Abstract
Exosomes are a specific population of extracellular vesicles (EVs) that originate from an endocytic process. Virtually every cell type secretes exosomes and their size ranges from 40 to 150 nm. Exosomes are surrounded by a lipid bilayer and contain functional cargo that comprises proteins, lipids and genetic material such as protein, RNA and DNA. In the recent years, several studies have reported the role of exosomes as mediators of intercellular communication. Exosomes serve as vehicles used by cancer cells and stromal cells to influence both local and distant metastatic sites, by reprogramming recipient cells. This chapter will focus on the mechanisms underlying the role of exosomes in tumor development, metastasis, immune escape, therapy resistance, microenvironment reprogramming and angiogenesis. Furthermore, we will also discuss the potential to target exosomes as a new therapeutic strategy in cancer.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Exosomes: Structure, Cargo and Origin
Normal and cancer cells release vesicles into the extracellular space. Typically, these vesicles have sizes in the range of hundreds to thousand nanometers and consist of a lipid bilayer that encloses proteins from the cytosol and from organelles, as well as nucleic acids such as RNA and DNA [1, 2]. Besides these common features, evidence shows that extracellular vesicles [3] are not homogeneous in their morphology and molecular content [4, 5]. This diversity is partially due to the fact that released vesicles derive from different subcellular locations. Larger vesicles are classically associated with a plasma-membrane origin [6], while smaller vesicles with a diameter inferior to 150 nm are associated with an endosomal origin [7]. Exosomes fall into this last category of EVs. With sizes ranging from 40 to 150 nm, they are produced within multivesicular bodies (MVB) that upon fusion with the plasma membrane release the exosomes to the extracellular space (Fig. 3.1). EVs heterogeneity is currently a highly discussed topic in the field and effort is being made to develop a systematic classification according to the already well-established features for each vesicle type [10]. In this chapter we discuss studies that respect the most accepted criteria to classify EVs, as it is size range, endosomal-origin associated markers and isolation methods.
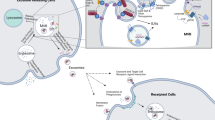
Exosomes-mediated communication in cancer. The biogenesis of exosomes initiates through the invagination of the cellular membrane into an early endosome. After the inward budding of the membrane of the endosome the ILVs are formed. The mature endosome with the ILVs are called MVB then fuses with the plasma membrane and releases its content to the extracellular space, as exosomes. In the extracellular space exosomes interact with nearby and distant cells, playing several roles that ultimately contribute to cancer formation and fuels disease progression. Exosomes are capable of modulating the expression and differentiation of fibroblasts [8, 9], degrading the ECM, and prepare the tissue for the arrival of cancer cells, thus promoting the formation of the pre-metastatic niche. The angiogenic and immunomodulatory features of exosomes have also been recently addressed which furthers implicates exosomes and its effectors in the carcinogenic process
Johnstone first observed exosomes in 1987; he was interested in understanding how the transferrin receptor (TfR) was secreted by reticulocytes [11]. By tracing this receptor with electron microscopy (EM) , they found that TfR co-localized with nanosized round-shaped structures inside MVBs of endosomal origin [11]. The endocytic pathway comprises the processes of internalization of extracellular components, lipids and membrane proteins [12]. Upon endocytosis, endosomes are formed by the inward folding of the PM resulting in sac-like structures commonly found in the cell cytoplasm [13]. These initial endosomes, also known as early endosomes, collect the endocytosed cargo and are responsible for its sorting. Early endosomes mature into late endosomes and during this maturation process, they form vesicles that bud into their lumen, intraluminal vesicles (ILVs) that correspond to future exosomes (Fig. 3.1). Due to their vesicular anatomy, these endosomes are called MVBs . MVBs can then fuse with lysosomes leading to their content degradation or fuse with the PM in an exocytosis process [13]. While ILVs are produced, they capture proteins and nucleic acids present in the cytoplasm [4], particularly the protein loading is carried by the endosomal sorting complex (ESCRT) , whose -0, -I and -II complexes recognize and sequestrate ubiquinated proteins in the endosomal membrane [16], while ESCRT-III is responsible for the inward budding of the plasma membrane [17]. Biogenesis of ILVs can also occur via an ESCRT-independent mechanism, for example through the action of sphingolipid ceramide [18]. Although these sorting mechanisms are not yet fully understood, it is believed that exosomes cargo is somewhat tailored by the cell. Therefore, the cargo of exosomes does not necessarily fully mimics the composition of the donor cell and can be enriched in certain components [19].
