Abstract
Purpose
This review aims to summarize the evidence that Notch signaling is associated with prostate development, tumorigenesis and prostate tumor progression.
Methods
Studies in PubMed database were searched using the keywords of Notch signaling, prostate development and prostate cancer. Relevant literatures were identified and summarized.
Results
The Notch pathway plays an important role in determining cell fate, proliferation, differentiation and apoptosis. Recent findings have highlighted the involvement of Notch signaling in prostate development and in the maintenance of adult prostate homeostasis. Aberrant Notch expression in tissues leads to dysregulation of Notch functions and promotes various neoplasms, including prostate cancer. High expression of Notch has been implicated in prostate cancer, and its expression increases with higher cancer grade. However, the precise role of Notch in prostate cancer has yet to be clearly defined. The roles of Notch either as an oncogene or tumor suppressor in prostate cancer hallmarks such as cell proliferation, apoptosis and anoikis, hypoxia, migration and invasion, angiogenesis as well as the correlation with metastasis are therefore discussed.
Conclusions
Notch signaling is a complicated signaling pathway in modulating prostate development and prostate cancer. Understanding and manipulating Notch signaling could therefore be of potential therapeutic value in combating prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Notch gene was originally discovered in 1917, when Thomas Hunt Morgan first observed a Notched-wing phenotype in Drosophila melanogaster mutants (Morgan 1917). Artavanis-Tsakonas and group later attributed this Notched-wing phenotype to gene haploinsufficiency (Artavanis-Tsakonas et al. 1999; Kidd et al. 1986; Wharton et al. 1985). The loss of Notch expression affected the morphology of the eye, wing and bristle, as well as contributed to the neurodevelopmental phenotype in this Drosophila strain. Fast forwarding nearly a century, it is now well-established that Notch signaling is involved in a variety of cellular processes, notably binary cell-fate determination, differentiation, proliferation and survival (Artavanis-Tsakonas et al. 1999; Rizzo et al. 2008; Weinmaster et al. 1991).
Dysregulation of Notch expression was first implicated in cancer through analysis of the chromosomal translocation t(7;9)(q34;q34.3) in patients with T cell acute lymphoblastic leukemia (T-ALL). The translocation of 3′ end of Notch1 on chromosome 9 to the Jb joining region of T cell receptor-β (TCR-β) on chromosome 7 resulted in overexpression of truncated Notch1 transcripts (Ellisen et al. 1991). Since then, aberrant Notch signaling has been linked to various human disorders. For example, mutations in Notch3 and a Notch ligand, Jagged1, respectively, lead to Alagille syndrome, an autosomal-dominant disorder (Li et al. 1997; Oda et al. 1997) and cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Joutel et al. 1996). Various human neoplasms associated with Notch signaling have also recently surfaced, including lymphoid neoplasms such as Hodgkin’s lymphoma (HL) and anaplastic large cell lymphoma (ALCL), breast cancer, lung cancer and cervical cancer, as well as prostate cancer (Allenspach et al. 2002).
To date, prostate cancer has become one of the most commonly diagnosed neoplasms in men, and the second leading cause of cancer-related mortality in the USA (Siegel et al. 2012). Because Notch signaling is evolutionarily conserved and is required for normal prostate development (Shou et al. 2001; Wang et al. 2006), it is expected that Notch signaling could also contribute to the development of prostate cancer. Despite Notch expression being up-regulated in prostate cancer and higher with increasing Gleason grade (Santagata et al. 2004; Zhu et al. 2013), the definitive role of the Notch pathway in human prostate cancer remains unclear. Several studies suggest that Notch signaling inhibits prostate cancer cell proliferation, and loss of Notch1 in prostate adenocarcinoma abolishes its capability to suppress tumor growth (Shou et al. 2001; Wang et al. 2006; Whelan et al. 2009), while others suggest that Notch promotes tumor growth and cancer progression (Li et al. 2007; Wang et al. 2010, 2011). Therefore, Notch’s role as a tumor suppressor or an oncogene in prostate adenocarcinoma requires further in-depth investigation to address these controversies. This review addresses the roles of Notch in prostate development, prostate tumorigenesis and prostate tumor progression.
