Abstract
Background
Microparticles (MPs) or ectosomes are small enclosed fragments (from 0.2 to 2 μm in diameter) released from the cellular plasma membrane. Several oncogenic molecules have been identified inside MPs, including soluble proteins XIAP, survivin, metalloproteinases, CX3CL1, PYK2 and other microRNA-related proteins; membrane proteins EGFR, HER-2, integrins and efflux pumps; and messenger RNAs and microRNAs miR-21, miR-27a, let-7, miR-451, among others. Studies have shown that MPs transfer their cargo to neoplastic or non-malignant cells and thus contribute to activation of oncogenic pathways, resulting in cell survival, drug resistance and cancer dissemination.
Discussion and Conclusion
This review summarizes recent findings on MP biogenesis and the role of the MPs cargo in cancer and discusses some of the RNAs and proteins involved. In addition, the discussion covers evidence of (1) how and which signaling pathways can be activated by MPs in recipient cells; (2) recipient cell-type selectivity in incorporation of proteins and RNAs transported by MPs; and (3) how upon stimulation, stromal cells release MPs, promoting resistance to chemotherapeutics and invasiveness in cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A comprehensive classification of the different types of shed vesicles is a main concern in the intercellular communication research field. However, the complexity of this task begins with confusing nomenclature, which includes oncosomes, microvesicles (MVs), microparticles (MPs), endosomes, exosomes and ectosomes. Usually, microvesicle is considered to be the collective term for both MPs and exosomes. MPs may be called ectosomes or, as recently proposed, oncosomes, in the case of cancer-derived MPs. The diameter of MPs may range between 0.2 and 2 µm (Piccin et al. 2007; Simak et al. 2004), and exosomes are usually approximately 50–100 nm in diameter. The methodologies available to distinguish MPs from exosomes by size may result in overlapping pools of the different microvesicles. Biochemically, MPs are derived from the plasma membrane and thus usually display membrane biomarkers such as PS or lipid raft proteins, whereas exosomes are derived from intracellular endosomes and present distinct biomarkers. In this review, we focus on studies that specifically isolate MPs to describe their role in cancer communication and their content.
Membrane microparticles: What are they?
MPs are small vesicles formed from the cytoplasmic membrane that can be released from normal or cancer cells (termed oncosomes) (El Andaloussi et al. 2013; Hugel et al. 2005). Commonly, MPs are characterized by exposure of phosphatidylserine (PS) residues on their outer surfaces. However, because MPs are preferentially released from membrane lipid rafts, they may also contain raft-related proteins, such as P-selectin glycoprotein ligand-1 (PSGL-1) and tissue factor (TF) in monocyte-derived MPs (Del Conde et al. 2005) and other markers, depending on the cell type, such as CD44 for breast cancer cell-derived MPs (BCC-MPs) and ezrin for leukemic cell-derived MPs and BCC-MPs (Jaiswal et al. 2013). Phospholipids are distributed in a specific manner in the bilayer membrane, but during MP shedding, an energy-dependent mechanism rearranges the PS to outer membrane. In this process, the flippase, floppase and scramblase enzymes respond to increasing intracellular levels of Ca2+ and promote phospholipid redistribution. In parallel, MP maturation is initiated by membrane restructuring and cytoplasmic protrusions with cytoskeletal rearrangement and degradation. These events are mediated by proteins such as gelsolin and calpain allowing MP shedding (Del Conde et al. 2005; Enjeti et al. 2008; Salzer et al. 2002).
In tumor cell-derived MPs, others proteins also contribute to MP shedding. The ADP-ribosylation factor (ARF), which belongs to ARF family of small GTPases, has been associated with membrane traffic, including internalization of ligands and the organization of the actin cytoskeleton (D’Souza-Schorey and Chavrier 2006). Muralidharan-Chari and colleagues demonstrated that ARF6 modulates MP shedding via the phospholipase D, extracellular-signal-regulated kinases (ERK) and myosin light chain pathway. They also observed that ARF6 inhibition blocks MP shedding (Muralidharan-Chari et al. 2009). In addition, Pasquier and colleagues showed the importance of ARF6 upregulation in MPs in mediating a vascular metastatic microenvironment. Inhibition or deletion of ARF6 triggered a reduction in MP shedding by endothelial and cancer cells (Pasquier et al. 2014). Furthermore, Schllienger et al. (2014) demonstrated that ARF1 activity is required for the shedding of MPs, invadopodia maturation and MMP-9 activity, suggesting that ARF1 is important not only to MP biogenesis but also to MP-mediated cancer cell invasiveness. They showed that ARF1 modulates RhoA and RhoC activity, which in turn activate myosin light chain (MLC) phosphorylation.
Another important protein is P2X7, a non-selective cation channel belonging to the family of P2X receptors. This family is associated with several cellular processes such as cell-to-cell communication, secretory activity and membrane excitability. Its activation induces a sustained Ca2+ influx and consequently changes in phospholipid asymmetry followed by the release of MPs (Roger et al. 2014). Several groups have previously demonstrated that P2X7 stimulation induces MP release in normal hematopoietic cells (Qu and Dubyak 2009). Qu et al. (2007) even suggested that P2X7 itself might be carried by MPs. In the context of cancer, Constantinescu et al. (2010) further demonstrated that P2X7 activity stimulates MP release from murine erythroleukemia cells, while inhibition of P2X7 activity significantly decreases MP release. Although they did not describe the contents of the MPs, they provided the first evidence that P2X7 is important for cancer-derived MP release.
Other proteins described as being involved in MP biogenesis are transglutaminase 2 (TG2) and neurokinin 1 receptor (NK1R). Transglutaminases (TGs) are enzymes involved in protein transamidation. TG2, also called tissue TG (tTG), is a member of the TG family that plays a role in cellular signaling and in cytoskeletal organization. TG2 mediates cell adhesion in association with integrins and fibronectin (Lorand and Graham 2003). In addition, van der Akker et al. (2012) suggested that cross-linking of TG2 with fibronectin and the association of this complex with cytoskeletal elements are required to form a structural foundation for the MP in smooth muscle cells. Conversely, Antonyak and colleagues (2011) demonstrated that TG2 was not necessary for cancer MP biogenesis and release, on the basis that its inhibition did not alter MP shedding (Antonyak et al. 2011). Regarding NK1R, activation of this protein induces rapid cell shape changes (membrane blebbing) (Meshki et al. 2009). Chen et al. (2012) observed that activation of NK1R in human embryonic kidney cells, but not in human glioblastoma cells, induces membrane blebbing followed by MP release via a ROCK and dynamic activity-dependent mechanism. Together, these studies suggest that tumor cell-derived MP biogenesis deserves further analysis. As few reports have investigated the role of TG2 and NK1R, at this point it is not clear whether tumor cells may present different mechanisms of MP biogenesis than normal cells.