Rab proteins are GTPases involved in intracellular vesicular transport and play an important role in guiding and processing the MVBs. Distinct Rabs act during exosomes biogenesis. Rab11 and Rab35 carry their role in the early endosomes promoting the docking and fusion of MVBs [20]. On the other hand, RAB27A and RAB27B are involved in the release of exosomes to the extracellular space by locating the MVBs close to the PM and managing its docking in order for the fusion to occur [21, 22]. As a result of their endosomal origin, exosomes are characterized by the presence of proteins involved in membrane transport and fusion processes, such as the mentioned Rabs, annexins and flotilins, components of the ESCRT complex , tumor susceptibility gene 101 (TGS101), heat shock proteins (HSP60, HSP70 and HSP90) [23], integrins and tetraspanins including CD81, CD63 and CD9 [7, 24]. Likewise, the double inward membrane invagination that originates the MVBs, allows the membrane-domains and receptors to retain the PM original orientation, meaning these proteins are capable of maintaining their molecular function.
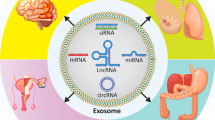
Regarding RNA cargo, exosomes are specially enriched in non-coding RNAs such as microRNAs. MicroRNAs consists of small RNA fragments of 20–22 nucleotides that imprecisely pair with mRNA silencing their expression and inhibiting their protein synthesis [25]. Because many microRNAs are located in cancer-associated genomic regions, there are several alterations in their expression that correlate with tumor initiation and progression [26]. Interestingly when analyzing human serum for microRNA content, the majority of the microRNAs are concentrated in the exosomes when compared with cell-free RNA [27]. Recent reports show that tumor-derived exosomes contain distinct microRNA profiles in many cancers, including gastric [28], hepatocellular carcinoma [29], breast [30], and ovarian cancer [31]. In glioblastoma-derived exosomes , the miR-21 is functionally active in host cells and positively impacts the proliferation of cancer cells [3].
Additionally to RNA and proteins, exosomes contain mitochondrial DNA [32], single stranded DNA, transposable elements [2] and more recently double stranded DNA [33]. Pancreatic cancer (PC) derived exosomes contain dsDNA fragments with a size >10-kb spanning all chromosomes and it is possible to detect mutations on TP53 and KRAS genes that are frequently found altered in these tumors [33]. Also using circulating exosomal DNA KRASG12D and TP53R273H mutations (Fig. 3.2) are detectable in both PC and precursor lesions. Up until now, the majority of the available studies show that exosomal DNA has great potential to be used as a biomarker, however unlike proteins and RNA, no biological function has been attributed to this cargo. Could exosomal DNA be delivered to a recipient cell and be transcribed? Is exosomal DNA degraded once is delivered? These are a few examples of the many questions that remain to be elucidated.
Cargo of cancer exosomes. Reported exosomal content includes both proteins and nucleic acids such as mRNA, microRNA and dsDNA. The surface markers CD63, CD9 and CD81 are known to be broadly expressed in exosomes and therefore are commonly used as exosomal biomarkers. Protein cargo specific for cancer exosomes is being currently explored; HIF1a [14] and GPC-1 [15] are examples of intra and surface proteins respectively, that are being explored as biomarkers
Exosomes-Mediated Communication Promotes Tumor Development
Cell-to-cell communication is a critical process that mediates homeostasis in a multicellular organism [34, 35]. Many physiological processes rely on a coordinate cellular response achieved by intercellular communication . Synapsis and gap junctions are classical examples of short-distant cellular communication that allow signals to travel fast and efficiently enough to allow a nervous response [36]. When it comes to cancer, it has been established that cancer cells communicate between themselves and with other cells from the tumor microenvironment . Several studies show that cancer cells secrete soluble factors such as chemokines, cytokines and growth factors that modulate immune cells activity and activate fibroblasts [37]. For example, transforming growth factor beta TGF-β secreted by cancer cells mediates a paracrine signaling between breast cancer cells and fibroblasts that activates and differentiates fibroblasts into cancer associated-fibroblasts (CAFs) , hence promoting tumor progression [38]. There are three main mechanisms proposed for exosomes-mediated intercellular communication: direct interaction of membrane proteins with receptors in the target cell activating intracellular signaling processes, through the cleavage of exosomal membrane proteins by proteases in the extracellular space, resulting in differently sized fragments that may act as ligands that can be internalized by the recipient cell or they can fuse with the target cell membrane and release their contents directly into the cytoplasm [39, 40]. Exosomes are found in every body fluid (blood, saliva, urine, cerebral fluid etc.) [41,42,43], meaning that these vesicles can travel long distances within the organism and they seem to be sufficiently stable to hold in circulation. This opens the possibility for exosomes to work as vehicles of inter-tissue/organ communication that can support cancer progression.