Canonical Notch signaling
Notch is a highly conserved family of single-pass transmembrane proteins, widely expressed in higher organisms including vertebrates. Canonical Notch signaling mediates a variety of cell–cell interactions and plays a pivotal role in determining cell-fate, proliferation, differentiation and apoptosis in C. elegans, Drosophila, zebrafish and mice (Artavanis-Tsakonas et al. 1999; Bray 2006).
In mammals, the Notch family consists of four distinct Notch receptor isoforms, namely Notch1/TAN-1, Notch2, Notch3 and Notch4/int-3, as well as five ligands, Jagged1, Jagged2, Delta-like ligand 1 (Dll1), Dll3 and Dll4 (Fleming 1998; Wang et al. 2008). Notch receptors are synthesized as single-pass transmembrane polypeptides in the endoplasmic reticulum. In the following synthesis, these 300 kDa unprocessed Notch proteins are transported to the cell surface via the trans-Golgi network. During the transfer to the cell surface, these full-length Notch proteins undergo post-translational modifications, such as glycosylation, and are cleaved by Furin, producing an inactive heterodimer that consists of a 180 kDa extracellular ligand-binding domain, a 120 kDa transmembrane domain and a cytoplasmic region (Blaumueller et al. 1997; Logeat et al. 1998).
Similar to Notch receptors, Notch ligands are single-pass transmembrane proteins, but have a specific Delta/Serrate/Lag (DSL) domain that binds Notch receptors (Bray 2006). The mutual binding of Notch ligand and receptor is the first step in modulating Notch signaling. Canonical Notch signaling is activated when a membrane-bound ligand on the sending cell binds to a Notch receptor on the receiving cell. This cell-to-cell interaction leads to two distinct proteolytic cleavages of the receptor that involve: (1) ADAM/TACE metalloprotease at the extracellular surface; and (2) a γ-secretase complex (composed of presenilin-1 and -2, Aph-1, Pen-2 and Nicastrin) at the phospholipid bilayer (Brou et al. 2000; Fraering et al. 2004; Mumm and Kopan 2000). This results in the release of a smaller transcriptional Notch transactivator, Notch intracellular domain (NICD), from the inner surface of plasma membrane. The cleaved NICD then translocates into the nucleus, where it forms a complex with the DNA-binding protein RBPJ (also known as CSL (CBF-1/Suppressor of Hairless (Su(H))/LAG-1)) (Andersson and Lendahl 2014). In the absence of NICD, RBPJ acts as a repressor of transcription, recruiting histone deacetylases and corepressors such as SMRT/NcoR, SHARP (or MINT) or CtIP/CtBP (Kao et al. 1998; Mulligan et al. 2011; Nagel et al. 2005; Yatim et al. 2012). The binding of NICD to the RBPJ complex displaces corepressor complexes and recruits coactivators, such as Mastermind-like protein (MAML) and histone acetyltransferase, to induce the expression of Notch downstream target genes, including Hes1, Hey1, cyclin D and others (Fischer and Gessler 2007; Iso et al. 2003; Miele 2006). In order to achieve precise temporal Notch regulation, Notch-induced transcription is eliminated immediately after the activation by NICD degradation and by the suppression from Hes1 and Hey1 (Frank and Miranti 2013). This canonical Notch signaling pathway is crucial for regulating cell homeostasis, cell-fate determination, cell proliferation and differentiation, as well as apoptosis in a variety of tissues (Fig. 1).
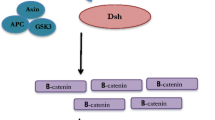
Canonical and non-canonical Notch signaling pathway in prostate cells. Canonical Notch signaling is initiated when Notch ligand–receptor binding triggers ADAM/TACE cleavage at the extracellular surface and γ-secretase cleavage at the phospholipid bilayer, producing Notch intracellular domain (NICD). NICD translocates into the nucleus and interacts with RBPJ, activating downstream target genes such as Hes1 and Hey1. Canonical Notch signaling in basal cells suppresses cell proliferation and promotes cell differentiation. However, in luminal cells, Notch signaling enhances cell proliferation, suppresses cell differentiation and inhibits apoptosis. Non-canonical Notch signaling occurs when Notch receptors bind to non-canonical ligands, such as Dlk1, inhibiting transcription of downstream genes, or it can also target Wnt/β-catenin signaling independent of ligand binding
Non-canonical Notch signaling
As with canonical Notch signaling, a fully functional Notch receptor is dependent on proper post-translational modifications and Furin cleavage. However, even when Furin cleavage is inhibited, Notch signaling is not completely diminished (Bush et al. 2001; Kidd and Lieber 2002). This observation has led to the discovery of other inputs and outputs of the Notch signaling pathway, collectively known as non-canonical Notch signaling.
