Abstract
Purpose
Pancreatic adenocarcinoma (PAC) represents one of the most fatal types of cancer with an exceptionally poor prognosis, underscoring the need for improved diagnostic and treatment approaches. An over-expression of somatostatin receptors (SST) as well as of the chemokine receptor CXCR4 has been shown for many tumour entities. Respective expression data for PAC, however, are scarce and contradictory.
Methods
Overall, 137 tumour samples from 70 patients, 26 of whom were diagnosed with PAC and 44 with pancreatic neuroendocrine tumour (PanNET), were compared in terms of SST and CXCR4 expression by immunohistochemical analysis using well-characterized rabbit monoclonal antibodies.
Results
Only SST1 and CXCR4 expression was detected in PAC tumours, with SST1 present in 42.3% and CXCR4 in 7.7% of cases. However, the overall staining intensity was very weak. In contrast to the tumour cells, in many PAC cases, tumour capillaries exhibited strong SST3, SST5, or CXCR4 expression. In PanNETs, SST2 was the most-prominently expressed receptor, observed in 75.0% of the tumours at medium–strong intensity. SST5, SST1, and CXCR4 expression was detected in 20.5%, 15.9%, and 11.4% of PanNET cases, respectively, but the staining intensity was only weak. SST2 positivity in PanNET, but not in PAC, was associated with favourable patient outcomes.
Conclusions
SST or CXCR4 expression in PAC is clearly of no therapeutic relevance. However, indirect targeting of these tumours via SST3, SST5, or CXCR4 on tumour microvessels may represent a promising additional therapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic adenocarcinoma (PAC) is the 12th most-common cancer in men and 11th most-common cancer in women worldwide, with 2018 incidence rates of 5.5 per 100,000 men and 4.0 per 100,000 women (Ilic and Ilic 2016; Bray et al. 2018; Rawla et al. 2019). Median age at diagnosis is about 70 years, and besides advanced age, PAC risk factors include genetic susceptibility, tobacco smoking, heavy alcohol consumption, dietary factors (e.g., increased consumption of red or processed meat), obesity, and diabetes mellitus (Ryan et al. 2014; Ilic and Ilic 2016; Kamisawa et al. 2016; Simoes et al. 2017; Rawla et al. 2019). Consequently, PAC incidence rates are higher in developed countries such as in North America and Europe compared with countries in Africa or Asia (Ilic and Ilic 2016; Kamisawa et al. 2016; Bray et al. 2018; Rawla et al. 2019). PAC is the seventh leading cause of cancer-related deaths worldwide and associated with an extremely poor prognosis (Ilic and Ilic 2016; Bray et al. 2018; Rawla et al. 2019). Overall, 5-year survival of PAC patients is only 9% and median survival ranges from 2 years for patients with local and resectable tumours to only a few months for patients with advanced disease (Ilic and Ilic 2016; Rawla et al. 2019). Reasons for this devastating situation are the lack of effective treatment options, early local recurrence, and absence of useful biomarkers that enable early detection of the tumour.
At diagnosis, only 20% of PAC patients are eligible for surgery. As adjuvant therapy or in the palliative situation combination chemotherapy regimens are recommended, primarily FOLFIRINOX (5-fluorouracil, folinic acid, irinotecan, oxaliplatin) or gemcitabine/nanoparticle albumin-bound (nab)-paclitaxel, depending on the patient’s age, comorbidities and health status. In the palliative situation, these therapies are associated with median overall survival of 11.1 or 8.5 months, respectively, which is still quite poor (Teague et al. 2015; Kamisawa et al. 2016; Waidmann et al. 2018). Therefore, new therapeutic options as well as methods that enable early detection of PAC are desperately needed.
Many tumour entities exhibit an over-expression of receptors for regulatory peptides, which can serve as the molecular basis for targeted diagnostics and treatment modalities. Somatostatin receptors (SSTs), for example, are well known for their over-expression, e.g., in well-differentiated G1 or G2 gastroenteropancreatic neuroendocrine tumours (GEP-NET) (Kaemmerer et al. 2015a; Mai et al. 2019), which enables functional imaging of these tumours by scintigraphy or PET/CT in addition to pharmacotherapy or peptide-receptor radionuclide therapy (PRRT) with (radiolabelled) somatostatin analogues (Smit Duijzentkunst et al. 2017). The chemokine receptor CXCR4, in contrast, has been shown to be mainly present in highly aggressive tumours such as G3 gastroenteropancreatic neuroendocrine carcinomas (GEP-NEC), small-cell lung cancer, or lymphomas (Kaemmerer et al. 2015a, b; Stollberg et al. 2016; Mai et al. 2019). In contrast to the antisecretory and antiproliferative effects mediated via SSTs (Rinke et al. 2009; Caplin et al. 2014), increased tumour expression of CXCR4 has been associated with rapid tumour progression, high invasive potential, early metastasis, and poor patient outcomes (Furusato et al. 2010; Zhao et al. 2015). As such, several peptide and non-peptide CXCR4 antagonists have been synthesized, and their antiproliferative activity has been tested both in vitro and in animal tumour models (Walenkamp et al. 2017). Based on promising results, several clinical studies on the efficacy of CXCR4 antagonists in cancer patients have been initiated, some of which are still ongoing (Domanska et al. 2013; Walenkamp et al. 2017). In addition, very recently, feasibility of both CXCR4-based imaging and PRRT in a variety of tumour types, including small-cell lung cancer, lymphoma, and multiple myeloma, has been demonstrated (Buck et al. 2017; Kircher et al. 2018).