Exosomes and Tumor Growth
Cancer derived-exosomes have oncogenic proteins as part of their cargo, as well as mRNA and pro-oncogenic microRNAs. Because exosomes can interact with and be internalized by surrounding cells, this means that their cargo can be directly delivered, constituting a new way of horizontal transfer of information between cells [1]. When oncogenic cargo is transferred from cancer cells via exosomes to less active cancer cells, it can ultimately reprogram the recipient cell into a more aggressive state. The oncogenic form of the epidermal growth factor receptor, EGFRvIII is particularly enriched in exosomes derived from glioma cells. Upon their cargo release, EGFRvIII is transferred to cancer cells with decreased tumorigenic activity, resulting in the activation of MAPK and AKT signaling pathways [44]. These cancer derived-exosomes constitute a mechanism for the propagation of oncogenic activity within tumor cells [44]. Exosomes derived from mutated cancer cells are also responsible for transmitting tumor-promoting proteins that include KRAS, EGFR, SRC family kinases, Amphiregulin (AREG) and integrins to non-mutated cancer cells [45, 46]. By delivering mutated proteins that are responsible for driving tumor progression, these exosomes are capable of enhancing three-dimensional cancer growth and promote invasion [45, 46]. But the simple transport of oncogenic material is not the only process that justifies the pro-tumor growth effect of cancer derived-exosomes on recipient cells. Breast cancer derived-exosomes are able to maturate miRNA in a cell independent fashion [47]. By selectively loading RISC-Loading Complex associated proteins such as DICER along with pre-miRNA into cancer exosomes, this allows the maturation of miRNA outside the cell of origin hence transforming pre-miRNA into functional miRNA that upon exosomes delivery silences genes expression on non-transformed cells [47]. DICER is also found associated to CD63, a well described exosomal marker, in colorectal cancer and its presence on exosomes is independent of the cells KRAS status [48]. Such a process occurring in cancer-exosomes points to a poorly explored exosomal feature of cell-independent activity. Are exosomes able to conduct even more complex cellular processes outside the cytoplasm? Exploring this topic can certainly add new knowledge to the biological significance of exosomes in cancer and even in normal physiological processes.
Exosomes and Tumor Microenvironment
A tumor is comprised of many cells and biological components besides the cancer cells themselves. Classically, the malignant mass that we call a tumor has also many non-malignant cells such as fibroblasts, endothelial cells, immune cells and other non-cellular components [49]. Depending on the cancer type and stage, the frequency and type of normal cells will vary [50, 51]. There is an evident interaction between cancer and tumor-microenvironment (TMC) that is based on the exchange of secreted factors such as cytokines, chemokines, growth factors and enzymes [49]. This intercellular communication strongly correlates with the promotion of malignant features like tumor growth, invasion and metastasis [52, 53]. Exosomes have also been pointed as mediators of the communication between cancer cells and TMC. Fibroblasts are commonly the most frequent cellular component of the tumor microenvironment. When associated to cancer, fibroblasts are also known as cancer-associated fibroblasts CAFs and present an activated phenotype similar to what happens in a wound healing process [54]. CAFs are well described for promoting tumor proliferation, production of ECM and for modulating metabolism [54]. Many studies support that CAFs-derived exosomes also have a positive impact on cancer proliferation and migration [1, 8, 47]. Breast cancer-associated fibroblasts secrete CD81-positive exosomes that activate Wnt-PCP pathway in recipient cancer cells through production of Wnt11, resulting in the overexpression of Fzd, Vangl and Dvl, which stimulate protrusive activity and mobility [9]. Exosomes derived from CAFs also play a role in metabolic reprogramming of cancer cells. Prostate and pancreatic cancer patient CAFs-derived exosomes are able to reduce oxygen-dependent metabolism by downregulating mitochondrial oxidative phosphorylation and promoting glycolysis [55]. Additionally, CAFs-derived exosomes contain pro-glycolysis metabolites such lactate, acetate, aminoacids, lipids and intermediates of the Krebs cycle. The recipient cancer cells in nutrient/oxygen-deprived environment continue to proliferate by using these metabolites.
On the other hand, cancer cells are also known to promote the recruitment and activation of fibroblasts. TGF-β is a well-established factor responsible for the differentiation of fibroblasts into CAFs (also known as myofibroblasts) (Fig. 3.2). Cancer-derived exosomes containing TGF-β can bind to type II receptor and ALK5 receptor, forming a complex that results in the phosphorylation of SMAD2 and 3 that can then bind to SMAD4 resulting in its activation. Following SMAD cascade, this protein translocates to the nucleus and activates the expression of several genes including αSMA that is associated with the myofibroblast phenotype [8]. This resulting activated phenotype that is driven by cancer-exosomes cargo is different from the one generated in response to soluble TGF-β [8]. Unlike the soluble form of TGF-β, cancer exosomes that carry this factor also activate the secretion of angiogenic factors by CAFs. Interestingly, downregulation of RAB27A gene, involved in the late stages of exosomes biogenesis, in cancer cells leads to a failure in triggering fibroblasts activation in vivo. Altogether, this demonstrates that exosomes play a crucial role on both sides of tumor-microenvironment communication. On one side cancer-derived exosomes promote the activated state of fibroblasts, on the other CAFs-exosomes enhance migration and support proliferation of cancer cells.