Although the mechanism of MP release has not been clearly characterized, the endosomal sorting complex required for transport (ESCRT) machinery has been shown to be required. Like other membrane-related processes, MP membrane fission and consequently MP release involve the core ESCRT machinery, assembled in three types of complexes on the membrane. These complexes are recruited by site-specific adaptors as well as other associated proteins such as the ATPase and vacuolar protein sorting 4 (VPS4). Mechanistic models have been proposed in which ESCRT-III filaments and VPS4 interact to catalyze membrane fission (Jouvenet 2012; McCullough et al. 2013).
The interaction between MPs and recipient cells is a fundamental process in cancer cell communication. Some studies have hypothesized that the exposed PS on the MP surface may bind to cellular PS receptors, leading to MP recognition and consequently, cargo transfer. Binding of PS to PS receptors has been described as an important process for the identification and removal of apoptotic cells and apoptotic bodies (large apoptotic vesicles) (Fadok et al. 1998; Hoffmann et al. 2001). Park and colleagues demonstrated that BAI1 receptor binds to PS on apoptotic cells and then promotes the phagocytic removal of their apoptotic bodies (Park et al. 2007). Tim4 and Tim1 were also identified as PS receptors associated with engulfment of cells undergoing apoptosis (Miyanishi et al. 2007).
Usually, membrane fusion events are dependent on ligand–receptor interactions. However, studies have suggested that MP may also be engulfed by the endosome pathway or the cargo may be transferred by a physical interaction between MP and the recipient cell, as reviewed by (Mause and Weber 2010).
Microparticles and receptor proteins
EGFR
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein member of the ErbB family, which consists of four members: EGFR (also known as ErbB1 or HER1), ErbB2 (HER2), ErbB3 (HER3) and ErbB4 (HER4). Similar to the other family members, EGFR activation culminates in changes in gene expression, rearrangement of the cytoskeleton, increased cell proliferation and inhibition of apoptosis. In several epithelial tumors, EGFR is constitutively activated. The EGFRvIII mutation produces a constitutively active receptor, which is associated with an unfavorable cancer prognosis. The EGFRvIII mutation is the most frequent genetic alteration in glioblastoma (GBM), accounting for 34–63 % of the cases. However, it has been shown to arise in a variety of other epithelial tumor types such as breast (20–78 % of cases) and lung carcinomas (16–39 % of cases). The EGFRvIII mutated protein can activate a variety of signaling pathways, such as Src/Stat3, PI3K/Akt, Raf/MEK1/2/ERK1/2, Beclin and NFκB, among others (Gan et al. 2013; Jutten and Rouschop 2014).
Al-Nedawi et al. (2008) were the first to detect EGRFvIII in GBM-derived MP. They observed that: (1) EGFRvIII expression in glioma cells induces the release of microvesicles containing flotillin-1, which is a common lipid raft protein. This observation indicates that these microvesicles are indeed MPs derived from lipid rafts-rich membrane regions, (2) these MP merge with the plasma membrane of cells that express the wild-type EGFR, and (3) this fusion further confers the oncogenic activity of EGFRvIII to the recipient cells, which begin to display activation of MAPK and Akt pathways, enhanced expression of EGFRvIII-upregulated genes, morphological transformation and increased anchorage-independent growth ability. The authors concluded that these glioma-derived MPs may be loaded with EGFRvIII and that blockage of MP exchange (for instance, using annexin derivatives) may have therapeutic potential (Al-Nedawi et al. 2008).
In a subsequent study, Al-Nedawi et al. (2009) further demonstrated that these EGFR-containing MPs may also be taken up by other cell types, such as non-malignant endothelial cells, and showed that (1) the recipient cells begin to exhibit EGFR-dependent signaling including MAPK and Akt phosphorylation and (2) MP transfer can indeed be inhibited by PS blockers such as annexin V. Furthermore, they observed that EGFR transfer through glioma-derived MPs is associated with the onset of VEGF (vascular endothelial growth factor) expression and autocrine activation of its receptor (VEGFR), consistent with previous findings that microvascular density was reduced by PS blockers. Diannexin (an annexin analogous) treatment even resulted in reduced tumor growth. Thus, they concluded that the uptake of glioma-derived MPs by normal endothelial cells renders these cells cooperative with the tumor, which in turn provides the endothelial cells with an angiogenic stimulus that favors tumor development (Al-Nedawi et al. 2009).
Together, these studies provided great insight into tumor cell communication within the tumor microenvironment because they demonstrated the lack of a requirement for cell–cell contact in order to transfer the EGFRvIII mutation phenotype horizontally. They argued that glioma cells can share EGFRvIII with wild-type tumor cells, via MPs converting them to a more aggressive phenotype, and with stromal cells, switching them to a tumor-prone phenotype.
HER2
Human epidermal growth factor receptor 2 (HER2/neu/Erb2) is also a member of the EGFR family. It shares many of its properties with EGFR. HER2 overexpression is a common feature of breast (15–30 % of cases) and ovarian (20–30 % of cases) cancer, but it also occurs in a number of others malignancies (head and neck, esophagus, stomach, colon, bladder, lung, etc.), and it may be associated with gene amplification (Iqbal and Iqbal 2014).
Recently, Liebhardt et al. (2010) identified HER2 expression in MPs from breast cancer patients. Although it did not reach statistical significance, the level of HER2-positive MPs were elevated in patients with lymph node metastasis, which suggests that the presence of this Erb family member may be involved in the metastatic ability of breast cancer cells (Liebhardt et al. 2010). This observation deserves further investigation.
Mac-1 integrins
Macrophage1 antigen, or Mac-1, is an αMβ2-integrin, also known as complement receptor 3 (CR3) or CD11/CD18b. It is a leukocyte adhesion receptor of the β2-integrin family, which consists of major players in the leukocyte adhesion cascade (Mitroulis et al. 2015). Although normally expressed only in neutrophils and monocytes, Mac-1 has been found to play an important role in MP-driven metastasis of murine hepatocarcinoma cells (Ma et al. 2013). Ma et al. (2013) observed that upon activation with PMA (phorbol myristate acetate), myeloid splenic cells produce and release MPs loaded with a variety of immune cell markers, including CD3, CD19, F4/80 and Gr-1. When co-incubated with hepatocarcinoma cells, these MPs are taken up by tumor cells, but the MPs do not co-localize with lysosomes, ER or Golgi, suggesting an endosome-independent pathway of uptake that preserves the MPs in the cytoplasm. Moreover, tumor cells that received MPs derived from PMA-treated tumor cells (PMA-MP) grow in several tissues and yield a worse prognosis. Furthermore, PMA-MP-treatment of both melanoma and hepatocarcinoma cells causes a tenfold increase in migration and invasion, suggesting that the MPs derived from activated immune cells could enhance the invasiveness of diverse tumor cell types. Blocking either the CD11b or CD18 subunits of Mac-1 inhibits transwell migration of most cells, diminishes adhesion of tumor cells to the endothelium and improves the overall survival of mice, suggesting that Mac-1 is a fundamental player in the metastasis promoted by activated immune cell-derived MPs. Thus, the authors suggested that activated immune cell-derived MPs may be taken up by tumor cells, providing the tumor with immune cells typical migration features, and facilitating metastasis. Interestingly, Mitroulis et al. (2015) recently reviewed endogenous inhibitors that modify integrin functions and could thus be implicated as promising therapeutic targets (Mitroulis et al. 2015).