In contrast to other tumour entities, studies on SST expression in human PAC are scarce. The available studies comprise only a small number of cases, and they were based on mRNA analyses, receptor autoradiography, or in vitro binding studies (Reubi et al. 1988; Buscail et al. 1996). However, to the best of our knowledge, comprehensive immunohistochemical studies on SST expression in PAC are lacking so far.
Regarding CXCR4 expression in PAC, several immunohistochemical studies are already available. However, results concerning correlations between CXCR4 expression and clinicopathologic data are contradictory. Whereas in some studies, CXCR4 expression has been shown to be associated with advanced tumour stage, the presence of lymph-node or distant metastases or poor patient outcomes (Maréchal et al. 2009; Cui et al. 2011; Bachet et al. 2012; Liao et al. 2012; Wang et al. 2013; Wu et al. 2015; Zhang et al. 2018a), in others no such interrelationships (Koshiba et al. 2000; Wehler et al. 2006; Gebauer et al. 2011) or even an inverse association with histological grade (Kure et al. 2012) has been found.
Based on the limited and in some cases conflicting data currently available, the aim of the present study was to comprehensively re-evaluate SST and CXCR4 expression in a large set of PAC samples by immunohistochemistry using well-characterized rabbit monoclonal antibodies. In addition, the expression patterns of SSTs and CXCR4 were compared directly to those of pancreatic neuroendocrine tumours (PanNETs), which are well known for the over-expression of SSTs in G1/G2 neoplasms and of CXCR4 in G3 carcinomas (Kaemmerer et al. 2015c; Mehta et al. 2015; Mai et al. 2019). PanNET account for only 1–2% of all pancreatic neoplasms, but exhibit a much better prognosis than PAC, with a 5-year survival rate of approximately 50% (Zhou et al. 2010; Dasari et al. 2017).
Methods
Tumour specimens
A total of 137 archived formalin-fixed, paraffin-embedded tumour samples from 70 patients were included in the present investigation (specifically, 1 sample each from 38 patients, 2 each from 16 patients, 3 each from 11 patients, 4 each from 2 patients, 6 each from 2 patients, and 14 from 1 patient; 90 samples were from primary tumours, and 47 represented metastases). Of the 70 patients, 26 were diagnosed with PAC and 44 with PanNET. Samples had been resected between 1999 and 2015 at the Department of General and Visceral Surgery, Zentralklinik Bad Berka, Bad Berka, Germany.
Immunohistochemical analyses
From the paraffin blocks, 4-µm sections were prepared and floated onto positively charged slides. Immunostaining was performed using an indirect peroxidase labeling method, as described previously (Kaemmerer et al. 2017a). Rabbit monoclonal antibodies (hybridoma cell culture supernatants) directed against the respective carboxyl-terminal tails of the different receptors were used to detect SSTs (except SST4) and CXCR4 (for detailed information regarding the clones, epitopes, and dilutions of the antibodies, see Table 1). These antibodies have been generated and extensively characterized by our group (Fischer et al. 2008a, b; Lupp et al. 2011, 2012, 2013), and have been validated by other researchers (e.g., Körner et al. 2012; Lambertini et al. 2013; Chinezu et al. 2014). For immunohistochemical detection of SST4, polyclonal antibodies were used. Sections obtained from normal human pancreas (islets; SST1, SST2, SST3, SST5), lymph nodes (germinal centres; SST2, SST5, CXCR4, Ki-67), and cortex (SST4) were used as positive controls. For negative controls, the primary antibody was either omitted or adsorbed for 2 h at room temperature with 10 µg/ml of the peptide used for rabbit immunizations (Kaemmerer et al. 2017a). Additional staining was performed with monoclonal mouse antibodies against the proliferation marker Ki-67 (Table 1).
Staining of receptors was scored in all sections using the semi-quantitative Immunoreactivity Score (IRS), as reported by Remmele and Stegner (1987). The percentage of positive tumour cells was stratified into five categories [no positive cells (0), < 10% positive cells (1), 10–50% positive cells (2), 51–80% positive cells (3), and > 80% positive cells (4)] and then multiplied by the staining intensity quantified in four categories [no staining (0), weak staining (1), moderate staining (2), and strong staining (3)]. Thus, IRS values ranging from 0 to 12 were obtained. Tumour samples with an average IRS value ≥ 3 were considered positive. For patients who had more than one tumour slide, the arithmetic mean was calculated from the IRS values of all slides for that patient, with primary tumour and metastasis/es taken together (per patient analysis). Only when primary tumours and metastases were compared, arithmetic means were calculated for the primary tumour samples and metastasis/es sample(s) separately. The antibodies against the SSTs and CXCR4 produced distinct immunostaining of not only the plasma membrane of tumour cells but also the cytoplasm, indicating receptor internalisation due to agonist stimulation. Both types of staining (cytoplasmic and cell surface) were evaluated equally. Staining of tumour blood vessels for SSTs and CXCR4 was evaluated separately by determining the percentage of positively stained vessels relative to all vessels. Tumours with ≥10% of vessels exhibiting staining for the respective receptor were considered positive. With respect to Ki-67 staining, the percentage of positive nuclei was determined. All immunohistochemical stainings were evaluated by two independent, blinded investigators (YK, AL). In the case of discrepant scores, the final decision was reached by consensus.