Exosomes and Immune Modulation
The immune escape in cancer includes processes that involve antigen masking. Transformation of cells into cancer cells originates a plethora of new antigens, the so-called neoantigens. Since tumors principally arise due to mutations in key oncogenic genes, they will express non-mutated and mutated antigens that will be recognized by the immune system. In order to become “invisible” to the immune selection, cancer cells often present loss of the major histocompatibility MHC complex and silence antigen presenting mechanisms. On the other hand, if the tumor retains antigenicity, it can reduce its immunogenicity instead by expressing immune inhibitor molecules such as PD-L1 and FASL [56].
Exosomes from both tumor and immune cells show expression of MHC class I and class II [57]. Cancer-derived exosomes express functional MHC-Peptides complexes that work similarly to an antigen-presenting cell by directly present or cross present the complexes to CD8+ T-Cells [57]. This process of exosomes-dependent antigen presentation is considered to be anti-cancer because it primes an immune response against the tumor. However, cancer-derived exosomes can also perform an immune inhibitory action (Fig. 3.2). In fact, this dual role can be observed in the response of subsets of T-Cells that react differently to carcinoma-derived exosomes [58]. Resting TRegs treated with tumor derived exosomes increase the expression of CD39 and CD73, while in activated TRegs there is an overexpression of immune-suppressive genes. T-cell CD39+CD73+ phenotype is associated with ATP catalysation into AMP that has pro-inflammatory effects and promotes secretion of IL-17 [59]. Additionally, uptake of tumor-derived exosomes in activated CD4+ T-cells leads to a decreased expression of immune suppressive genes like COX2, CTLA-4, Fas, Fas Ligant (FASL) and TGF-β. Exosomes from the same tumor-origin potentiate contrary responses when it comes to immune cells. Depending on the recipient cell type, tumor-derived exosomes can potentiate an immune response or suppress an anti-tumor reaction. The suppression of an immune reaction is also achieved by cancer-derived exosomes that express FASL. By binding to FAS, this ligand induces apoptosis in CD8+ T-Cells when they are treated with these exosomes [60]. However, this effect can be reversed when exosomes are treated with antibodies against FASL that block the interaction with T-Cells FAS. Besides lymphocytes, tumors are also classically associated with inflammatory pro-oncogenic environments modulated by myeloid cells like macrophages [61]. Several studies show that macrophages uptake exosomes ex-vivo and in-vivo [62, 63]. Particularly, cancer-derived exosomes stimulate NF-KB that results in the production of pro-inflammatory cytokines such as IL-6, TNFα, GCSF, and CCL2 [62]. When genetically abrogated in breast cancer cells Toll-like receptor 2 (TLR2), part of NF-KB signaling, cancer-derived exosomes do no longer show this effect. On the other hand, engineered PC-derived exosomes that express miRNA-155 and miR-125b2, are able to invert M2 polarized macrophages into M1 polarization [64]. M1 polarization state corresponds to the activated state that reacts to immune stimuli such as interferon γ, while M2 macrophages promote inflammation by secretion of angiogenesis and fibrosis. This means that cancer-exosomes can themselves be used to alter the immune composition of the tumor microenvironment when it comes to macrophages. Due to the complex role of immune cells in cancer, it is crucial to further address the immunomodulatory properties of cancer derived-exosomes in the context of tumor progression and explore how these apparent contradictory exosomes-mediated effects occur in vivo.
Exosomes and Extracellular Matrix (ECM)
ECM is composed by a network of macromolecules such as proteins, collagen and proteoglycans frequently modified with sugar chains that are secreted by stromal and cancer cells. It is well established that ECM not only provides support and mechanic cues but its composition is also an authentic reservoir of growth factors and signaling molecules that have great impact on the tumor growth [65]. Fibronectin (FN) is one of the most frequent ECM constituents and it is released via fibrosarcoma-derived exosomes that promote the adhesion assembly of cancer cells to the ECM. This FN exosomes-mediated release also directs cancer cell mobility, since cells attach their protrusions to FN that is deposited according to integrins orientation that directly interact with the exosomes [66]. This exosomal-mediated process comprehends an autocrine mechanism of directional mobility and it also influences the migration speed of cancer cells. Cancer cells with higher mobility are known to transmit its migratory phenotype to non-motile cells. Uptake of exosomes derived from hepatocellular carcinoma leads to the activation of PI3K/AKT and MAPK pathways that result in the expression of metalloproteinases, particularly MMP-2 and MMP-9 [67]. This group of enzymes is responsible for degrading proteins and collagen part of the ECM . Traditionally, degradation of the ECM promotes migration leading to invasion and ultimately to metastization. Evidence shows that due to their cargo, cancer-derived exosomes are able to directly interplay with ECM via protein-interaction or indirectly by activating the expression of ECM degrading proteins.