CXCR4
CXCR4 is a membrane receptor for CXCL12 (stromal cell-derived factor-1), which is a potent chemoattractant for human progenitor cells. CXCL12 mediates homing to the bone marrow, survival, proliferation and even egress to the circulation (Lapidot et al. 2005). Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration and repopulation in mice (Kahn et al. 2004), whereas disruption of CXCL12/CXCR4-mediated cell anchorage induces the release of hematopoietic progenitors as well as mature cells from the bone marrow into the circulation (Lapidot et al. 2005). In cancer, the CXCL12/CXCR4 interaction has been implicated in acute myeloid leukemia (AML) progression and in solid tumor growth and metastasis (Juarez and Bendall 2004; Zlotnik 2004).
Kalinkovich et al. (2006) described CXCR4 as well as its ligand, CXCL12, in acute myeloid leukemia (AML)-derived MP. CXCR4 molecules could be transferred from MPs to AML cells, enhancing migration toward CXCL12 in vitro and homing to the bone marrow of mice. CXCR4 inhibition diminished these effects, suggesting that CXCR4-loaded MPs are involved in the progression of AML. The levels of CXCR4 and CXCL12 in MPs were also enhanced in the serum of AML patients compared with normal subjects, indicating that these proteins could be used as biomarkers for the disease (Kalinkovich et al. 2006).
Osteopontin
Osteopontin (OPN) is a small integrin-binding ligand N-linked glycoprotein (SIBLING). SIBLING proteins bind to cell surface integrins and CD44 in normal tissues and function as signal transducers to promote cell adhesion, motility and survival through the activation of kinase cascades and transcription factors. They can also regulate processes such as inflammation, immune response and cancer (Bellahcene et al. 2008).
In both non-malignant and cancer cells, OPN has been shown to activate the PI-3K/AKT/NFκB pathway, which promotes cellular proliferation and differentiation, and inhibits apoptosis (Gimba and Tilli 2013). Interaction with CD44 and integrins has been shown to activate this OPN-dependent signaling. In cancer, OPN is also involved in invasion, extracellular matrix degradation, metastasis, inflammation/complement evasion and angiogenesis (Bellahcene et al. 2008; Ramchandani and Weber 2015).
Recently, Fremder et al. (2014) detected a high OPN content in tumor cell-derived MP. Although OPN depletion from MP is not sufficient to reduce tumor growth, they observed that when bone marrow-derived pro-angiogenic cells (BMDC) from paclitaxel-treated mice were injected into OPN-depleted tumor-bearing mice, tumor growth and BMDC infiltration were greatly inhibited (Fremder et al. 2014). These data suggest that OPN-loaded MPs may play important roles in tumor homing and BMDC mobilization, which facilitates angiogenesis.
Microparticles and cancer resistance
Multidrug resistance (MDR) is one of the major obstacles for chemotherapy in several types of neoplasia. The main mechanism of MDR is the overexpression of P-glycoprotein (Pgp/ABCB1), a transporter protein involved in the efflux of anticancer drugs. Pgp belongs to the ABC superfamily of transporters, and others members of this family including multidrug resistance-associated protein 1 (MRP1 or ABCC1) and breast cancer resistance protein (BCRP or ABCG2) are also associated with the efflux of chemical compounds in cancer (Gottesman et al. 2002; Vasconcelos et al. 2011).
In 2009, Bebawy and colleagues demonstrated that Pgp is carried by MPs derived from drug-resistant cancer cells and can be transferred to drug-sensitive cells. Their work also showed transfer of functional Pgp and consequently MDR phenotype acquisition in recipient cells after MPs interaction (Bebawy et al. 2009). In another study, the same group identified the Pgp mRNA and also ABCC1 mRNA in MPs derived from resistant cancer cells and their transfer to recipient cells (Lu et al. 2013). They also showed MDR-related miRNAs in the contents of drug-resistant cell-derived MPs (Jaiswal et al. 2012a).
Jaiswal and co-workers (2013) hypothesized that Pgp transfer by MPs can occur selectively. They showed that MPs derived from resistant breast cancer cells could only transfer their MDR protein content to malignant breast cells and not to non-malignant cells, while resistant leukemic cell-derived MPs could transfer MDR proteins to both malignant and non-malignant cells. These results suggest MPs derived from breast cancer cells display cell-type selectivity on the transfer of MDR features. In this regard, the authors suggested that the CD44 carried by MPs derived from breast cancer cells could contribute to the horizontal transfer of MDR proteins (Jaiswal et al. 2013). Additionally, our group showed that Pgp protein and mRNA as well as miRNAs related to the MDR phenotype are carried in MPs derived from myeloid leukemia cells and that these molecules can be transferred to sensitive breast and lung cancer cells, displaying no cell-type selectivity (de Souza et al. 2015). Moreover, Pasquier et al. (2014) showed that endothelial cells are recipient cells for the MPs derived from both epithelial and mesenchymal cancer cells, suggesting that endothelial cells might be targets for MPs of both origins.
A proteomic study demonstrated that nineteen cytoskeletal proteins and ten proteins involved in regulation of the actin cytoskeletal pathway were detected in the MPs derived from MDR breast cancer cells. These results suggest that these proteins are important for stabilizing Pgp in MPs (Pokharel et al. 2014). Among the identified proteins, CD44, ezrin, radixin and moesin appeared to be involved in the resistance phenotype and metastasis (Donatello et al. 2012). Moreover, CD44 has been demonstrated to interact with Pgp and also to be associated with cell migration and invasiveness (Miletti-Gonzalez et al. 2005), suggesting that the presence of CD44 in MPs may be required at least to facilitate the MDR phenotype transfer from solid tumor cells to drug-sensitive recipient cells (Pokharel et al. 2014).