Statistical analyses
IBM SPSS statistics software, version 25.0 (Armonk, NY, USA), was used for statistical analyses. Because the data were not normally distributed (Kolmogorov–Smirnov test), the Mann–Whitney test, Chi-square test, Kendall’s τ-b test, or Spearman’s rank correlation test was performed. For survival analyses, the Kaplan–Meier method with log-rank test was used. A p value ≤ 0.05 was considered indicative of statistical significance.
Results
Patient characteristics
Tumours from 35 male and 35 female patients (PAC: 12 males, 14 females; PanNET: 23 males, 21 females) were evaluated (Table 2).
Median age at diagnosis was 59.5 years overall (range: 14.6–80.1 years), with a significant difference between the PAC and PanNET groups. PAC patients were diagnosed at a significantly higher age (median age: 67.4 years) compared to PanNET patients (median age: 55.8 years; p = 0.002; Table 2).
Median survival was 1.84 years overall (minimum: 0.03 years; maximum: 15.32 years). Patients with PAC had significantly worse outcomes relative to those with PanNET (median survival: PAC: 1.28 years; PanNET: 3.35 years; p = 0.001; Table 2; Kaplan–Meier survival analysis: p < 0.001; Fig. 1).
At diagnosis, 3.8% (1/26) of PAC patients had UICC (Union Internationale Contre le Cancer) stage I disease, whereas 65.4% (17/26) had stage II, 7.7% (2/26) stage III, and 23.1% (6/26) stage IV disease. Of the PanNET samples examined, 11.4% (5/44) were classified as stage I, 25.0% (11/44) as stage II, 4.5% (2/44) as stage III, and 59.1% (26/44) as stage IV. For one PanNET patient, the stage of the disease was not known (Table 2).
With respect to histological grading, 16 PAC samples (61.5%) were grade 2, whereas 10 tumours (38.5%) exhibited grade 3 histology. PanNETs were graded as follows: 36.4% (16/44) as G1, 52.3% (23/44) as G2, and 11.3% (5/44) as G3 histology (Table 2).
Of the (corresponding) primary tumours, 1 PAC tumour (3.8%) was classified as T2, whereas 19 (73.1%) were classified as T3 and 4 (15.4%) as T4. In two cases (7.7%), the extent of the primary tumour was unknown. Among PanNETs, 6 (13.6%) were classified as T1, 11 (25.0%) as T2, 20 (45.5%) as T3, and 4 (9.1%) as T4. The T status was unknown for three PanNET tumours (6.8%) (Table 2). Eleven PAC patients (42.4%) exhibited no lymph-node metastases at diagnosis, whereas lymph-node metastases were already present in 14 cases (53.8%). Lymph-node status was unknown for one PAC patient (3.8%). Among the PanNET patients, 18 (40.9%) had no lymph-node metastases, 25 cases (56.8%) presented already with lymph-node metastases at diagnosis, and in 1 (2.3%) case, the existence of lymph-node metastases was not known (Table 2). No distant metastases were detected in 21 of the PAC patients (80.8%); in 4 cases (15.4%), distant metastases were already present. The presence of distant metastases was not reported in the files of one patient (3.8%). Among PanNET patients, 18 (40.9%) exhibited no distant metastases, but tumours had already metastasized to other organs in 26 patients (59.1%) (Table 2).
Receptor expression patterns and correlations with clinical data
Figure 2 shows typical examples of staining in PAC and PanNET samples obtained with the monoclonal antibodies against SST1, 2 and 5 and CXCR4. With these antibodies, as well as with the anti-SST3 antibody, distinct immunostaining of the plasma membrane, but also of the cytoplasm of the tumour cells was observed. The polyclonal anti-SST4 antibody, in contrast, yielded only cytoplasmic staining (data not shown). In addition to and independent of the staining of tumour cells, in many cases, SST3, SST5, and CXCR4 were found to be strongly expressed on the tumour capillaries.
Somatostatin receptor (SST) and chemokine receptor CXCR4 expression pattern in pancreatic adenocarcinoma (PAC) and pancreatic neuroendocrine tumour (PanNET) tissues. Depicted are typical examples for the staining patterns of SST1, SST2, SST5, and CXCR4. Immunohistochemistry (red–brown colour), counterstaining with haematoxylin; original magnification: 400×
For all receptors, but especially for SST1 and the SST2, expression levels varied considerably between individual patients and sometimes also between different samples from the same tumour, which is mirrored by the length of the respective boxes and whiskers in Fig. 3b.
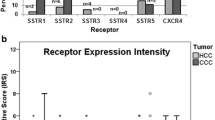
Expression profiles of the different somatostatin receptor (SST) subtypes and the chemokine receptor CXCR4 in pancreatic adenocarcinomas (PAC) and pancreatic neuroendocrine tumours (PanNET). a Percentage of positive cases for the different SSTs and CXCR4. Tumours were only considered positive with an immunoreactivity score (IRS) ≥ 3. b Box plots of the expression levels (IRS values) of SSTs and CXCR4. Depicted are median values, upper and lower quartiles, minimum and maximum values, as well as outliers. The outliers are defined as follows: circles: mild outliers; data that fall between 1.5 and 3 times above the upper quartile or below the lower quartile; asterisks: extreme outliers; data that fall more than three times above the upper quartile or below the lower quartile
SST1 was the most-prominently expressed receptor in PAC specimens. Of the 26 PAC tumours examined in the present study, 11 (42.3%) exhibited an IRS ≥3 for SST1 and were, therefore, considered SST1-positive. In some cases, even a strong expression with IRS values up to 9 was observed. However, with a median IRS of 2, the overall intensity of expression was quite low. SST2, SST3, SST4, and SST5 were not positively expressed in PAC specimens. In addition, median IRS values were only 0. CXCR4 was positively expressed in 2 (7.7%) PAC specimens, but also here, median IRS was 0 (Fig. 3a, b).