Exosomes and Angiogenesis
Tumor growth implies an increased supply of oxygen, nutrients and a constant replacement of extracellular fluid that allows waste excretion. To support cells proliferation and metastization, tumors promote a pro-angiogenic environment leading to the formation of new vessels. Based on chemical factors secreted by cancer cells, angiogenic factors, endothelial cells are recruited to regions were the basement membrane was disrupted, where they proliferate and stabilize [68]. Tumors often present regions of hypoxia and the exosomes released from these hypoxic cancer cells are able to reach endothelial cells [69, 70]. Exosomes derived from hypoxic cancer cells promote endothelial proliferation via cytokines and growth factors that also stimulate pericytes and lead to the activation of PI3K/AKT pathway [71]. Hypoxia seems to have a great influence on exosomes biogenesis and their composition [72]. Cells under hypoxia produce a significant higher number of exosomes [71, 72]. Moreover, under hypoxic conditions, lung cancer exosomes specifically express miR-23a, that is known to target PHD2 [73]. PHD protein family members control HIF1α action. When HIF1α is downregulated in endothelial cells, these present tight junction loss and are more easily recruited [73]. By turning back on HIF1α, it increases endothelial cells proliferation and tube formation. Additionally, in hypoxic conditions, miR-23a besides targeting PHD2 also downregulates ZO-1, a tight junction protein, allowing endothelial barrier to be disrupted hence promoting cancer cells extravasation [73]. Cancer-derived exosomes are considerably enriched in angiogenic friendly-cargo and this particular cargo results from the hypoxic state of their cell of origin.
Exosomes and Metastasis
Metastasis corresponds to the process by which cancer cells leave the primary tumor, reach a secondary location and proliferate originating a new tumor mass [74]. The mechanisms used by cancer cells to gain a migratory phenotype, such as epithelial-to-mesenchymal transition, to survive in circulation and create a metastatic niche are far from being totally explored. In fact, the probability of a tumor cell that enters circulation, a circulating tumor cell (CTC), to actually give rise to a mass is below 0.02% [75]. Therefore, cancer cells should probably have other tricks under their sleeve to help them seed to different organs. The concept of pre-metastatic niche arises from the observation that bone marrow-derived hematopoietic progenitor cells (BMDCs) are recruited previous to the cancer cells arrival to a distant organ [76, 77]. These BMDCs express vascular endothelial factor receptor-1 and VLA-4 while maintaining their progenitor phenotype (CD133+, CD34+ and c-Kit expression). The role of these cells is to create a permissive “soil” for cancer cells to seed [76]. Interestingly, melanoma-derived exosomes re-educate BMDCs and enhance their recruitment through MET signaling. When treating mice with melanoma-derived exosomes it is possible to simulate BMDCs recruitment, which after cancer cells inoculation show to significant metastasis enhancement [6]. The protein cargo of exosomes derived from highly metastatic cells compared with poor metastatic melanoma cells is actually enriched in MET cascade proteins that are responsible for the BMDCs recruitment [6]. Melanoma patient’s exosomes are also known to contain a proteomic signature that includes TYRP2, VLA-4, HSP70, HSP90 and MET [6]. The presence of this cargo also correlates with the patients’ metastatic disease and tumor burden [6].
Cancer derived-exosomes are responsible for the formation of the liver pre-metastatic niche that is a typical metastasis site for PC [6]. PC-derived exosomes are uptaken by the liver Kupffer cells (KC) and induce TGF-β signaling [78]. This activation leads to ECM remodeling by hepatic stromal cells and deposition of FN that in turn promotes bone marrow derived-macrophages migration to the liver [78]. All of these reprogramming effects construct a favorable environment in the liver that helps cancer cells to proliferate and form a metastasis. PC-exosomes contain MIF, an anti-fibrotic factor that most likely is the molecular effector behind this niche formation. In fact, MIF-positive exosomes bind more frequently to Kupffer cells in the liver, increasing TGF-β expression that then will increase FN production and deposition [78]. FN also works as an anchor for bone marrow-derived macrophages to settle and create an inflammatory reaction that is also advantageous for the cancer cells [78].
Exosomes can also be behind a very peculiar characteristic of tumors, the so called metastatic organotropism. This term refers to the predisposition of cancer cells to metastasize only to certain organs. PC-derived exosomes express integrins that target specific niche cells, ITGαvβ5 is associated with KC cells in the liver, while ITGα6β4 and ITGα6β1 are associated with the uptake of these exosomes by lung resident fibroblasts and epithelial cells [79]. When interacting with the target cells, these exosomes promote a pro-migratory and inflammatory reaction mediated by the S100 gene family overexpression [79]. By injecting specific integrin-positive exosomes it is possible to recapitulate and simulate PC-metastization organotropism, also by using antibodies to block these integrins exosomes uptake is significantly reduced [79]. Altogether, these observations support that exosomes derived from tumors have a predominant role in promoting metastasis, however these observations need a deeper evaluation using better animal cancer-models that recapitulate the human disease.