Ezrin, radixin and moesin proteins were shown to be important for the interaction between the plasma membrane and cytoskeletal proteins (Arpin et al. 2011). The presence of all of these proteins in MPs may facilitate the transfer and intracellular trafficking of Pgp. Probably, other membrane proteins are also involved in Pgp transfer to drug-sensitive cancer cells via MPs (Pokharel et al. 2014). In addition, ezrin plays an important role in Pgp membrane insertion. This protein is carried in MPs, and its expression is unchanged in recipient cells. Therefore, it has been suggested that ezrin is related to Pgp stabilization in MPs and incorporation by the drug-sensitive recipient cells (Brambilla et al. 2012; Jaiswal et al. 2013; Luciani et al. 2002).
Pokharel et al. (2014) also observed CD73 loaded in the MPs derived from MDR breast cancer cells. Their data suggest that Pgp interacts with CD73 along with CD44 in MPs, which further suggests that CD73 may also participate in the MDR phenotype transfer from solid tumor cells to recipient cells (Pokharel et al. 2014). Increased levels of CD73 protein expression were found in several MDR cell lines, and inhibition of its enzymatic activity reverses the MDR phenotype and inhibits tumor cell growth, suggesting that this enzyme is also involved in drug resistance (Ujhazy et al. 1996). Moreover, in human breast cancer cells, CD73 has been shown to promote tumor angiogenesis (Wang et al. 2013), invasion, migration, adhesion and growth (Wang et al. 2008; Zhi et al. 2007).
The aforementioned studies demonstrate that MPs derived from cancer cells are potent disseminators of the drug-resistant phenotype, because MPs can interact with sensitive cancer cells and non-malignant cells as well as with endothelial cells. Studies have also reported the presence of MPs in the plasma of cancer patients (Fleitas et al. 2012; Savasan et al. 2004; Toth et al. 2008). Liebhardt et al. (2010) analyzed the MPs in the plasma of breast cancer patients and identified four major subpopulations of MPs including a group positive for BCRP (BCRP+). The authors observed that patients with lymph node metastases showed elevated levels of BCRP+ MPs compared to patients with initial disease and attributed this increase to enhanced cancer growth and dissemination in these patients (Liebhardt et al. 2010).
The clinical relevance of the MPs role on pathological processes has been comprehensively discussed by several researchers. Gong and co-workers (2015) emphasized the clinical importance of MPs on regarding drug resistance and metastasis. On their review, the MPs potential on cancer diagnosis and prognosis was also discussed (Gong et al. 2015). In addition, El Andaloussi and co-workers (2013) largely reviewed the role of MPs in tumor biology. They argued about the importance of MPs in promoting cancer progression, because MPs transport and transfer molecules associated with tumor growth, immune system escape by tumors and angiogenesis (El Andaloussi et al. 2013).
Microparticles and microRNAs
MicroRNAs (miRNAs) are small non-coding RNAs (18–24 nucleotides) that regulate gene expression by binding to mRNAs, leading to mRNA degradation or impairment of translation. Briefly, miRNAs are processed by a complex machinery composed by the endonucleases Drosha and Dicer, and by a protein complex called RISC (RNA silencing complex). The central components of RISC are Argonaute proteins (Ago), primarily Ago-2. Ago proteins mediate the binding of mature miRNAs to mRNA (Winter et al. 2009). Each miRNA has hundreds of predicted targets, and it is believed that 60 % of mRNAs are regulated by miRNAs (Jansson and Lund 2012). In cancer, they are deregulated and involved in tumorigenesis, tumor progression, metastasis and resistance to treatment. Furthermore, miRNAs have recently been proposed as promising biomarkers for cancer diagnosis and follow-up studies on disease progression, because they can be detected in body fluids, carried by MPs or associated with proteins that protect them from degradation (Fujiwara et al. 2014; Inns and James 2015; Jackson et al. 2014; Lindner et al. 2015; Liu and Xiao 2014; Pimentel et al. 2014). Several groups are attempting to uncover the underlying mechanisms involved in miRNA selection, release and transport to blood fluids and their role in cancer (Arroyo et al. 2011; Li et al. 2012; Turchinovich et al. 2011). However, the majority of the studies that have attempted to elucidate the mechanism of miRNAs transfer among cells did not differentiate between MPs and exosomes, analyzing them together as MVs. Arroyo et al. (2011) demonstrated that only 15 % of circulating miRNAs, including let-7a and miR-142-3p, are found in MVs (exosomes and MPs). The majority of the circulating miRNAs is found in non-vesicular fractions associated with ribonucleoprotein complexes containing Ago-2 protein (Arroyo et al. 2011). The association of miRNAs and Ago-2 in MPs was also identified in cancer cells undergoing apoptosis and in those in which differentiation had been induced, suggesting that Ago-2 confers higher stability to miRNAs molecules (Li et al. 2012).
However, distinguishing between these types of vesicles is very important because they transport different cargos, as demonstrated by Ji and colleagues (2014). These authors separated exosomes from MPs derived from a colon carcinoma cell line and demonstrated that some miRNAs including Let-7 family members and miR-451a are common to all vesicles, but other miRNAs are specific for either MPs or exosomes. They found four miRNAs selectively carried in MPs: miR-675-5p, miR-7704, miR-98-5p and miR-664a-3p (Ji et al. 2014).
Some studies have investigated the role of MPs in the resistance to cancer treatment. Using parental acute lymphocytic leukemia (ALL) and breast cancer cells along with their MDR counterparts, Jaiswal and colleagues (2012a) demonstrated the effect of MDR cell-derived MPs on their respective parental cell lines. The authors identified transcripts of Drosha, Dicer and Ago-2 in MPs derived from both parental and MDR cell lines. This study supports the general MVs findings that suggest that miRNA machinery proteins may be important to guarantee the activity of the miRNA molecules (Jaiswal et al. 2012a). Additionally, the authors also identified miR-1228*, miR-1246, miR-1308, miR-149*, miR-455-3p, miR-638 and miR-923 in MPs derived from both MDR cell lines. These miRNAs are transferred to drug-sensitive cells and can activate important pathways associated with the vesiculation of MPs, including the calcium signaling pathway and regulation of the actin cytoskeletal pathway, as well as pathways associated with MDR phenotype and oncogenesis (Jaiswal et al. 2012a; b).