In PanNET, SST2 was by far the most often and most pronounced expressed receptor. SST2 was detected in 33/44 (75.0%) of the tumours with a median IRS of 7.75, representing medium–strong expression. SST5 was present in 9 (20.5%) PanNET specimens, whereas SST1 was detected in 7 (15.9%) and CXCR4 in 5 (11.4%) of the PanNET cases. SST3 was detected in only one tumour. The median IRS values for these receptors were only 0, but for SST1 and SST5 in some of the tumours up to 8 and for CXCR4 up to 7 IRS points were reached.
Regarding receptor combinations, in most PAC specimens, SST1 was the only SST detected. In one case, the SST1 was present in combination with the CXCR4, and in another tumour, only CXCR4 was observed. In the majority of the PAC specimens (14 tumours; 53.8%), however, neither an expression of the SSTs nor of the CXCR4 could be detected.
Most of the PanNETs (20 tumours; 45.5%) showed an expression of SST2 only, five neoplasms (11.4%) expressed SST2 together with SST5. In two tumours each, co-expression of SST2 plus CXCR4 with SST1 or with SST1 plus SST5 was observed. One tumour each expressed SST2 in combination with SST5 plus CXCR4 or in association with SST1 plus SST3. Only SST1, only CXCR4 or SST1 together with SST5 and CXCR4 were detected in one tumour each. Eight tumours did not exhibit expression of any of the receptors investigated.
Furthermore, in PAC samples, a significant correlation between SST5 and CXCR4 expression as well as a tendency toward an association between SST1 and SST2 expression was observed (Table 3). In PanNET specimens, by contrast, SST1 expression was significantly correlated with the incidence of SST3 and SST5, and SST3 expression was significantly associated with the occurrence of CXCR4 (Table 4).
Regarding correlations with clinical data, in PAC, a tendency toward a positive association between SST2 expression and patient overall survival was observed (Table 3). There was also a positive association between SST5 expression and patient age (rsp = 0.508, p < 0.001). Analysis of the clinical data revealed additionally a significant negative association between tumour stage and overall survival (τ = − 0.318, p = 0.048).
In PanNET, a significant negative correlation between the expression of SST2 and tumour grading was observed (τ = − 0.282, p = 0.026) and a positive association between the expression of SST2 or SST5 and overall survival (Table 4). This latter association was also evident in the respective Kaplan–Meier analyses, showing a significantly better outcome for patients with SST2-positive than for those with SST2-negative tumour (p < 0.001; Fig. 4). Furthermore, SST1 expression was negatively correlated with tumour size (rsp = − 0.272, p = 0.004), whereas between Ki-67 values and tumour size, a positive association was found (rsp = 0.264, p = 0.031). As in PAC, also in PanNET, a significant negative correlation between tumour stage and overall survival was noticed (τ = − 0.327, p = 0.007).
Aside from the above-mentioned findings, in both tumour entities, no other correlations between receptor expression intensities and either patient sex, tumour grading, staging, size, or the presence of lymph-node or distant metastases or within the clinical data could be detected.
In contrast to the tumour cells, in PAC a strong SST3, SST5, and CXCR4 expression was observed on the tumour capillaries. SST3 and SST5 were present on tumour microvessels in 34% and CXCR4 in 58% of the PAC cases, respectively. In PanNET, only CXCR4 expression could be detected on the tumour capillaries, being present in 44% of the neoplasms. However, in both tumour entities, no associations between SST or CXCR4 expression on tumour capillaries and any of the clinical parameters were observed.
Differences in receptor expression between PAC and PanNET specimens
Between the two tumour entities, differences were noted in the intensity of expression of SST1, SST2, and SST3, with a higher SST1 but lower SST2 and SST3 IRS in PAC compared to PanNET (SST1: p = 0.067; SST2: p < 0.001; SST3: p = 0.009; Fig. 3b). Furthermore, PAC exhibited significantly higher Ki-67 values than PanNET (median: 15.2 vs. 4.7; mean: 19.0 vs. 8.1; p < 0.001).
Discussion
Regarding SST and CXCR4 expression in PanNET, the present results completely correspond both with our previous data obtained with different cohorts of patients (Kaemmerer et al. 2015c; Mai et al. 2019) and with the findings reported by other groups (Mehta et al. 2015). Our results also unequivocally corroborate data on SST2 expression as positive predictor in GEP-NETs (Corleto et al. 2009; Okuwaki et al. 2013; Mehta et al. 2015; Kaemmerer et al. 2015a; Qian et al. 2016; Song et al. 2016; Mai et al. 2019) and provide further clear evidence for the utility of SST-based diagnostics and treatment modalities in PanNET patients (Falconi et al. 2016; Bozkurt et al. 2017; Smit Duijzentkunst et al. 2017).
