by using R programming version 3.4.3 (R development Core Team, Vienna, Austria). In all levels, 2-tailed p < 0.05 was considered statistically significant.
Results
Patient and treatment characteristics
The baseline characteristics are summarized in Table 1. Among 144 enrolled patients, 77 patients underwent WBRT, 39 patients underwent WBRT + boost, and 28 patients underwent SIB-IMRT. The mean patient age was 59 years (standard deviation [SD], ± 10.5) at first BM diagnosis; 63.9% (92/144) were under 65 years. 62.5% (90/144) patients were male, 75.0% (108/144) were urban, 52.8% (76/144) were adenocarcinoma, and 51.4% (74/144) were smoking. 61.8% (89/144) patients had no initial symptom, 75.0% (108/144) had multiple BMs, 63.2% (91/144) had no extracranial metastasis. 65.3% (90/144) patients underwent systematic chemotherapy, 20.8% (30/144) underwent surgical pulmonary resection, and 25.0% (36/144) underwent thoracic radiotherapy. 21.5% (31/144) patients belonged to RPA 1 class, 36.8% (53/144) to RPA 2 class, and 41.7% (60/144) to RPA 3 class. 31.3% (45/144) patients belonged to DS-GPA 1 class, 55.6% (80/144) to DS-GPA 2 class, and 13.2% (19/144) to DS-GPA 3 class. The median BMFI was 6 months, therefore, we stratified patients into three classes as shown.
Survival outcomes
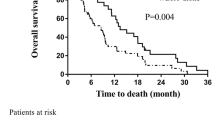
The OS rate at 1- and 2- year were as follows: 32.5 and 5.7% in WBRT group, 48.7 and 6.8% in WBRT + boost group, 64.3 and 35.4% in SIB-IMRT group. The median OS in three groups were 7 (95% CI 5.5–8.5), 11 (95% CI 8.3–13.7) and 14 (95% CI 8.8–19.1) months respectively. The survival differences among three groups were significant (log-rank p = 0.002). Besides, SIB-IMRT showed a survival benefit compared with both WBRT group (log-rank p < 0.001) and WBRT + boost group (log-rank p = 0.037) (Fig. 1).
Analysis of survival factors
In univariate Cox regression analysis, gender (HR = 1.56; 95% CI 1.09–2.24, p = 0.016), smoking status (HR = 1.60; 95% CI 1.13–2.28, p = 0.009), squamous carcinoma (HR = 1.63; 95% CI 1.10–2.40, p = 0.014), extracranial metastasis (HR = 1.60; 95% CI 1.13–2.27, p = 0.009), RPA class (class 2, p = 0.002; class 3, p < 0.001), DS-GPA class (class 2, p < 0.001; class 3, p < 0.001), 0 < BMFI ≤ 6 months (HR = 1.83; 95% CI 1.12–2.99, p = 0.016) and SIB-IMRT (HR = 0.44; 95% CI 0.28–0.70, p < 0.001) were significantly associated with OS (Table 2). To further analyze the prognostic impact of radiotherapy modalities, three multivariate-adjusted Cox regression models were constructed. Model 1 was adjusted for age and gender. Model 2 was adjusted for gender, smoking status, lung cancer histology, extracranial metastasis status, RPA class, DS-GPA class and BMFI. Model 3 was adjusted for gender, age, smoking status, lung cancer histology, extracranial metastasis status, RPA class, DS-GPA class and BMFI. It was showed that mortality risk of patients treated with SIB-IMRT decrease 59, 64 and 64% in model 1 (HR = 0.41; 95% CI 0.26–0.65, p < 0.001), model 2 (HR = 0.36; 95% CI 0.21–0.61, p < 0.001), and model 3 (HR = 0.36; 95% CI 0.21–0.61, p < 0.001) (Fig. 2). Besides, multivariate analysis in model 2 and model 3 showed that age, smoking status, RPA class, DS-GPA class, and 0 < BMFI ≤ 6 months were independent prognostic factors for OS (Table 2).