miR-503 downregulation and the resistance phenotype
Gong et al. (2014) identified miR-22-3p, miR-185-5p, miR-503-5p, miR-652-3p, miR-1280 in MPs associated with the acquisition of the MDR phenotype. Among these, miR-503 was overexpressed in drug-sensitive cells and downregulated in drug-resistant cells. The authors observed that MPs derived from drug-resistant cells can inhibit miR-503 expression in recipient cells and promote migration and invasion (Gong et al. 2014). It was shown that miR-503 inhibits PI3Kp85 and IKKβ and consequently, Akt phosphorylation and NFκB activation (Yang et al. 2014). In agreement with this, our group and others have demonstrated that activation of these signaling pathways by MPs confers a resistance phenotype (de Souza et al. 2015; Pasquier et al. 2014; Wysoczynski and Ratajczak 2009). miRNA-503 downregulation was also observed in lung and hepatocellular cancers and in highly invasive breast cancer cells, and its overexpression induces G1 arrest, reduces cell migration and invasiveness in vitro and in vivo (Gong et al. 2014; Yang et al. 2014; Zhou and Wang 2011). One study demonstrated that in response to a microbial challenge, epithelial cells overexpress CX3CL1 in a NFκB- and Dicer-dependent manner. This effect was associated with the downregulation of miR-503, which directly inhibits CX3CL1 expression (Zhou et al. 2013). CX3CL1 is a membrane-bound chemokine that can be shed in a soluble form or through release of MPs (Bazan et al. 1997; Castellana et al. 2009). In addition, Castellana et al. (2009) showed that fibroblasts activated by tumor MPs in turn release MPs containing CX3CL1 and induce a migratory phenotype in recipient prostate cancer cells. Together, these findings suggest that miR-503 downregulation in cancer cells may be mediated by Dicer, CX3CL1 and probably by other MP cargo.
PYK2 regulation by CD44, miR-494 and miR-303-3p
As miR-503 was transported by MPs but was not detected in recipient cells, the opposite situation was observed for proline-rich tyrosine kinase 2 (PYK2) (Gong et al. 2014). PYK2 is a cytoplasmic tyrosine kinase of the focal adhesion kinase subfamily (Fan and Guan 2011). PYK2 promotes cell migration and drug resistance in many tumor types including hepatocellular and breast cancer and is activated by the PI3K/Akt signaling pathway (Datta et al. 2015; Geng et al. 2011). PYK2 mRNA and protein were detected in recipient cells after co-culture with the MDR counterpart cell line, although PYK2 was not detected in MPs. These observations suggest that MPs carry and transfer intermediates that result in the inhibition of miR-503 and PYK2 expression in the recipient cells (Gong et al. 2014). Jaiswal and colleagues demonstrated that CD44 is carried by the MPs but is not detected in the recipient cells and that CD44 promotes PYK2 phosphorylation. This observation suggests that somehow the interaction with CD44 stimulates activation of endogenous PYK2 in the recipient cells. miR-494 and miR-330-3p are regulators of PYK2 expression (Gong et al. 2014) and were modulated in recipient cells. miR-494, an inhibitor of a repressor of PYK2, was transported by MPs and incorporated into the recipient cells. Conversely, miR-330-3p is a repressor of PYK2, and its expression was inhibited after co-culture, suggesting that PYK2 expression might be regulated by these miRNAs in association with CD44 through cellular signaling pathways (Jaiswal et al. 2013). In conclusion, the regulatory role of MPs in cancer cells is a remarkable, complex and modestly understood intercellular communication mechanism.
microRNAs involved with Efflux pumps
Likewise, the miRNAs involved with Pgp and MRP1 regulation are transferred by MPs to drug-sensitive cancer cells. miR-27a and miR-451 indirectly promote Pgp overexpression, while miR-326 inhibits MRP1 expression (Liang et al. 2010). Zhu et al. (2008) demonstrated that miR-27a and miR-451 positively regulate Pgp/ABCB1 expression. However, other reports have demonstrated that miR-27a is downregulated in Pgp-positive leukemia cells (Feng et al. 2011). Jaiswal et al. (2012b) demonstrated that a MDR leukemia cell line transfers miR-326 to the sensitive counterpart which is associated with MRP1 downregulation in the cells. Moreover, the breast cancer MDR-derived MPs transfer miR-326 and miR-27a, but the miR-326 expression did not correlate with MRP1 downregulation. MPs carry miRNAs from the donor cells, but not all of them are incorporated by the recipient cells. These results suggest that the MP regulatory mechanisms that control MP formation and incorporation differ among cell types (Jaiswal et al. 2012b). Corroborating these findings, our group demonstrated that the chronic myeloid leukemia (CML) cell lines K562 and Lucena (a MDR-positive line derived from K562 by continuous exposure to vincristine) release MPs and that the Lucena-derived MPs carried Pgp protein and mRNA (ABCB1). Furthermore, the co-cultures of Lucena cell line or the Lucena-derived MP with recipient cells (breast and lung cancer models) transferred miR-27a and miR-451. However, an important oncogenic miRNA, miR-21, was transferred only to the breast cancer cells, reinforcing the concept that transfer of cargo by MPs differs among the recipient cell lines because it occurs in a cell-type-dependent manner (de Souza et al. 2015; Jaiswal et al. 2013).
Signaling pathways and resistance mediated by MPs
Several reports have demonstrated that the molecules carried in cancer cell vesicles can activate diverse signaling pathways including STAT, PI3K-Akt and MAPK, and promote angiogenesis, metastasis and treatment resistance in the recipient cells (Lam et al. 2013). However, the majority of these studies do not differentiate between MPs and other vesicles. Here, we focus specifically on MP-related mechanisms.
Uptream pathways: STAT, PI3K-Akt and MAPK
STAT activation has been strongly associated with the induction of angiogenesis, and this activation may be mediated by MPs containing miRNAs. MPs released by pancreatic adenocarcinoma, colorectal adenocarcinoma and lung carcinoma cells induce STAT3 activation in monocytes, an important pathway for cytokine production and activation of these cells (Baj-Krzyworzeka et al. 2007).
A non-pure fraction of MPs derived from human and murine lung cancer cell lines activates the Akt and MAPK pathways in human umbilical vein endothelial cells (HUVEC) and in tumor-associated fibroblasts (TAF) inducing the expression of pro-angiogenic factors, cytokines, adhesion molecules and metalloproteinases (MMPs), without inducing HUVEC proliferation. Once stimulated by MPs, endothelial cells and fibroblasts, in turn, influence tumor cells by inducing STAT3, Akt and MAPK activation and by enhancing the metastatic potential of lung tumor cells in vivo (Wysoczynski and Ratajczak 2009). Corroborating these findings, MPs derived from prostate cancer cells transfer MMPs to fibroblasts, activate ERK1/2 and consequently induce an increase in active MMP-9. MMPs are involved in extracellular matrix degradation allowing cancer cell migration and dissemination. Additionally, MP-induced chemoresistance in fibroblasts was shown to be reversed by ERK1/2 inhibitors, which demonstrates the role of this pathway in the acquired resistance. In turn, the activated fibroblasts induce cancer cell migration and invasion in a fibroblast MP-dependent manner (Castellana et al. 2009).