In contrast to PanNET, PAC displayed only negligible SST expression overall, except a few cases in which medium–high SST1 expression was observed. These data are in good agreement with reports from the literature, indicating that both at the mRNA and at the protein level, SSTs are lost from pancreas tissue during PAC development (Buscail et al. 1996; Chalabi-Dchar et al. 2015). Consequently, SSTs are not expressed in human PAC tumours, as demonstrated by receptor autoradiography and in vitro binding studies using human tumour samples as well as in vivo 111In-octreotide scintigraphy analyses (Reubi et al. 1988; Van Eijck et al. 1996). In addition, SSTRs are only rarely detectable in human PAC xenografts (Singh et al. 1991). Our results are also consistent with the failure of clinical trials using somatostatin analogues in PAC patients (Reubi et al. 1988; Klijn et al. 1990; Canobbio et al. 1992; Huguier et al. 1992; Friess et al. 1993; Fazeny et al. 1997; Sulkowski et al. 1999;). In contrast to human tumours, antiproliferative effects of somatostatin analogues have been reported in the PAC cell line MIA PaCa-2 as well as MIA PaCa-2 xenografts in nude mice (Radulovic et al. 1993; Fisher et al. 1996). However, it should be noted that MIA PaCa-2 (and PANC-1) cells exhibit neuroendocrine differentiation and therefore express SST2, which renders them susceptible to SST-based therapies (Gradiz et al. 2016). This particular feature of these two cell lines was corroborated by another study (Fisher et al. 1998) involving nine different PAC cell lines: AsPC-1, BxPC-3, Capan-1, Capan-2, HS766T, Panc-1, SU.86.86, CAV, and MIA PaCa-2. Although many of these cell lines expressed SSTs at the mRNA level, in that study, only MIA PaCa-2 cells displayed SST protein expression on the cell surface, as assessed by 125I-labeled somatostatin-14 binding assays, and only MIA PaCa-2 cells demonstrated susceptibility to the antiproliferative effect of somatostatin (Fisher et al. 1998).
Regarding CXCR4, only in a few PAC tumours, noticeable expression was detected. This result is in contrast to the literature data demonstrating the presence of this receptor in 45–100% of the PAC cases (Koshiba et al. 2000; Wehler et al. 2006; Maréchal et al. 2009; Cui et al. 2011; Gebauer et al. 2011; Bachet et al. 2012; Kure et al. 2012; Liao et al. 2012; Wang et al. 2013; Zhang et al. 2018a). One reason for this discrepancy could be the use of different antibodies and rating methods. Whereas well-characterized monoclonal antibodies were employed in the present study, in the literature, a wide variety of polyclonal and monoclonal antibodies from different commercial and non-commercial sources have been used. The array of antibodies used could also explain the high variability in the CXCR4 expression profiles described in the literature as well as the divergent results between the different studies regarding potential associations between CXCR4 expression and clinicopathologic parameters such as patient outcomes. We rated receptor staining using the IRS, which takes both frequency and intensity of expression into account, and only samples exhibiting an IRS ≥3 were considered positive. Less-differentiated classifications of staining results were used in most studies reported in the literature. In addition, in the vast majority of previously reported studies, staining was rated positive at intensities lower than those considered positive in the present study. Nevertheless, studies have shown that CXCR4 is primarily expressed in highly aggressive tumours such as lymphomas or small-cell lung cancer (Kaemmerer et al. 2015b; Stollberg et al. 2016), with median Ki-67 values well above 20. With a median IRS of 15.2, as observed in the present investigation, PAC probably does not belong with that group of tumours. This conclusion is supported by similar findings indicating quite low CXCR4 expression rates in other aggressive (but not highly aggressive) tumour entities, such as hepatocellular and cholangiocellular carcinomas (Kaemmerer et al. 2017b) or grade IV astrocytic brain tumours (Lange et al. 2018).
More than half of the PAC specimens examined in the present study exhibited strong CXCR4 expression on the tumour capillaries. Similar observations have been previously described both in PAC (Koshiba et al. 2000; Cui et al. 2011; Gebauer et al. 2011) and in other tumour entities (Ingold et al. 2010; Kaemmerer et al. 2017a; Kaemmerer et al. 2017b; Lange et al. 2018). Neo-angiogenesis plays an important role in tumour development, progression, and metastasis, and this holds true for PAC, as well (Zhang et al. 2018b; Annese et al. 2019). Therefore, targeting tumour microvessels using anti-CXCR4 therapies represents a potentially promising additional therapeutic strategy.
Conclusions
According to our results, the very low SST or CXCR4 expression on the PAC tumour cells has clearly no therapeutic relevance. In contrast, indirect targeting of these tumours via the CXCR4, but also via the SST3 or SST5, which are strongly expressed on the tumour capillaries, represents a potentially useful additional therapeutic strategy.