Non-canonical Notch signaling can occur independently of Notch ligand stimulation, can be regulated by non-canonical ligands or can even occur without the RBPJ transcription factors (Andersen et al. 2012; D’Souza et al. 2010). An example of a non-canonical ligand is Delta-like 1 homolog (Dlk1). Dlk1 is highly related to Delta-Notch family, but lacks the DSL activating domain, which is considered to be crucial for binding with Notch family of receptors. Nonetheless, Dlk1 has been proposed to be an antagonist of Notch signaling whereby it interacts with Notch receptors, competes with canonical Notch ligands and negatively regulates Notch signaling (Baladron et al. 2005; Bray et al. 2008; Ceder et al. 2008). Baladron et al. demonstrated that transfection of Dlk1 into Notch1-positive Balb/c14 cells down-regulated the expression of Hes1 (Baladron et al. 2005). However, Dlk1 expression could also be modulated by Notch signaling. A study showed that during prostate luminal differentiation, activation of Notch signaling down-regulated the protein expression level of Dlk1 in the luminal transit amplifying cell (Ceder et al. 2008). Therefore, these studies suggested reciprocal regulation between canonical Notch and Dlk1 (Ceder et al. 2008).
Several reports have also suggested that Notch can exert its function via ligand- and transcription-independent mechanisms by post-translationally targeting Wnt/β-catenin signaling (Andersen et al. 2012). Uncleaved, membrane-bound Notch suppressed the active form of β-catenin in a GSK3β-independent manner, which suggested that Notch could interact with canonical Wnt signaling without the presence of its ligands (Hayward et al. 2005). The detailed mechanisms, however, remain unclear and are worth further investigations as this pathway could have physiological significance in development and tumorigenesis.
General role of Notch
With its highly conserved signaling mechanism that operates across multicellular organisms, Notch signaling is critical for the development and homeostasis of most tissues. In normal cellular processes, Notch signaling impinges mainly on the participation of cell-fate decision during development. For instance, the role of Notch has been implicated in the differentiation of various cell types in the heart, maintenance of neural stem cells, regulation of lipid content in the liver, lineage specification during pancreas development and differentiation of mammary epithelial cells that undergo morphogenetic changes during puberty, pregnancy and lactation (Gaiano and Fishell 2002; Grego-Bessa et al. 2007; Murtaugh et al. 2003; Pajvani et al. 2013; Raouf et al. 2008). Loss of Notch signaling consequently impairs heart development (Grego-Bessa et al. 2007), depletes neural stem cell population and disrupts brain function (Ables et al. 2011; Imayoshi et al. 2010), causes fatty liver (Pajvani et al. 2013), prevents the differentiation of pancreas progenitors (Murtaugh et al. 2003) and disturbs ductal morphogenesis in the breast (Raouf et al. 2008).
With its participation in the normal development of tissues, it is not surprising that dysregulation of Notch plays a part in tissue disorders or diseases with Notch exhibiting either oncogenic or tumor suppressor activities. Studies have shown that a truncated constitutively active form of Notch1 is capable of inducing T cell leukemia (Ellisen et al. 1991), while a similar truncated form of Notch4 results in poorly differentiated mammary adenocarcinoma in mice (Robbins et al. 1992). Overexpression of Notch3 by a copy number variation gained at chromosome 19p13.12 contributes to tumor progression in ovarian cancer (Park et al. 2006). In addition, Notch3 has been reported to form a juxtacrine loop with its primary ligand, Jagged1, in ovarian cancer, thus promoting its malignancy (Choi et al. 2008).