In a similar way, Antonyak and colleagues (2011) co-cultured glioblastoma- and breast cancer cell-derived MPs with non-tumoral cells. Cancer cell-derived MPs induced Akt and ERK activation, promoting enhanced cellular survival, growth in medium with low serum concentration and anchorage-independent growth, all of which are characteristics of transformed cells. TG2 was found in the outer leaflet of the MP membranes. The authors associated the oncogenic transformation of the non-tumoral cells with the transfer of TG2 by the MPs. Nevertheless, TG2 alone was not sufficient for cell transformation; TG2 cross-linking with fibronectin was required. In addition, the breast cancer cell-derived MPs promoted aberrant growth of non-malignant mammary epithelial cells. Finally, breast cancer cells from murine tumors secrete MPs and induce transformation of fibroblasts in vivo (Antonyak et al. 2011).
Pasquier et al. (2014) observed that MPs derived from cells with a mesenchymal phenotype (M–MPs) induce proliferation, motility and activation of endothelial HUVEC cells, while MPs derived from cells with an epithelial phenotype (E-MPs) could not induce the same effect. M–MPs induced Akt phosphorylation and activation of this pathway by upregulation of Arf-6 expression. In addition, using an endothelial cell model in which Akt was constitutively activated, the authors demonstrated that MPs induced cellular resistance to doxorubicin and taxol. The M–MPs also induced the formation of spheres and epithelial-mesenchymal transition (EMT) and as a consequence, an increase in the pro-metastatic phenotype of tumor cells (Pasquier et al. 2014). In agreement with this study, we have previously observed that after co-culture with MPs derived from an MDR cell line, breast or lung cancer recipient cells become resistant to paclitaxel and cisplatin. To further clarify the pathways modulated in recipient cells by the MDR-positive MPs, activation of Akt pathway in breast cancer cells and NFκB transcription factor nuclear localization in both recipient cell lines were demonstrated (de Souza et al. 2015). Collectively, these studies strengthen the evidence for a role of Akt pathway in the acquired resistance, demonstrating once more that tumor-derived MPs can induce a drug-resistant phenotype (de Souza et al. 2015; Pasquier et al. 2014).
Inhibitor of apoptosis proteins
Furthermore, MPs derived from fibrosarcoma cells activate the NFκB signaling pathway in recipient mesenchymal stem cells. This activation is associated with the upregulation of several targets of this pathway that are involved in cell survival including MMP-1, MMP-3, BIRC3, NFκB1 and TNF. It is noteworthy that the authors of this work studied MPs in a plasmatic membrane vesicle-enriched fraction, containing approximately 25 % exosomes. Among the activated genes, BIRC3 encodes a member of the inhibitor of apoptosis protein (IAP) family that is important for cancer cell survival (Lozito and Tuan 2014; Verhagen et al. 2001).
Survivin and XIAP are the most studied and best characterized IAP family members. These proteins are overexpressed in several types of cancer and are associated with treatment resistance and poor prognosis (Faccion et al. 2012; Fulda and Vucic 2012). Our group was the first to demonstrate that MPs transfer IAP proteins and mRNAs including survivin, XIAP and cIAP1 to recipient cells (de Souza et al. 2015). Honegger and colleagues demonstrated that cervical cancer-derived extracellular vesicles carry IAPs including survivin, cIAP1, XIAP and livin. However, these vesicles were not a pure MP fraction. (Honegger et al. 2013). Our group also observed that after co-culture, lung cancer cells expressed higher amounts of survivin, XIAP and cIAP protein and mRNA (BIRC5, BIRC4 and BIRC2, respectively), while breast cancer cells expressed higher levels of XIAP with no increase in cIAP1 mRNA (BIRC2), suggesting a selectivity with respect to MP cargo transfer (de Souza et al. 2015). In addition, because XIAP mRNA (BIRC4) was not detected in the MDR cell line-derived MP, but was detected in recipient cells, others intermediates carried by MPs may regulate the gene expression in recipient cells.
Activation of cellular signaling pathways by MPs is also associated with miRNAs. The oncomiR (oncogenic miRNA) miR-21 inhibits proteins associated with cell survival and apoptosis pathways, including PDCD4 (programmed cell death 4) and PTEN. This inhibition induces Akt and NFκB activation and results in cellular transformation (Iliopoulos et al. 2010). Our group identified miR-21 as a content of leukemic MDR cell-derived MPs. We analyzed miR-21 in recipient cells and observed an association of this miRNA with NFκB activation mediated by Akt phosphorylation in these cells (de Souza et al. 2015). In summary, MPs can mediate activation of signaling pathways associated with cancer survival in recipient cells by carrying and transferring miRNAs or other regulatory molecules that modify the cellular gene/protein expression patterns.
Conclusion and future perspectives
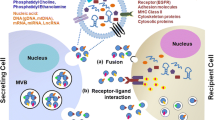
In this review, we have described recent findings regarding the contents of MPs and how these vesicles influence the acquisition of an MDR or metastatic phenotype in recipient cells (Fig. 1). Gaining better understanding of MPs role in cancer cell communication and its distinct role regarding each cell type will provide future tools for cancer therapy. Although MPs study and understanding are developing fast, many issues concerning its role and properties have not being clarified yet.
Schematic representation of a membrane microparticles (MPs) budding from donor cell and cargo content transference to recipient cell. MPs are released from donor cells after increase in intracellular Ca2+ which promotes phospholipids translocases response and consequently cytoskeleton disruption. MPs cargo can include membrane and solubles proteins, and microRNAs. MPs content can be transferred to recipient cells and promote oncogenic pathways activation
First, a notable and major challenge in the study of MPs is the similarity between MPs and other cellular vesicles, such as exosomes; because these are all small microvesicles, their profiles may overlap, depending on the methodology employed to study them. Thus, properly differentiating the types of MVs is primordial to improve our understanding of their specific roles. However, up until now few studies analyze microvesicles properly, and in most, a mixture of very different types of microvesicles are included in the same analysis. In addition, terms such as oncosomes, microvesicles, microparticles, endosomes, exosomes and ectosomes present a challenge, as they often appear in the nomenclature as synonyms when indeed they are not. Thus, many controversial data are obtained because the literature lacks standardized nomenclature. Markers to identify and differentiate these vesicles and refined protocols for extraction and purification are also urgently needed. To move forward and to fill the empty gaps with reliable results, it is required to properly differentiate MVs structures. Cellular MVs are in fact the collective of MPs and exosomes, and they may have some common cargo molecules. In this review, we made an effort to select studies that exclusively focus on MPs on the basis that there are major biological differences between these and other MVs and that studies on single forms of microvesicles are required to properly understand the contribution of each microvesicle type to cellular communication.