Discussion
For patients with malignant tumor, the presence of BM is associated with a dismal prognosis, and it means an advanced stage (clinical stage IV). Hence, the treatment planning should focus on maximizing the survival time, reducing neurological deteriorations, and improving the QoL. Previous studies showed that effective treatment included but were not limited to surgical resection, WBRT and SRS/SRT single or combined. Among them, SRS could achieve a similar outcome compared with surgery resection and with a higher local control rate (Muacevic et al. 2008). The RTOG 9502 randomized trial had reached a consensus that the addition of SRS to WBRT resulted in better local control rate and higher KPS score in patients with 1–3 BMs, and with a survival benefit in patients with single BM (Andrews et al. 2004). By analyzing the results of RTOG 9502, Sperduto et al. concluded that the addition of SRS to WBRT offer a significant survival advantage in patients with 1–3 BMs and high GPA score (Sperduto et al. 2014). However, due to the necessities of an independent treatment planning system and relatively high technical requirements, as well as greater cost, it is difficult for SRS to be applied routinely in many institutions. In recent years, many clinicians were attempting to find a way to replace WBRT + SRS, and SIB-IMRT was the strongest competitor. To date, several studies had preliminarily confirmed the dosimetric advantage and radiotherapy-related safety of SIB-IMRT compared with WBRT + SRS (Borghetti et al. 2016; Ferro et al. 2017; Giaj Levra et al. 2016; Oehlke et al. 2015; Yang et al. 2017). But the study of the impact on prognosis remained limited. Herein, we retrospectively compared the effects of WBRT, WBRT + boost and SIB-IMRT on survival.
In this study, we found that OS in SIB-IMRT group were significantly longer than those in WBRT and WBRT + boost groups. This finding was in line with a previous study, but inadequately, they did not directly compare the survival difference between WBRT + boost and SIB-IMRT (Dobi et al. 2020). Another study demonstrated SIB-IMRT could prolong the survival time in patients with brain oligometastases, but there were only 29 patients enrolled and no statistical significance in lung cancer subgroup (Tiwari et al. 2015). In addition, the results of our study showed that OS in SIB-IMRT group were significantly longer than WBRT + boost group. To the best of our knowledge, this might be a novel finding. According to linear quadratic model (L-Q model, BED = nd [1 + d/(α/β)]), different BED was caused by different total dose and dose-fractionation regimens or both. In general, this survival difference was due to different BED. But in our study, WBRT + boost group and SIB-IMRT group received a quite similar BED in both whole brain (40.0 Gy vs. 35.40–40.45 Gy, based on EQD2) and BMs (59.6–66.0 Gy vs. 59.73–65.52 Gy, based on EQD2). And there was no interval between WBRT planning and subsequent boost delivery, that could reduce the influence from 4Rs (repair of sublethal damage, reoxygenation, repopulation and redistribution). Taken together, we speculated this could be attributed to the additional dose to critical structures caused by an individual subsequent boost planning overlapping a completed whole brain dose. On the other hand, SIB-IMRT technique had much steeper dose gradients and optimal dose distribution, which would reduce the morbidity of radiation-related damage (Borghetti et al. 2016).
We also analyzed potential prognostic factors in patients receiving radiotherapy to BM of lung cancer. Historically, several factors had been proven to be associated with survival which including age, KPS, treatment, neurocognitive status, extracranial metastasis status, number of BMs, RPA class and GPA score (Sperduto et al. 2017; Tsakonas et al. 2018). Among them, RPA class and GPA index were widely practiced. RPA index was first proposed by Gaspar et al. in 1997, in which, patients with BMs were divided into three classes according to KPS (< 70, ≥ 70), age (< 65 years, ≥ 65 years), primary tumor control status and extracranial metastasis status (Gaspar et al. 1997). In subsequent studies, RPA index was reconfirmed to be valid and reliable (Gaspar et al. 2000; Videtic et al. 2007). In 2008, Sperduto et al. considered that there were still some shortcomings in RPA index, and established GPA index according to age (< 50 years, 50–59 years, > 65 years), KPS (< 70, 70–80, 90–100), number of BMs (> 3, 2–3, 1), and extracranial metastasis status (Sperduto et al. 2008). Thereafter, this team presented DS-GPA index in 2012 and GPA for lung cancer using molecular markers (Lung-molGPA) index in 2017 as update (Sperduto et al. 2012, 2017). In Lung-molGPA index, gene status (EGFR and ALK alterations in patients with adenocarcinoma) were included. We still use DS-GPA index in this study because patients with squamous carcinoma and small cell cancer were also enrolled. The number of BMs, as mentioned above, was usually considered as a prognostic factor as well as an important component in GPA index. But unexpectedly, we did not prove it is one of the predictors of survival both in univariate and multivariate analyses. Some researchers revealed that total volume of BMs might be a greater factor rather than the number of BMs, which would be meaningful to traditional views and GPA index (Routman et al. 2018; Yamamoto et al. 2017).