As for the tumor suppressive role of Notch, a study reported that Notch signaling induced growth arrest in small cell lung cancer cells (Sriuranpong et al. 2001). Overexpression of the active forms of Notch1 and Notch2 in small cell lung cancer cells up-regulated p21Cip1 and p27Kip1 expression, resulting in G1 cell cycle arrest (Sriuranpong et al. 2001). Another group reported that, as Notch1 activation up-regulated p21Cip1 expression and inhibited cell growth in mouse keratinocytes, Notch1 inactivation enhanced epidermal and corneal hyperplasia, followed by skin tumor development in an experimental mouse model (Nicolas et al. 2003; Rangarajan et al. 2001). Loss of Notch1 in the epidermis and in keratinocytes further leads to inappropriate activation of the β-catenin pathway, which is usually associated with various malignancies (Nicolas et al. 2003; Polakis 2000). Even in acute myeloid leukemia (AML) patients, it was found that the Notch signaling pathway was silenced. Knocking-in of NICD in a mouse model of AML reversed the disease phenotype by apoptosis induction in AML blasts (Kannan et al. 2013; Lobry et al. 2013).
Correlation between Notch and prostate formation, development and differentiation
The prostate is a complex, branched ductal organ surrounding the urethra at the base of the bladder of a mammalian male. Formation of the prostate occurs during late embryogenesis through branching morphogenesis from the epithelium and mesenchyme of the urogenital sinus (UGS) and is dependent on testicular androgen synthesis (Abate-Shen and Shen 2000; Cunha et al. 2002). Histologically, both mouse and human prostates are composed of branching glands, with ducts made up of three differentiated epithelial cell types: luminal, basal and neuroendocrine (Abate-Shen and Shen 2000). The androgen-dependent columnar luminal epithelial cells, characterized by expression of cytokeratin (CK) 8, CK18 and Nkx3.1 markers, form a layer just above the basal cells (Marker et al. 2003; Wu et al. 2007). The androgen-independent cuboidal basal cells express CK5, CK14, and p63 markers (Marker et al. 2003; Shen and Abate-Shen 2010). Neuroendocrine cells are dispersed throughout the basal layer and are believed to provide paracrine signals that support the growth of luminal cells (Shen and Abate-Shen 2010). Surrounding the gland is the stroma containing fibroblasts, myofibroblasts and the smooth muscle cells. Although stromal–epithelial interactions are poorly understood, it has been suggested that stromal cells play an important role in the growth and differentiation of the epithelium, as well as prostate cancer (Lawson and Witte 2007). The adult prostate retains the ability to support cell replication, differentiation and morphogenesis, suggesting the existence of stem/progenitor cells. A subpopulation of prostate cells which co-express CK8, CK18, CK5 and CK14 was found present within the basal compartment of adult prostatic epithelium. These cells were speculated to undergo lineage commitment and formed either CK8/CK18-expressing luminal cells or the CK5/CK14-expressing basal cells (Wang et al. 2001). However, recent studies suggest that both the basal and luminal cells are self-sustained in adult prostate (Choi et al. 2012). The transdifferentiation between lineages is rare in rodents, while it has been observed in human tissues (Blackwood et al. 2011; Choi et al. 2012; Gaisa et al. 2011; Ousset et al. 2012). Although basal cells are more resistant to direct transformation, it has been proven that both lineages could serve as the source of tumorigenesis population (Choi et al. 2012; Wang et al. 2009).
The regulation of prostate morphogenesis is mediated, in part, by androgens, but also involves numerous signaling pathways, including Sonic hedgehog (SHH) (Berman et al. 2004; Freestone et al. 2003; Podlasek et al. 1999), Fibroblast Growth Factor 10 (FGF10) (Donjacour et al. 2003) and Bone Morphogenic Protein 4 and 7 (BMP4 and BMP7) (Grishina et al. 2005; Lamm et al. 2001). SHH and FGF10 have been shown to promote prostate growth, while BMP4 and 7 suppress prostate branching (Berman et al. 2004; Donjacour et al. 2003; Freestone et al. 2003; Grishina et al. 2005; Lamm et al. 2001; Podlasek et al. 1999). Recent studies have also discovered the involvement of Notch in normal prostate development (Shou et al. 2001; Valdez et al. 2012; Wang et al. 2004, 2006).