Second, modulation of MPs release, uptake and its surface molecules have emerged as a promising therapeutic option and several groups are trying to reduce MPs cancer cells release and their uptake by recipient cells in order to impair cancer progression as recently reviewed by El Andaloussi (El Andaloussi et al. 2013). Furthermore, cellular engineering using MVs as a drug delivery system can be a less toxic treatment option because MVs are biocompatible, can cross biological barriers such as the blood–brain barrier (BBB), do not activate immune responses against them and can deliver nucleic acids and drugs, becoming an promissing tool for gene therapy (Alvarez-Erviti et al. 2011; Zhuang et al. 2011). Finally, further understanding the role of these vesicles in the communication between cancer cells and the microenvironment (such as extracellular matrix elements, stromal and immune cells) will contribute to elucidate an important gap in our current understanding of the cancer cells network. However, there is still much more to be learnt in each one of these fields. In conclusion, MPs play a remarkable, complex and incompletely understood role in cancer cell communication.
References
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10(5):619–624
Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA 106(10):3794–3799
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
Antonyak MA et al (2011) Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA 108(12):4852–4857
Arpin M, Chirivino D, Naba A, Zwaenepoel I (2011) Emerging role for ERM proteins in cell adhesion and migration. Cell Adhes Migr 5(2):199–206
Arroyo JD et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108(12):5003–5008
Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Zembala M (2007) Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett 113(2):76–82
Bazan JF et al (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385(6617):640–644
Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE (2009) Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 23(9):1643–1649
Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS (2008) Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer 8(3):212–226
Brambilla D et al (2012) P-glycoprotein binds to ezrin at amino acid residues 149–242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int J Cancer 130(12):2824–2834
Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, Kunzelmann C (2009) Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1–CX3CR1 axis. Cancer Res 69(3):785–793
Chen P, Douglas SD, Meshki J, Tuluc F (2012) Neurokinin 1 receptor mediates membrane blebbing and sheer stress-induced microparticle formation in HEK293 cells. PLoS ONE 7(9):e45322
Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJ, Wiley JS, Sluyter R (2010) P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta 1798(9):1797–1804
Datta A et al (2015) Selective targeting of FAK-Pyk2 axis by alpha-naphthoflavone abrogates doxorubicin resistance in breast cancer cells. Cancer Lett 362(1):25–35
de Souza PS, Cruz AL, Viola JP, Maia RC (2015) Microparticles induce multifactorial resistance through oncogenic pathways independently of cancer cell type. Cancer Sci 106(1):60–68
Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA (2005) Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106(5):1604–1611
Donatello S, Babina IS, Hazelwood LD, Hill AD, Nabi IR, Hopkins AM (2012) Lipid raft association restricts CD44-ezrin interaction and promotion of breast cancer cell migration. Am J Pathol 181(6):2172–2187
D’Souza-Schorey C, Chavrier P (2006) ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7(5):347–358
El Andaloussi S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347–357
Enjeti AK, Lincz LF, Seldon M (2008) Microparticles in health and disease. Semin Thromb Hemost 34(7):683–691
Faccion RS, Rezende LM, Romano Sde O, Bigni Rde S, Mendes GL, Maia RC (2012) Centroblastic diffuse large B cell lymphoma displays distinct expression pattern and prognostic role of apoptosis resistance related proteins. Cancer Invest 30(5):404–414
Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM (1998) The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ 5(7):551–562
Fan H, Guan JL (2011) Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J Biol Chem 286(21):18573–18582
Feng DD et al (2011) Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med 15(10):2164–2175
Fleitas T et al (2012) Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS ONE 7(10):e47365
Fremder E et al (2014) Tumor-derived microparticles induce bone marrow-derived cell mobilization and tumor homing: a process regulated by osteopontin. Int J Cancer 135(2):270–281
Fujiwara T, Kunisada T, Takeda K, Uotani K, Yoshida A, Ochiya T, Ozaki T (2014) MicroRNAs in soft tissue sarcomas: overview of the accumulating evidence and importance as novel biomarkers. BioMed Res Int. Article ID 592868
Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11(2):109–124
Gan HK, Cvrljevic AN, Johns TG (2013) The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J 280(21):5350–5370
Geng W et al (2011) The role of proline rich tyrosine kinase 2 (Pyk2) on cisplatin resistance in hepatocellular carcinoma. PLoS ONE 6(11):e27362
Gimba ER, Tilli TM (2013) Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Lett 331(1):11–17
Gong J, Luk F, Jaiswal R, Bebawy M (2014) Microparticles mediate the intercellular regulation of microRNA-503 and proline-rich tyrosine kinase 2 to alter the migration and invasion capacity of breast cancer cells. Front Oncol 4:220
Gong J, Jaiswal R, Dalla P, Luk F, Bebawy M (2015) Microparticles in cancer: a review of recent developments and the potential for clinical application. Semin Cell Dev Biol 40: 35–40
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58
Hoffmann PR et al (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 155(4):649–659
Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F (2013) Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int J Cancer 133(7):1631–1642
Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM (2005) Membrane microparticles: two sides of the coin. Physiology 20:22–27
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K (2010) STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 39(4):493–506
Inns J, James V (2015) Circulating microRNAs for the prediction of metastasis in breast cancer patients diagnosed with early stage disease. Breast 24(4):364–369
Iqbal N, Iqbal N (2014) Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int 2014:852748
Jackson BL, Grabowska A, Ratan HL (2014) MicroRNA in prostate cancer: functional importance and potential as circulating biomarkers. BMC Cancer 14:930
Jaiswal R et al (2012a) Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB J 26(1):420–429
Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M (2012b) Microparticle conferred microRNA profiles–implications in the transfer and dominance of cancer traits. Mol Cancer 11:37
Jaiswal R, Luk F, Dalla PV, Grau GE, Bebawy M (2013) Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PLoS ONE 8(4):e61515
Jansson MD, Lund AH (2012) MicroRNA and cancer. Mol Oncol 6(6):590–610
Ji H, Chen M, Greening DW, He W, Rai A, Zhang W, Simpson RJ (2014) Deep sequencing of RNA from three different extracellular vesicle (EV) subtypes released from the human LIM1863 colon cancer cell line uncovers distinct miRNA-enrichment signatures. PLoS ONE 9(10):e110314
Jouvenet N (2012) Dynamics of ESCRT proteins. Cell Mol Life Sci 69(24):4121–4133
Juarez J, Bendall L (2004) SDF-1 and CXCR4 in normal and malignant hematopoiesis. Histol Histopathol 19(1):299–309