Abbreviations
- CXCR4:
-
CXC motif chemokine receptor 4
- GEP-NEC:
-
Gastroenteropancreatic neuroendocrine carcinomas
- GEP-NET:
-
Gastroenteropancreatic neuroendocrine tumours
- IRS:
-
Immunoreactivity score
- PAC:
-
Pancreatic adenocarcinoma
- PanNET:
-
Pancreatic neuroendocrine tumour
- SST:
-
Somatostatin receptor
References
Annese T, Tamma R, Ruggieri S, Ribatti D (2019) Angiogenesis in pancreatic cancer: pre-clinical and clinical studies. Cancers (Basel) 11:381
Bachet JB, Maréchal R, Demetter P, Bonnetain F, Couvelard A, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet A, Louvet C, Paye F, Rougier P, Penna C, Vaillant JC, André T, Closset J, Salmon I, Emile JF, Van Laethem JL (2012) Contribution of CXCR2 and SMAD4 in predicting disease progression pattern and benefit from adjuvant chemotherapy in resected pancreatic adenocarcinoma. Ann Oncol 23:2327–2335
Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, Ambrosini V, Kjaer A, Delgado-Bolton R, Kunikowska J, Oyen WJG, Chiti A, Giammarile F, Fanti S (2017) Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F–DOPA. Eur J Nucl Med Mol Imaging 44:1588–1601
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Buck AK, Stolzenburg A, Hänscheid H, Schirbel A, Lückerath K, Schottelius M, Wester HJ, Lapa C (2017) Chemokine receptor—directed imaging and therapy. Methods 130:63–71
Buscail L, Saint-Laurent N, Chastre Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C (1996) Loss of SST2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res 56:1823–1827
Canobbio L, Boccardo F, Cannata D, Gallotti P, Epis R (1992) Treatment of advanced pancreatic carcinoma with the somatostatin analogue BIM 23014. Preliminary results of a pilot study. Cancer 69:648–650
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P, For the CLARINET Investigators (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. New Engl J Med 371:224–233
Chalabi-Dchar M, Cassant-Sourdy S, Duluc C, Fanjul M, Lulka H, Samain R, Roche C, Breibach F, Delisle MB, Poupot M, Dufresne M, Shimaoka T, Yonehara S, Mathonnet M, Pyronnet S, Bousquet C (2015) Loss of somatostatin receptor subtype 2 promotes growth of KRAS-induced pancreatic tumors in mice by activating PI3 K signaling and overexpression of CXCL16. Gastroenterology 148:1452–1465
Chinezu L, Vasiljevic A, Jouanneau E, François P, Borda A, Trouillas J, Raverot G (2014) Expression of somatostatin receptors, SSTR2A and SSTR5, in 108 endocrine pituitary tumors using immunohistochemical detection with new specific monoclonal antibodies. Hum Pathol 45:71–77
Corleto VD, Falconi M, Panzuto F, Milione M, De Luca O, Perri P, Cannizzaro R, Bordi C, Pederzoli P, Scarpa A, Delle Fave G (2009) Somatostatin receptor subtypes 2 and 5 are associated with better survival in well-differentiated endocrine carcinomas. Neuroendocrinology 89:223–230
Cui K, Zhao W, Wang C, Wang A, Zhang B, Zhou W, Yu J, Sun Z, Li S (2011) The CXCR12-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J Surg Res 171:143–150
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342
Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EGE, Walenkamp AME (2013) A review on CXCR14/CXCL12 axis in oncology: no place to hide. Eur J Cancer 49:219–230
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT, All other Vienna Consensus Conference participants (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103:153–171
Fazeny B, Baur M, Prohaska M, Hudec M, Kremnitzer M, Meryn S, Huber H, Grunt T, Tuchmann A, Dittrich C (1997) Octreotide combined with goserelin in the therapy of advanced pancreatic cancer—results of a pilot study and review of the literature. J Cancer Res Clin Oncol 123:45–52
Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S (2008a) Reassessment of SST2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab 93:4519–4524
Fischer T, Nagel F, Jacobs S, Stumm R, Schulz S (2008b) Reassessment of CXCR18 chemokine receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-2. PLoS One 3:e4069
Fisher WE, Muscarella P, O’Dorisio TM, O’Dorisio MS, Kim JA, Doran TA, Sabourin CL, Schirmer WJ (1996) Expression of the somatostatin receptor subtype-2 gene predicts response of human pancreatic cancer to octreotide. Surgery 120:234–241
Fisher WE, Doran TA, Muscarella P II, Boros LG, Ellison EC, Schirmer WJ (1998) Expression of somatostatin receptor subtype 1-5 genes in human pancreatic cancer. J Natl Cancer Inst 90:322–324
Friess H, Buchler M, Beglinger C, Weber A, Kunz J, Fritsch K, Dennler HJ, Beger HG (1993) Low-dose octreotide treatment is not effective in patients with advanced pancreatic cancer. Pancreas 8:540–545
Furusato B, Mohamed A, Uhlén M, Rhim JS (2010) CXCR22 and cancer. Pathol Int 60(497):505
Gebauer F, Tachezy M, Effenberger K, von Loga K, Zander H, Marx A, Kaifi JT, Sauter G, Izbicki JR, Bockhorn M (2011) Prognostic impact of CXCR23 and CXCR23 expression in pancreatic adenocarcinoma. J Surg Oncol 104:140–145
Gradiz R, Silva HC, Carvalho L, Botelho MF, Mota-Pinto A (2016) MIA PaCa-2 and PANC-1 pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci Rep 6:21648