In earlier reports using transgenic mice, which express GFP in the Notch1 promoter, Notch1 was expressed selectively in the prostate basal cell lineage (Shou et al. 2001; Wang et al. 2004). However, Valdez et al. recently found that Notch1 and Notch2 receptors were not only expressed in basal but also in luminal cells of murine prostate (Valdez et al. 2012). Notch ligands Jagged1, Jagged2 and Dll1 were also preferentially expressed in the basal cells, although Jagged2 and Dll1 were expressed at a much lower level. Other Notch ligands such as Dll3 and Dll4 were undetectable (Valdez et al. 2012). However, expressions of Notch receptors and ligands in the normal human prostate are less known. To date, only Notch1, Jagged1 and its non-canonical ligand, Dlk1, have been found in the normal human prostate epithelium. Notch1 is expressed in both the basal and luminal layers, Jagged1 in the luminal cells, and Dlk1 in basal cells (Carvalho et al. 2014). Even though more detailed analyses are needed, the presence of Notch receptors and ligands in human prostate suggests that Notch plays a role in prostate development and homeostasis.
In an experiment designed to determine the role of Notch in prostate biology, the expression patterns of Notch1 receptors during rodent prostatic development were examined. RT-PCR analysis from different developmental stages of the rat prostate showed high levels of Notch1 expression in the developing prostate, whereas its expression was significantly reduced in the adult prostate (Shou et al. 2001). Selective ablation of Notch1-expressing cells led to impairment of early prostate development and inhibition of re-growth of prostate following castration and androgen replacement (Wang et al. 2004). Therefore, these Notch1-expressing cells are believed to represent the stem/progenitor cells in prostate epithelium, and they are indispensable for proper prostatic morphogenesis, growth, differentiation and regeneration. An in-depth study further showed that Notch activation induced the proliferation of p63+ prostate progenitor cells (Wu et al. 2011). Gain of Notch function promoted proliferation and increased progenitor cell numbers in embryonic prostate as well as in postnatal prostate, and conversely, knockout of RBPJ, an effector of Notch signaling, in mice decreased progenitor cell proliferation and survival (Wu et al. 2011). However, contradicting findings were reported by Wang et al. who showed that Notch inhibition caused a significant proliferation of epithelial cells co-expressing CK8 and CK14, and impaired luminal and basal layer segregation in postnatal prostate (Wang et al. 2006). Hyperplastic phenotypes that were observed in Notch1 knockout mice were thus a consequence of the impaired epithelial differentiation (Wang et al. 2006). These controversies require careful interpretation as the differences in gene manipulation and the choice of cell markers might influence the experimental outcome.
In a more comprehensive study, Valdez et al. demonstrated that knockout of RBPJ led to enhanced proliferation and suppression of differentiation in prostate basal cells (Valdez et al. 2012). They further proved that Notch activation mediated a positive feedback loop by up-regulating TGF-β signaling to suppress basal progenitor activity (Valdez et al. 2012). Another study supported the link between TGF-β and Notch in inhibiting prostate branching morphogenesis. Treatment with recombinant BMP7, a member of the TGF-β superfamily, in urogenital sinus explants significantly decreased cleaved Notch1 and its downstream target, Hes1 expression (Grishina et al. 2005).
Interestingly, Kwon et al. also found that ectopic activation of Notch signaling in luminal cell promoted cell growth in vitro and induced prostate sphere formation ability (Kwon et al. 2014). These luminal cells, which possess short-term self-renewal capacities, are speculated to be the putative transit amplifying luminal progenitors (Kwon et al. 2014). Therefore, these data suggest that the activation of Notch signaling in luminal cells preserves the transit amplifying luminal progenitor population in the prostate (Kwon et al. 2014). In addition, Ceder et al. also demonstrated that Notch1 signaling was activated in the transit amplifying luminal progenitor cell population in human prostate tissue culture and further showed that this activation led to the down-regulation of Dlk1, which is a prostate basal cell marker and an inhibitory non-canonical Notch ligand (Ceder et al. 2008).
Notably, another group shifted the focus of Notch functions in the developing prostate to stromal cells. They observed that Notch2 and Dlk1 were co-expressed in the inductive prostatic mesenchyme of the ventral mesenchymal pad and were partially colocalized in the smooth muscle layer of the urethral stroma (Orr et al. 2009). Inhibition of Notch signaling with a γ-secretase inhibitor resulted in the loss of stromal tissue, and the loss of Notch signaling disrupted epithelial and stromal differentiation (Orr et al. 2009).