Exosomes and Therapy Resistance Mechanisms
In cancer, a failed response to therapy can derive from intrinsic or acquired resistance. The first one corresponds to pre-existing factors that unable the drug action, while acquired resistance results from cellular response to the therapy [80]. The cellular mechanisms behind acquired resistance are extremely complex and rely on varied signaling pathways that are not fully understood [81]. The dissemination of therapy resistance due to communication between resistant and sensitive cancer cells is one of the processes that can help explain how a tumor becomes rapidly resistant to a certain drug. Exosomes derived from a breast cancer cell line resistant to docexatel express P-glycoprotein [82, 83]. P-glycoprotein works as a drug efflux pump that allows a cell to reduce the drug intracellular level. Upon exosomes transfer to sensitive cells with low levels of P-glycoprotein, these become resistant to docexatel [83]. Exosomes can efficiently transfer molecular cargo from resistant to sensitive cells thereby transferring resistance to therapy.
Together with cancer cells, TMC particularly fibroblasts also play a role in the overall tumor response to therapy [84]. Paracrine signaling and fibroblasts-derived exosomes communication towards cancer cells constitutes important mechanisms that promote therapy resistance. In response to radiotherapy, breast cancer stroma derived-exosomes transfer large non-coding RNA and transposable elements that activate anti-viral response in cancer cells via RIG-1 receptor and STAT pathway [85]. This results in the expansion of tumor initiating cells (TICs) that are therapy resistant thereby leading to tumor growth . Likewise, CAFs exosomes isolated from colorectal cancer also promote an increased number of cancer stem cells (CSCs) as well as their clonogenicity [86]. Cancer stem cells are known to be intrinsically resistant to therapy and their presence promotes tumor recurrence after treatment [87]. Alongside with the mentioned indirect promotion of therapy resistance via TICs or CSCs proliferation, fibroblasts exosomes also seem to directly support anti-drug response. When PC associated fibroblasts are exposed to therapy (gemcitabine) there is a significant increase in their exosomes production that leads to increase proliferation and survival of PC cells [88]. Upon gemcitabine treatment PC-associated fibroblasts show increased levels of SNAIL1 and microRNA-146a, hence modulating the cancer cells response to therapy [88]. Exosomes mediated-transfer of therapy resistance can constitute an important cellular response mechanism that tumors rely on to rapidly bypass the aggressions provoked by conventional chemo and radiotherapy.
Oncogenic Transformation and Its Impact on Exosomes
Cancer derived-exosomes have oncogenic proteins and mutated genetic material as part of their cargo [3]. Additionally, many studies have shown that this cargo is effectively delivered to neighbor cells and reprogram them, most frequently in favor of the tumor progression. Could this mean that cancer cells have the ability to use exosomes in their advantage and that this was a result of their malignant transformation they suffered? Currently, there is not enough evidence to fully support this hypothesis, however some studies are showing that oncogenic drivers have direct implications on the cargo and biogenesis of exosomes. Upon DNA damage, p53 is activated and promotes or represses the transcription of certain genes. This gene is frequently mutated in many cancers. Non-small cell lung cancer cell lines that are p53 wild type increase exosomes secretion when submitted to γ radiation [89]. On the other hand, p53 mutant cell lines do not show increased exosomes production after radiation treatment [89]. Interestingly, expression of TSAP6 gene allows the cells to produce exosomes after stress, independently of p53 status [89]. By comparing microRNA expression of colorectal cancer cells that express wild type or mutant KRAS, it is observed that KRAS mutant cells show an enrichment of miR-100 and miR-10b [90]. Additionally, KRAS activating mutations of MEK-ERK signaling regulate AGO2 secretion into exosomes by promoting a phosphorylation process that prevents AGO2 interaction with MVB, also affecting the sorting of specific miRNAs such as let-7a, miR-100 and miR-320a into exosomes [48]. There is also increasing evidence that oncogenic transformation affects exosomes uptake. Stimulation of EGFR increases exosomes uptake via macropinocytosis [91]. Also, PC cell lines with activating KRAS mutations have increased macropinocytosis when compared with KRAS wild type cancer cells [91, 92]. If oncogenic transformation deeply alters exosomes processing it is relevant to consider which and how different biological functions can be carried by exosomes in cancer compared to the normal physiological role of these vesicles.
Therapeutic Potential of Exosomes: Targets and Vehicles
Due to their pro-oncogenic properties, cancer derived-exosomes could constitute an important target when considering anti-tumor therapy. At the moment, there are no genes described exclusively dedicated to the biogenesis of exosomes. Nonetheless, several proteins associated with MVB and exosomes release show promising results as targets to block exosomes secretion by cancer cells. Most importantly, when blocking exosomes production many studies show that it is possible to inhibit the pro-oncogenic effects caused by these vesicles. GW4869, a neutral sphingomyelinase inhibitor that acts on ceramide, can effectively block the production of exosomes. Treating PC associated CAFs with GW4869 exosomes production is reduced both in the presence and absence of gemcitabine [88]. Since exosomes derived from PC-CAFs enhance cancer cell proliferation, especially when the CAFs are treated with gemcitabine, cancer cells significantly reduce their survival once CAFs-derived exosomes are blocked [88]. Similarly, exosomes produced by metastatic breast cancer cells lines induce invasion by miR-10b regulation of non-metastatic cells. GW4869 treatment reduces exosomes production in metastatic cells, and minimizes the invasion potential of the non-metastatic recipient cells [93]. RAB proteins family are implicated in MVB processing. RAB27A and B are involved in the late stages of MVB fusion and exosomes release. Melanoma-derived exosomes are able to potentiate the recruitment of BMDCs to metastatic niches [6]. Moreover, these cells show an increased expression of RAB27A that points to an increased exosomes secretion [6]. Using RNA interference to shut down this gene, exosomes release is significantly reduced [6]. This also prevents the recruitment of BMDCs via exosomes [6].