Huguier M, Samama G, Testart J, Mauban S, Fingerhut A, Nassar J, Houry S, Jaeck D, De Mestier P, Favre JP, Michot F, Vidrequin A, Mantion G, Veyrières M, Fourtanier G, Lointier P, Gignoux M (1992) Treatment of adenocarcinoma of the pancreas with somatostatin and gonadoliberin (luteinizing hormone-releasing hormone). The French association for surgical research. Am J Surg 164:348–353
Ilic M, Ilic I (2016) Epidemiology of pancreatic cancer. World J Gastroenterol 22:9694–9705
Ingold B, Simon E, Ungethüm U, Kuban RJ, Müller BM, Lupp A, Neumann U, Ebert MP, Denkert C, Weichert W, Schulz S, Röcken C (2010) Vascular CXCR27 expression—a novel antiangiogenic target in gastric cancer? PLoS One 5:e10087
Kaemmerer D, Träger T, Hoffmeister M, Sipos B, Hommann M, Sänger J, Schulz S, Lupp A (2015a) Inverse expression of somatostatin and CXCR28 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 6:27566–27579
Kaemmerer D, Reimann C, Specht E, Wirtz RM, Sayeg M, Baum RP, Schulz S, Lupp A (2015b) Differential expression and prognostic value of the chemokine receptor CXCR29 in bronchopulmonary neuroendocrine neoplasms. Oncotarget 6:3346–3358
Kaemmerer D, Wirtz RM, Fischer EK, Hommann M, Sänger J, Prasad V, Specht E, Baum RP, Schulz S, Lupp A (2015c) Analysis of somatostatin receptor 2A immunohistochemistry, RT-qPCR, and in vivo PET/CT data in patients with pancreatic neuroendocrine neoplasm. Pancreas 44:648–654
Kaemmerer D, Sänger J, Arsenic R, D’Haese JG, Neumann J, Schmitt-Graeff A, Wirtz RM, Schulz S, Lupp A (2017a) Evaluation of somatostatin, CXCR31 chemokine and endothelin A receptor expression in a large set of paragangliomas. Oncotarget 8:89958–89969
Kaemmerer D, Schindler R, Mußbach F, Dahmen U, Altendorf-Hofmann A, Dirsch O, Sänger J, Schulz S, Lupp A (2017b) Somatostatin and CXCR32 chemokine receptor expression in hepatocellular and cholangiocellular carcinomas: tumor capillaries as promising targets. BMC Cancer 17:896
Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388:73–85
Kircher M, Herhaus P, Schottelius M, Buck AK, Werner RA, Wester HJ, Keller U, Lapa C (2018) CXCR34-directed theranostics in oncology and inflammation. Ann Nucl Med 32:503–511
Klijn JG, Hoff AM, Planting AS, Verweij J, Kok T, Lamberts SW, Portengen H, Foekens JA (1990) Treatment of patients with metastatic pancreatic and gastrointestinal tumours with the somatostatin analogue Sandostatin: a phase II study including endocrine effects. Br J Cancer 62:627–630
Körner M, Waser B, Schonbrunn A, Perren A, Reubi JC (2012) Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. A J Surg Pathol 36:242–252
Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S (2000) Expression of stromal cell-derived factor 1 and CXCR37 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res 6:3530–3535
Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, Ishiwata T (2012) Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol 41:1314–1324
Lambertini C, Barzaghi-Rinaudo P, D’Amato L, Schulz S, Nuciforo P, Schmid HA (2013) Evaluation of somatostatin receptor subtype expression in human neuroendocrine tumors using two sets of new monoclonal antibodies. Regul Pept 187:35–41
Lange F, Kaemmerer D, Behnke-Mursch J, Brück W, Schulz S, Lupp A (2018) Differential somatostatin, CXCR40 chemokine and endothelin A receptor expression in WHO grade I-IV astrocytic brain tumors. J Cancer Res Clin Oncol 144:1227–1237
Liao WC, Wang HP, Huang HY, Wu MS, Chiang H, Tien YW, Lin YL, Lin JT (2012) CXCR41 expression predicts early liver recurrence and poor survival after resection of pancreatic adenocarcinoma. Clin Transl Gastroenterol 3:e22
Lupp A, Hunder A, Petrich A, Nagel F, Doll C, Schulz S (2011) Reassessment of sst5 somatostatin receptor expression in normal and neoplastic human tissues using the novel rabbit monoclonal antibody UMB-4. Neuroendocrinology 94:255–264
Lupp A, Nagel F, Doll C, Röcken C, Evert M, Mawrin C, Saeger W, Schulz S (2012) Reassessment of SST3 somatostatin receptor expression in normal and neoplastic human tissues using the novel rabbit monoclonal antibody UMB-4. Neuroendocrinology 96:301–310
Lupp A, Nagel F, Schulz S (2013) Reevaluation of sst1 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-7. Regul Pept 183:1–6
Mai R, Kaemmerer D, Träger T, Neubauer E, Sänger J, Baum RP, Schulz S, Lupp A (2019) Different somatostatin and CXCR45 chemokine receptor expression in gastroenteropancreatic neuroendocrine neoplasms depending on their origin. Sci Rep 9:4339
Maréchal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Devière J, Salmon I, Van Laethem JL (2009) High expression of CXCR46 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer 100:1444–1451
Mehta S, de Reuver PR, Gill P, Andrici J, D’Urso L, Mittal A, Pavlakis N, Clarke S, Samra JS, Gill AJ (2015) Somatostatin receptor SSTR-2a expression is a stronger predictor for survival than Ki-67 in pancreatic neuroendocrine tumors. Medicine (Baltimore) 94:e1281
Okuwaki K, Kida M, Mikami T, Yamauchi H, Imaizumi H, Miyazawa S, Iwai T, Takezawa M, Saegusa M, Watanabe M, Koizumi W (2013) Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor 2A to outcomes. Cancer 119:4094–4102
Qian ZR, Li T, Ter-Minassian M, Yan J, Chan JA, Brais LK, Masugi Y, Thiaglingam A, Brooks N, Nishihara R, Bonnemarie M, Masuda A, Inamura K, Kim SA, Mima K, Sukawa Y, Dou R, Lin X, Christiani DC, Schmidlin F, Fuchs CS, Mahmood U, Ogino S, Kulke MH (2016) Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 45(1386):1393