Based on the existing evidence, Notch signaling appears to have diverse functions in different stages of development, as well as different cell populations. Notch signaling promotes prostate progenitor cell proliferation during embryonic and postnatal development. In the adult, Notch activation may inhibit the proliferation but promote the differentiation of adult basal cells, whereas it may enhance the proliferation and suppress the differentiation of luminal progenitors (Figs. 1, 2). It is also worth noting that the dominant Notch ligands in basal and luminal layer are different. As discussed earlier, with the expression of Jagged1 in luminal cells and the inhibitory Notch ligand Dlk1 in basal cells of the human prostate (Carvalho et al. 2014), it may indicate the delicate modulation of Notch signaling in the maintenance of homeostasis. Taken together, we can conclude that Notch signaling is indeed necessary for the regulation of stromal survival, epithelial differentiation, prostate development and for the maintenance of progenitor cell populations.
Notch signaling in prostate epithelial cell lineage development and tumorigenesis. Notch signaling inhibits basal cell proliferation, while it promotes basal cell differentiation. On the contrary, Notch signaling preserves the transit amplifying luminal progenitor by promoting proliferation and inhibiting differentiation. Both basal and luminal lineages can serve as a tumorigenic cell population, although the role of Notch signaling as an oncogene or a tumor suppressor remains unclear
Notch and androgen receptor in prostate cells
The normal development, maintenance and function of the prostate are dependent upon androgen, requiring the activation of the transcriptional activity of the androgen receptor (AR) (Marker et al. 2003). The androgen-induced AR transactivation recruits various cofactors or corepressors that either enhance or inhibit the rate of gene transcription, respectively (Gelmann 2002). Like normal prostate tissue, prostate cancer is also dependent on androgen for its growth. AR is expressed in cancer cells, and corruption of its downstream growth control mechanism, such as p53, plays a significant role in tumorigenesis and metastasis of prostate cancer (Chang et al. 2014; Lavery et al. 2011; Nantermet et al. 2004). Therefore, inhibiting the activities of AR is a crucial step in treating primary prostate cancer to prevent tumor progression (Karantanos et al. 2013). Unfortunately, prostate cancer may become resistant to androgen deprivation and may initiate metastasis (Miki and Rhim 2008; Siegel et al. 2013); however, the mechanisms of disease progression are not yet fully understood.
Recent investigations revealed the interaction between AR and Notch signaling. Belandia et al. demonstrated that the Notch target, Hey1, acted as an AR corepressor (Belandia et al. 2005). Hey1 interacted with both transcription coactivators, SRC1 and AR, repressing AR activation and AR-dependent gene expression. As Hey1 is a Notch effector, overexpression of the constitutively active form of Notch, NICD, also showed repression of AR activity in myoblast, prostate and breast cancer cells (Belandia et al. 2005). In addition, HEYL, the third member of Hey family and also an effector of Notch signaling, was found to be a more potent repressor of AR signaling. Similar to Hey1, HEYL bound to AR activation function-1 and blocked AR activity, leading to the suppression of androgen-dependent prostate cancer cell growth (Lavery et al. 2011). In contrast, a study also suggested that AR signaling repressed Notch signaling (Nantermet et al. 2004). In a genomic profiling study, when prostate cell proliferation was induced by 5α-dihydrotestosterone (DHT) treatment, Notch1 and Jagged1 were found to be repressed upon AR activation (Nantermet et al. 2004). Therefore, these studies suggested that Notch and AR signaling suppressed each other in prostate cells. However, Belandia et al. and Lavery et al. also discovered that the interaction between Notch and AR signaling could be disrupted in prostate cancers whereby both Hey1 and HEYL were found to be excluded from the nucleus by unknown mechanisms (Belandia et al. 2005; Lavery et al. 2011). This disruption was correlated with the malignancy of prostate cancer.
A recent report showed that AR promoted the oncogenic property of Jagged1, a Notch ligand whose overexpression is associated with prostate cancer progression, in prostate cancer cell lines (Yu et al. 2014). It was shown that AR-negative prostate cancer cell lines, DU145 and PC3, when overexpressed with AR and Jagged1, significantly increased their proliferation rates to a level similar to that of an AR-positive prostate cancer cell line, LNCaP, which overexpressed Jagged1 (Yu et al. 2014). They reported that AR together with Jagged1 increased the phosphorylation of Akt and the expression level of cyclin B1, promoting prostate cancer cell growth. Furthermore, high expression of AR and Jagged1 predicted a worse prognosis in prostate cancer patients (Yu et al. 2014).