We found 0 < BMFI ≤ 6 months was one of the independent prognostic factors of survival. The patients in BMFI = 0 group were diagnosed with BM and lung cancer simultaneously. Median OS of this group was obviously longer than the other two groups. The reason might be that most patients in this group were diagnosed and treated earlier than diagnosed lung cancer patients who did not received surveillance head CT/MRI. In BMFI > 0 patients, longer BMFI was associated with favorable prognosis, which was consistent with a recent study (Smith et al. 2019). We also found squamous carcinoma was associated with poor prognosis compared with adenocarcinoma. We considered it was probably because there were 17 adenocarcinoma patients underwent molecular targeted therapy. As is well known, numerous molecular targeted agents were confirmed to be able to prolong the survival time in NSCLC patients with BM in past decade (Cross et al. 2014; Iuchi et al. 2013; Mok et al. 2017; Nishio et al. 2018; Wu et al. 2015). We hope that more future clinical trials will continue to examine whether molecular targeted therapy combined with BM radiotherapy could bring greater survival benefits for patients. Moreover, radiomics is evolving rapidly in diagnosis and treatment of BM. It could distinguish treatment-related changes from BM recurrence, distinguish BM from glioblastoma, predict BM origin (in cancer of unknown primary, CUP), and predict treatment response (Lohmann et al. 2020a, b). We look forward that radiomics could be able to predict which kind of patients with BM would benefit from WBRT + boost and which kind of patients would benefit from SIB-IMRT.
While there were some significant findings in this study, some limitations could not be neglected. First and notably, the results were concluded by a single-institution retrospective observational study. Though we had designed the inclusion criteria and exclusion criteria relatively strictly, selection bias and heterogeneity in enrolled patients were inevitable because of the retrospective nature. Second, the sample size especially in SIB-IMRT group was small, we were unable to further control the potential confounders by statistical methods like inverse probability of treatment weighting. Third, we included eight covariates in model 3, hence we could not exclude the possibility of overfitting given the limited simple size. Fourth, the data spanned the period from 2007 to 2017, we acknowledged our inability to assess radiotherapy toxicity and local tumor control among three modalities. Considering these limitations, our study warrants a large sample size multi-institution randomized clinical trial to reappraise.
Conclusions
Although deficient and limited, the results of our study showed that SIB-IMRT is one of the important and independent prognostic factors for patients with BMs of lung cancer, and SIB-IMRT seems to be associated with a more favorable prognosis compared with both WBRT and WBRT + boost.
References
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Borghetti P, Pedretti S, Spiazzi L, Avitabile R, Urpis M, Foscarini F, Tesini G, Trevisan F, Ghirardelli P, Pandini SA, Triggiani L, Magrini SM, Buglione M (2016) Whole brain radiotherapy with adjuvant or concomitant boost in brain metastasis: dosimetric comparison between helical and volumetric IMRT technique. Radiat Oncol 11:59
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ et al (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4(9):1046–1061. https://doi.org/10.1158/2159-8290.CD-14-0337
Dobi Á, Fodor E, Maráz A, Együd Z, Cserháti A, Tiszlavicz L, Reisz Z, Barzó P, Varga Z, Hideghéty K (2020) Boost irradiation integrated to whole brain radiotherapy in the management of brain metastases. Pathol Oncol Res 26(1):149–157
Ferro M, Chiesa S, Macchia G, Cilla S, Bertini F, Frezza G, Farioli A, Cammelli S, Balducci M, Ianiro A, Angelini AL, Compagnone G, Valentini V, Deodato F, Morganti AG (2017) Intensity modulated radiation therapy with simultaneous integrated boost in patients with brain oligometastases: a phase 1 study (ISIDE-BM-1). Int J Radiat Oncol Biol Phys 97:82–90
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Gaspar LE, Scott C, Murray K, Curran W (2000) Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 47:1001–1006
Giaj Levra N, Sicignano G, Fiorentino A, Fersino S, Ricchetti F, Mazzola R, Naccarato S, Ruggieri R, Alongi F (2016) Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for brain metastases: a dosimetric volumetric-modulated arc therapy study. Radiol Med 121:60–69
Iuchi T, Shingyoji M, Sakaida T, Hatano K, Nagano O, Itakura M et al (2013) Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 82(2):282–287. https://doi.org/10.1016/j.lungcan.2013.08.016