Jutten B, Rouschop KM (2014) EGFR signaling and autophagy dependence for growth, survival, and therapy resistance. Cell Cycle 13(1):42–51
Kahn J et al (2004) Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 103(8):2942–2949
Kalinkovich A et al (2006) Functional CXCR4-expressing microparticles and SDF-1 correlate with circulating acute myelogenous leukemia cells. Cancer Res 66(22):11013–11020
Lam D, Barre B, Guette C, Coqueret O (2013) Circulating miRNAs as new activators of the JAK-STAT3 pathway. JAK-STAT 2(1):e22996
Lapidot T, Dar A, Kollet O (2005) How do stem cells find their way home? Blood 106(6):1901–1910
Li L et al (2012) Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS ONE 7(10):e46957
Liang Z et al (2010) Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol 79(6):817–824
Liebhardt S et al (2010) CEA-, Her2/neu-, BCRP- and Hsp27-positive microparticles in breast cancer patients. Anticancer Res 30(5):1707–1712
Lindner K, Haier J, Wang Z, Watson DI, Hussey DJ, Hummel R (2015) Circulating microRNAs: emerging biomarkers for diagnosis and prognosis in patients with gastrointestinal cancers. Clin Sci 128(1):1–15
Liu HS, Xiao HS (2014) MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol 20(34):12007–12017
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4(2):140–156
Lozito TP, Tuan RS (2014) Endothelial and cancer cells interact with mesenchymal stem cells via both microparticles and secreted factors. J Cell Mol Med 18(12):2372–2384
Lu JF, Luk F, Gong J, Jaiswal R, Grau GE, Bebawy M (2013) Microparticles mediate MRP1 intercellular transfer and the re-templating of intrinsic resistance pathways. Pharmacol Res 76:77–83
Luciani F et al (2002) P-glycoprotein-actin association through ERM family proteins: a role in P-glycoprotein function in human cells of lymphoid origin. Blood 99(2):641–648
Ma J et al (2013) Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin alpha(M)beta(2) to tumor cells. J Immunol 191(6):3453–3461
Mause SF, Weber C (2010) Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107(9):1047–1057
McCullough J, Colf LA, Sundquist WI (2013) Membrane fission reactions of the mammalian ESCRT pathway. Ann Rev Biochem 82:663–692
Meshki J, Douglas SD, Lai JP, Schwartz L, Kilpatrick LE, Tuluc F (2009) Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J Biol Chem 284(14):9280–9289
Miletti-Gonzalez KE et al (2005) The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res 65(15):6660–6667
Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T (2015) Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther 147:123–135
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450(7168):435–439
Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C (2009) ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19(22):1875–1885
Park D et al (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450(7168):430–434
Pasquier J et al (2014) Microparticles mediated cross-talk between tumoral and endothelial cells promote the constitution of a pro-metastatic vascular niche through Arf6 up regulation. Cancer Microenviron 7(1–2):41–59
Piccin A, Murphy WG, Smith OP (2007) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21(3):157–171
Pimentel F et al. (2014) Technology in MicroRNA Profiling: Circulating MicroRNAs as Noninvasive Cancer Biomarkers in Breast Cancer. J Lab Autom [Epub ahead of print]
Pokharel D, Padula MP, Lu JF, Tacchi JL, Luk F, Djordjevic SP, Bebawy M (2014) Proteome analysis of multidrug-resistant, breast cancer-derived microparticles. J Extracell Vesicles. doi:10.3402/jev.v3.24384
Qu Y, Dubyak GR (2009) P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal 5(2):163–173
Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179(3):1913–1925
Ramchandani D, Weber GF (2015) Interactions between osteopontin and vascular endothelial growth factor: implications for cancer. Biochim Biophys Acta 1855(2):202–222
Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang L (2014) Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochimica et Biophysica Acta. doi:10.1016/j.bbamem.2014.10.029
Salzer U, Hinterdorfer P, Hunger U, Borken C, Prohaska R (2002) Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 99(7):2569–2577
Savasan S, Buyukavci M, Buck S, Ravindranath Y (2004) Leukaemia/lymphoma cell microparticles in childhood mature B cell neoplasms. J Clin Pathol 57(6):651–653
Schlienger S, Campbell S, Claing A (2014) ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol Biol Cell 25(1):17–29
Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG (2004) Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol 125(6):804–813
Toth B et al (2008) Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res 28(2A):1107–1112
Turchinovich A, Weiz L, Langheinz A, Burwinkel B (2011) Characterization of extracellular circulating microRNA. Nucleic Acids Res 39(16):7223–7233
Ujhazy P, Berleth ES, Pietkiewicz JM, Kitano H, Skaar JR, Ehrke MJ, Mihich E (1996) Evidence for the involvement of ecto-5’-nucleotidase (CD73) in drug resistance. Int J Cancer 68(4):493–500
van den Akker J et al (2012) Transglutaminase 2 is secreted from smooth muscle cells by transamidation-dependent microparticle formation. Amino Acids 42(2–3):961–973
Vasconcelos FC, Silva KL, Souza PS, Silva LF, Moellmann-Coelho A, Klumb CE, Maia RC (2011) Variation of MDR proteins expression and activity levels according to clinical status and evolution of CML patients. Cytom Part B Clin Cytom 80(3):158–166
Verhagen AM, Coulson EJ, Vaux DL (2001) Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol 2(7):3009
Wang L et al (2008) Ecto-5’-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol 134(3):365–372
Wang L et al (2013) Ecto-5’-nucleotidase (CD73) promotes tumor angiogenesis. Clin Exp Metastasis 30(5):671–680
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234
Wysoczynski M, Ratajczak MZ (2009) Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer 125(7):1595–1603
Yang Y et al (2014) MiR-503 targets PI3K p85 and IKK-beta and suppresses progression of non-small cell lung cancer. Int J Cancer 135(7):1531–1542
Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L (2007) RNA interference of ecto-5’-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis 24(6):439–448
Zhou J, Wang W (2011) Analysis of microRNA expression profiling identifies microRNA-503 regulates metastatic function in hepatocellular cancer cell. J Surg Oncol 104(3):278–283
Zhou R, Gong AY, Chen D, Miller RE, Eischeid AN, Chen XM (2013) Histone deacetylases and NF-kB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. PLoS ONE 8(5):e65153
Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM (2008) Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 76(5):582–588
Zhuang X et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19(10):1769–1779
Zlotnik A (2004) Chemokines in neoplastic progression. Semin Cancer Biol 14(3):181–185
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação para Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ). PSS and RSF were supported by postdoctoral fellowships from Ministério da Saúde/Instituto Nacional de Câncer. PSB was supported by a “Nota 10” Ph.D. scholarship from (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflict of interest.
Additional information
Paloma Silva de Souza, Roberta Soares Faccion and Paula Sabbo Bernardo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
de Souza, P.S., Faccion, R.S., Bernardo, P.S. et al. Membrane microparticles: shedding new light into cancer cell communication. J Cancer Res Clin Oncol 142, 1395–1406 (2016). https://doi.org/10.1007/s00432-015-2029-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2029-8