In all, these reports suggest that Notch and AR signaling suppress each other in prostate cells and maintain balanced signaling during prostate development and homeostasis. However, for unknown reasons, restricting activity of the Hey family of transcription factors could abolish the inhibitory effect of Notch on AR signaling. The disrupted balance between Notch and AR signaling could therefore lead to the tumorigenesis of prostate cancer.
Prostate cancer hallmarks and correlation with Notch
The loss of basal cells and reduced matrix diversity marks the initiation of tumor formation in the prostate (Frank and Miranti 2013). It has been suggested that the accumulation of prostatic intraepithelial neoplasia (PIN) lesions may eventually lead to the development of adenocarcinoma (Lawson and Witte 2007). The mechanisms of prostate tumor initiation and progression are, however, poorly understood. As mentioned earlier, it is believed that there are stem/progenitor cells in the prostate to maintain prostate cell replication, differentiation and morphogenesis. Recently, it was speculated that these progenitors could be transformed into cancer-initiating cells by suppressing PTEN and p53 (Choi et al. 2012). Classically, it was thought that cancer-initiating cells originated from the luminal cell, as the majority of the tumor cells expressed luminal cell specific markers such as CK8 and CK18 (Long et al. 2005). Basal cell markers such as CK5, CK14 and p63 were rarely observed, and thus, mature luminal cells or luminal progenitors were suggested to be the origin of prostate cancer (Lawson and Witte 2007). Conversely, another study reported colocalization of basal and luminal cell markers in human prostate cancer (Verhagen et al. 1992). In androgen-independent prostate cancer (AIPC) cells, basal cell characteristics, such as expression of protooncogene Bcl-2, were observed (McDonnell et al. 1992). It was suggested that prostate cancer cells might obtain or regain the basal characteristics during tumor growth (Long et al. 2005).
According to Hanahan and Weinberg, the hallmarks of a cancer comprise of the capabilities to sustain proliferative signaling, evade growth suppressors, resist cell death, enable replicative immortality, induce angiogenesis and activate invasion and metastasis (Hanahan and Weinberg 2011). As much as it is crucial for maintaining normal prostate development and homeostasis, Notch signaling has also been associated with prostate adenocarcinoma. Understanding the hallmarks and the balance between the oncogenic and tumor suppressor roles of Notch in prostate cancer could assist in the development of novel therapies.
Emerging evidence has demonstrated that Notch expression is significantly higher in prostate cancer and dysregulation of Notch signaling contributes to tumor development and cancer metastasis. In prostate cancer cell lines, such as DU145, LNCaP and PC3, Notch1 is expressed at various levels (Shou et al. 2001). In human prostate samples, among 218 prostate cancer samples examined, Jagged1 mRNA expression levels were significantly higher in primary tumor and metastasis samples compared to normal samples (Yu et al. 2014). Likewise, Zhu et al. reported that Jagged1 and Notch1 protein levels were elevated in advanced prostate cancers (Zhu et al. 2013). Moreover, higher Jagged1 expression is significantly associated with recurrence (Santagata et al. 2004). However, in the context of cancer etiology, the role of Notch proteins, their ligands and regulation are diverse (Li et al. 2007; Shou et al. 2001; Wang et al. 2006, 2010, 2011; Whelan et al. 2009). As reported by Wang et al. Notch inactivation might be associated with prostate tumorigenesis (Wang et al. 2006). By comparing gene expression patterns in wild type and Notch1 knockout mouse prostate, c-Fos, c-Jun, PSCA and FGF18 were shown to be significantly up-regulated in Notch1-deficient prostate (Wang et al. 2006). In clinical studies, c-Fos, c-Jun and PSCA levels are significantly elevated in human prostate adenocarcinoma specimens (Aoyagi et al. 1998; Reiter et al. 1998). Although the association of FGF18 in prostate cancer was unclear, two other closely related FGFs, FGF8 and FGF17, were elevated in prostate tumor samples (Heer et al. 2004; Valve et al. 2001). Moreover, prostate tumor overexpressed-1 (PTOV1), the adapter protein up-regulated in prostate cancer, promotes prostate cancer progression by down-regulation of Notch downstream targets Hes1 and Hey1, indicating a tumor suppressing role of Notch in prostate cancer (Alana et al. 2014). Contrarily, numerous reports have also indicated that Notch signaling promotes cell proliferation, prevents apoptosis, augments cell migration and invasion and facilitates cell metastasis, which will be discussed in detail later. Despite the controversies of Notch being a tumor suppressor or an oncogene, these results collectively show that disruption of the Notch pathway potentiates neoplastic alterations in the prostate.