On the other hand, if considering exosomes as specialized vehicles of intercellular communication , they can constitute a way of delivering therapy to cancer or other cells because they are well described for delivering genetic information capable of reprogramming recipient cells. Interference RNA can be electroporated into exosomes and efficiently shutdown gene expression on target cells [94, 95]. Moreover, is also possible to redirect exosomes to certain cell types [96]. Exosomes can be directed to neural cells by using dendritic cells that are engineered to express Lamp2b fused with an RVG peptide that is neuron specific [96]. In this study engineered exosomes were filled with siRNA against GADPH and BACE-1 genes and were delivered in vivo via intravenous injection and inhibited the target genes expression by 60%. Studies like this one illustrate how exosomes are biocompatible and stable in vivo, and open up the possibility to engineer exosomes to target them to specific cell populations. Epidermal growth factor receptor, EGFR, is frequently overexpressed in many cancers such breast, lung and kidney, and its signaling, which is activated by EGF, promotes cell division and therefore proliferation. A small peptide called GE11 specifically binds to EGFR, but because it is significantly less mitogenic than EGF, GE11 can work as its competitor [97]. Exosomes engineered to express GE11 at their surface is done using a platelet-derived growth factor receptor transmembrane domain fused with the GE11 sequence, hence promoting its expression on the plasma membrane and consequently on exosomes [97]. Using this strategy, GE11 positives exosomes are able to target EGFR expressing cancer cells, both in vitro and in vivo. Additionally, it is possible to deliver microRNA let-7a in a breast cancer model via GE11 exosomes [97]. Let-7a inside modified exosomes reduces also tumor growth [97].
Recently, it was demonstrate that fibroblasts-derived exosomes can be engineered to target one of the most common mutated genes in PC, KRAS [92]. The activated KRAS gene is a well-known driver of PC, and up until now no therapy system to target KRAS was successfully developed. In this study, exosomes were engineered to carry siRNA against KRASG12D, a mutation that is present in about 80% of PC cases [98]. Curiously, fibroblasts-derived exosomes express CD47 that is a ligand for the signal regulatory protein alpha SIRPα present in macrophages, that upon binding inhibits their destruction, working like a ‘don’t eat me signal’ [99, 100]. Moreover, this activated form of KRAS promotes micropinocytosis that constitutes a process of exosomes uptake, hence enhancing the delivery of these exosomes to PC tumors. In vivo studies using genetically engineered mouse models (GEMMs) that spontaneously develop PC driven by KRASG12D mutation, show that the modified exosomes significantly decrease pancreatic tumors by suppressing cell proliferation , enhancing apoptosis and blocking RAS signaling [92]. Altogether, studies like the ones mentioned in this chapter illustrate the potential of exosomes to be used as tailored therapy deliver vehicles with very promising results in vivo. Due to their biocompatible and engineering properties, modified exosomes will soon be tested in a clinical setting.
Conclusion
Accumulating evidence points to the pro-oncogenic role of tumor and stroma-derived exosomes. As discussed, these vesicles seem to actively participate in very crucial processes that sustain tumor development such cell proliferation, activation of the microenvironment, immunosuppressive action and formation of the pre-metastatic niche. However, many of these effects are described based on in vitro observations, or based on in vivo models that do not fully recapitulate the natural behavior of exosomes in an organism. We hope in the future that the field will provide new demonstrations of the role of exosomes in cancer using models that allow to track exosomes secreted from the tumor and/or stroma in new animal models. Moreover, very few studies explore how blocking exosomes could hamper tumor progression; this is also a very crucial aspect that will define how important exosomes are for cancer development. On the other hand, the potential of exosomes as therapy delivery systems is being highly explored with great success. With the advent of more comprehensive and promising studies being published we predict that engineered exosomes can reach clinical studies in a near future.
References
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–30.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56.
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608.
Elkin SR, Lakoduk AM, Schmid SL. Endocytic pathways and endosomal trafficking: a primer. Wien Med Wochenschr. 2016;166(7–8):196–204.
Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–22.
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177.
Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106(2):145–55.
Schuh AL, Audhya A. The ESCRT machinery: from the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol. 2014;49(3):242–61.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7.
Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980.
Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–32.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30.
Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010;12(1):3–4.
Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280(24):23349–55.
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–77.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66.
Gallo A, Tandon M, Alevizos I, Illei GG. The majority of MicroRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679.
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10):e13247.
Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–48.
Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–28.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21.
Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316(12):1977–84.
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–75.
Classen L, Tykocinski LO, Wiedmann F, Birr C, Schiller P, Tucher C, et al. Extracellular vesicles mediate intercellular communication: transfer of functionally active microRNAs by microvesicles into phagocytes. Eur J Immunol. 2017;47:1535.
Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–35.
Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 2011;15(7):1458–73.
Calvo F, Sahai E. Cell communication networks in cancer invasion. Curr Opin Cell Biol. 2011;23(5):621–9.
Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–14.
Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. https://doi.org/10.3402/jev.v3.24641.
Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–95.
Pan J, Ding M, Xu K, Yang C, Mao LJ. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget. 2017. 10.18632/oncotarget.18532.
Yan S, Han B, Gao S, Wang X, Wang Z, Wang F, et al. Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget. 2017;8:60149.
Giusti I, Di Francesco M, Dolo V. Extracellular vesicles in glioblastoma: role in biological processes and in therapeutic applications. Curr Cancer Drug Targets. 2017;17(3):221–35.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24.
Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12(2):343–55.
Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21(9):779–86.
Melo Sonia A, Sugimoto H, O’Connell Joyce T, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microrna biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–21.
McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, et al. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15(5):978–87.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–6.
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf Anna C, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–95.
Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13(6):227.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. elife. 2016;5:e10250.
Kearney CJ, Lalaoui N, Freeman AJ, Ramsbottom KM, Silke J, Oliaro J. PD-L1 and IAPs co-operate to protect tumors from cytotoxic lymphocyte-derived TNF. Cell Death Differ. 2017;24:1705.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72.
Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254.
Wang LL, Tang HP, Shi GC, Wan HY, Tang W, Hou XX, et al. CD39/CD73 and the imbalance of Th17 cells and regulatory T cells in allergic asthma. Mol Med Rep. 2013;8(5):1432–8.
Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cell Mol Dis. 2005;35(2):169–73.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750.
Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. 2016;6:30110.
Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10(7):1517–27.
Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224.
Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164.
He M, Qin H, Poon TCW, Sze S-C, Ding X, Co NN, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36(9):1008–18.
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–9.
Huang Z, Feng Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced beta-catenin signaling in endothelial cells. Oncol Res. 2017;25(5):651–61.
Hsu DH, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, et al. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26:440.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7.
King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12(1):421.
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–91.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92.
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–17.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Lippert TH, Ruoff HJ, Volm M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung. 2008;58(6):261–4.
Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92.
Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35(11):10773–9.
Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23(9):1643–9.
McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12(3):217–28.
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513.
Hu Y, Yan C, Mu L, Huang K, Li X, Tao D, et al. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One. 2015;10(5):e0125625.
Maitland NJ, Collins AT. Cancer stem cells – a therapeutic target? Curr Opin Mol Ther. 2010;12(6):662–73.
Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36(13):1770–8.
Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–801.
Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, et al. KRAS-dependent sorting of miRNA to exosomes. elife. 2015;4:e07197.
Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep. 2015;5:10300.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503.
Singh R, Pochampally R, Watabe K, Lu Z, Mo Y-Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13(1):256.
Wahlgren J, Statello L, Skogberg G, Telemo E, Valadi H. Delivery of small interfering RNAs to cells via exosomes. Methods Mol Biol. 2016;1364:105–25.
El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7(12):2112–26.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotech. 2011;29(4):341–5.
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Miglio U, Oldani A, Mezzapelle R, Veggiani C, Paganotti A, Garavoglia M, et al. KRAS mutational analysis in ductal adenocarcinoma of the pancreas and its clinical significance. Pathol Res Pract. 2014;210(5):307–11.
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–64.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer. 2017;76:100–9.
Acknowledgements
This work was supported by: FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the projects “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274), and “Papel dos Exosomas na Heterogeneidade Tumoral: Mais do Que Simples Vesículas” (PTDC/BIM-ONC/2754/2014); and Maratonas da Saúde; and by NORTE-01-0145-FEDER-000029, supported by Norte Portugal Regional Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). SAM is supported by FCT – Foundation for Science and Technology (IF/00543/2013). C. F. Ruivo is supported by FCT (PTDC/BIM-ONC/2754/2014). We thank Dr. Nuno Barros for the help with the design of the figures included in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ruivo, C.F., Melo, S.A. (2018). The Emerging Role of Exosomes in Cancer Progression and Their Potential as Therapy Targets. In: Fayyaz, S., Farooqi, A. (eds) Recent Trends in Cancer Biology: Spotlight on Signaling Cascades and microRNAs. Springer, Cham. https://doi.org/10.1007/978-3-319-71553-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-71553-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71552-0
Online ISBN: 978-3-319-71553-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)