Radulovic S, Comaru-Schally AM, Milovanovic S, Schally AV (1993) Somatostatin analogue RC-160 and LH-RH antagonist SB-75 inhibit growth of MIA-PaCa-2 human pancreatic cancer xenografts in nude mice. Pancreas 8:88–97
Rawla P, Sunkara T, Gaduputi V (2019) Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 10:10–27
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140
Reubi JC, Horisberger U, Essed CE, Jeekel J, Klijn JGH, Lamberts SWJ (1988) Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology 95:760–763
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R, PROMID Study Group (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27:4656–4663
Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371:1039–1049
Simoes P, Olson SH, Saldia A, Kurtz RC (2017) Epidemiology of pancreatic adenocarcinoma. Chin Clin Oncol 6:24
Singh P, Townsend CM Jr, Poston GJ, Reubi JC (1991) Specific binding of cholecystokinin, estradiol and somatostatin to human pancreatic cancer xenografts. J Steroid Biochem Mol Biol 39:759–767
Smit Duijzentkunst DA, Kwekkeboom DJ, Bodei L (2017) Somatostatin receptor 2-targeting compounds. J Nucl Med 58:54S–60S
Song KB, Kim SC, Kim JH, Seo DW, Hong SM, Park KM, Hwang DW, Lee JH, Lee YJ (2016) Prognostic value of somatostatin receptor subtypes in pancreatic neuroendocrine tumors. Pancreas 45:187–192
Stollberg S, Kaemmerer D, Neubauer E, Schulz S, Simonitsch-Klupp I, Kiesewetter B, Raderer B, Lupp A (2016) Differential somatostatin and CXCR1 chemokine receptor expression in MALT-type lymphoma of gastric and extragastric origin. J Cancer Res Clin Oncol 142:2239–2247
Sulkowski U, Buchler M, Pederzoli P, Arnold R, Dinse P, Kay A, Haus U, Beger HG (1999) A phase II study of high-dose octreotide in patients with unresectable pancreatic carcinoma. Eur J Cancer 35:1805–1808
Teague A, Lim KH, Wang-Gillam A (2015) Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol 7:68–84
van Eijck CH, Lamberts SW, Lemaire LC, Jeekel H, Bosman FT, Reubi JC, Bruining HA, Krenning EP (1996) The use of somatostatin receptor scintigraphy in the differential diagnosis of pancreatic duct cancers and islet cell tumors. Ann Surg 224:119–124
Waidmann O, Pelzer U, Boeck S, Waldschmidt DT (2018) Sequence therapy in metastatic pancreatic cancer. Z Gastroenterol 56:578–581
Walenkamp AME, Lapa C, Herrmann K, Wester HJ (2017) CXCR65 ligands. The next big hit? J Nucl Med 58:77S–82S
Wang Z, Ma Q, Li P, Sha H, Li X, Xu J (2013) Aberrant expression of CXCR66 and beta-catenin in pancreatic cancer. Anticancer Res 33:4103–4110
Wehler T, Wolfert F, Schimanski CC, Gockel I, Herr W, Biesterfeld S, Seifert JK, Adwan H, Berger MR, Junginger T, Galle PR, Moehler M (2006) Strong expression of chemokine receptor CXCR67 by pancreatic cancer correlates with advanced disease. Oncol Rep 16:1159–1164
Wu H, Zhu L, Zhang H, Shi X, Zhang L, Wang W, Xue H, Liang Z (2015) Coexpression of EGFR and CXCR68 predicts poor prognosis in resected pancreatic ductal adenocarcinoma. PLoS One 10:e0116803
Zhang J, Liu C, Mo X, Shi H, Li S (2018a) Mechanisms by which CXCR69/CXCL12 cause metastatic behaviour in pancreatic cancer. Oncol Lett 15:1771–1776
Zhang Z, Ji S, Zhang B, Liu J, Qin Y, Xu J, Yu X (2018b) Role of angiogenesis in pancreatic cancer biology and therapy. Biomed Pharmacother 108:1135–1140
Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B (2015) CXCR71 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget 6:5022–5040
Zhou J, Enewold L, Stojadinovic A, Clifton GT, Potter JF, Peoples GE, Zhu K (2010) Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes Control 21:853–861
Funding
The Theranostic Research Center, Zentralklinik Bad Berka, 99437 Bad Berka, Germany, provided funding for this research.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Daniel Kaemmerer and Amelie Lupp. Provided the tumour samples: Jörg Sänger. Provided the antibodies: Stefan Schulz. Acquired the clinical data: Daniel Kaemmerer. Performed the experiments: Ylberta Kajtazi and Amelie Lupp. Analysed the data: Ylberta Kajtazi and Amelie Lupp. Interpreted the data: Amelie Lupp. Wrote the paper: Amelie Lupp. Critically revised the manuscript: Ylberta Kajtazi, Daniel Kaemmerer, Jörg Sänger, Stefan Schulz, and Amelie Lupp. Each of the authors has approved the manuscript and acknowledges that he or she participated sufficiently in the work to take public responsibility for its content.
Corresponding author
Ethics declarations
Conflict of interest
Daniel Kaemmerer received funding and support for travel to meetings from IPSEN and PFIZER. All other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Permission was gained from the local ethics committee (Ethikkommission der Landesärztekammer Thüringen) for this retrospective analysis. For this type of study, formal consent was not required. All data were recorded and analysed anonymously.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kajtazi, Y., Kaemmerer, D., Sänger, J. et al. Somatostatin and chemokine CXCR4 receptor expression in pancreatic adenocarcinoma relative to pancreatic neuroendocrine tumours. J Cancer Res Clin Oncol 145, 2481–2493 (2019). https://doi.org/10.1007/s00432-019-03011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03011-0