Lohmann P, Galldiks N, Kocher M, Heinzel A, Filss CP, Stegmayr C et al (2020a) Radiomics in neuro-oncology: basics, workflow, and applications. Methods. https://doi.org/10.1016/j.ymeth.2020.06.003
Lohmann P, Kocher M, Ruge MI, Visser-Vandewalle V, Shah NJ, Fink GR et al (2020b) PET/MRI Radiomics in patients with brain metastases. Front Neurol 11:1. https://doi.org/10.3389/fneur.2020.00001
Mok TS, Y-L W, M-J A, Garassino MC, Kim HR, Ramalingam SS, et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640. https://doi.org/10.1056/NEJMoa1612674
Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW (2008) Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol 87:299–307
Nishio M, Nakagawa K, Mitsudomi T, Yamamoto N, Tanaka T, Kuriki H et al (2018) Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 121:37–40. https://doi.org/10.1016/j.lungcan.2018.04.015
Oehlke O, Wucherpfennig D, Fels F, Frings L, Egger K, Weyerbrock A, Prokic V, Nieder C, Grosu AL (2015) Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: local tumour control and survival. Strahlenther Onkol 191:461–469
Preusser M, Winkler F, Valiente M, Manegold C, Moyal E, Widhalm G, Tonn JC, Zielinski C (2018) Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: summary of a multidisciplinary roundtable discussion. ESMO Open 3:e000262
Routman DM, Bian SX, Diao K, Liu JL, Yu C, Ye J, Zada G, Chang EL (2018) The growing importance of lesion volume as a prognostic factor in patients with multiple brain metastases treated with stereotactic radiosurgery. Cancer Med 7:757–764
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Smith DR, Bian Y, Wu CC, Saraf A, Tai CH, Nanda T, Yaeh A, Lapa ME, Andrews J, Cheng SK, McKhann GM, Sisti MB, Bruce JN, Wang T (2019) Natural history, clinical course and predictors of interval time from initial diagnosis to development of subsequent NSCLC brain metastases. J Neurooncol 143:145–155
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425
Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, Bahary JP, Souhami L, Won M, Mehta M (2014) Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1–3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 90:526–531
Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, Sneed P, Boyle J, Kirkpatrick JP, Mak KS, Shih HA, Engelman A, Roberge D, Arvold ND, Alexander B, Awad MM, Contessa J, Chiang V, Hardie J, Ma D, Lou E, Sperduto W, Mehta MP (2017) Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 3:827–831
Tiwari V, Pande SC, Verma K, Goel S (2015) Simultaneous integrated boost with intensity modulated radiation therapy in brain oligometastases: a feasible technique for developing countries. South Asian J Cancer 4:11–14
Tsakonas G, Hellman F, Gubanski M, Friesland S, Tendler S, Lewensohn R, Ekman S, de Petris L (2018) Prognostic factors affecting survival after whole brain radiotherapy in patients with brain metastasized lung cancer. Acta Oncol 57:231–238
Videtic GM, Adelstein DJ, Mekhail TM, Rice TW, Stevens GH, Lee SY, Suh JH (2007) Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys 67:240–243
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z et al (2015) First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label ENSURE study. Ann Oncol 26(9):1883–1889. https://doi.org/10.1093/annonc/mdv270
Yamamoto M, Serizawa T, Higuchi Y, Sato Y, Kawagishi J, Yamanaka K, Shuto T, Akabane A, Jokura H, Yomo S, Nagano O, Aoyama H (2017) A Multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): irradiation-related complications and long-term maintenance of Mini-Mental State Examination Scores. Int J Radiat Oncol Biol Phys 99:31–40
Yang J, Zhan W, Zhang H, Song T, Jia Y, Xu H, Lin B, Lv S, Liang X (2017) Intensity-modulated radiation therapy for patients with 1 to 3 brain metastases in recursive partitioning analysis class 3. Medicine (Baltimore) 96:e7715
Funding
The authors did not receive any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This single-institution retrospective study was conducted in accordance with ethical standards of Helsinki Declaration and its amendments. Meanwhile, this study was authorized by Fourth Affiliated Hospital of China Medical University ethics committee as well.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, TQ., Li, X., Zhong, WS. et al. Brain metastases of lung cancer: comparison of survival outcomes among whole brain radiotherapy, whole brain radiotherapy with consecutive boost, and simultaneous integrated boost. J Cancer Res Clin Oncol 147, 569–577 (2021). https://doi.org/10.1007/s00432-020-03359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03359-8