Notch and prostate cancer cell proliferation
Notch1 is highly expressed in several prostate cancer cell lines, and comparably, Notch1 mRNA expression is up-regulated in malignant and metastatic prostate epithelial cells of the transgenic adenocarcinoma of mouse prostate (TRAMP) model (Shou et al. 2001; Wang et al. 2011). Interestingly, the expression of Notch ligands is low or undetectable either in prostate cancer cells or in TRAMP mice, suggesting minimal to no physiological activation of Notch signaling. However, once activated, Notch1 inhibits the proliferation of prostate cancer cells (Shou et al. 2001), implicating that Notch signaling is suppressed in malignant prostate cells.
Wang et al. reported that inactivation of Notch1 in prostate led to enhanced cell proliferation, tufting, bridging and localized clusters of epithelial cells, which resembled the phenotype of genetically engineered mouse models for prostate cancer (Wang et al. 2006). Upon further examination, microarray analysis revealed that Notch1 and Hey1 genes were significantly down-regulated in prostate adenocarcinoma compared to normal prostate, suggesting that dysregulation of Notch signaling pathway might facilitate prostatic tumorigenesis (Wang et al. 2006). In line with this report, Whelan et al. reported loss of total and cleaved Notch1 and Hey1 immunohistochemistry in prostate adenocarcinoma foci (Whelan et al. 2009). To further investigate the role of Notch1 signaling in prostate cancer cells, they overexpressed constitutively active Notch1 in the DU145 prostate cancer cell line. Activation of Notch1 showed no significant effect on cell proliferation rate, but reduced cell migration. They also found that expression of PTEN, a tumor suppressor gene, was up-regulated through Notch signaling. Therefore, they suggested that Notch signaling suppresses cancer progression by up-regulating PTEN tumor suppressor gene expression (Whelan et al. 2009).
Although some studies support the anti-proliferative role of Notch, others have suggested Notch involvement in tumor growth. Zhang et al. reported that down-regulation of Jagged1 inhibited cell growth in prostate cancer cell lines, including PC3, DU145, LNCaP and C4-2B (Zhang et al. 2006). Another group also recently reported that knocking down Jagged1 by siRNA transfection in LNCaP, LAPC4, DU145 and PC3 greatly decreased cell proliferation; conversely, overexpression of Jagged1 significantly increased cell proliferation by twofold in LNCaP and LAPC4 cells, whereas Jagged1 showed little effect on the proliferation of DU145 or PC3 cell lines (Yu et al. 2014). Furthermore, knockdown of RBPJ led to reduced proliferation in PC3 cells (Yong et al. 2011). These results indicate that Notch signaling promotes prostate cancer cell growth, although Notch signaling may have different effects on different prostate cancer cell lines. In addition, when non-canonical Notch inhibitory ligand Dlk1 is overexpressed in cancer-associated fibroblasts, which are the main components of cancer microenvironment, the proliferation rate and tumor growth are suppressed (Orr et al. 2013). These results further support the requirement of active Notch signaling in prostate cancer cell proliferation.
Proliferation is tightly controlled by cell cycle regulation. It has been reported that down-regulation of Notch1 and/or its ligand, Jagged1, retards S phase cell cycle progression and inhibits cell growth in PC3 prostate cancer cell line (Zhang et al. 2006). S phase cell cycle progression is dependent upon CDK2 activity as well as S phase cyclins. In Jagged1 knockdown in PC3 cells, CDK2 expression was reduced by 50 % and cyclin A decreased by 90 %. In addition, the protein expression of p27, a CDK inhibitor, was up-regulated by tenfold, corroborating that Jagged1 is required for cell cycle progression in prostatic tumorigenesis (Zhang et al. 2006). Intriguingly, although knockdown of both Jagged1 and Notch1 induced cell growth inhibition, knockdown of Jagged1 exhibited a much stronger inhibitory effect on prostate cancer cell lines when compared to knockdown of Notch1 (Zhang et al. 2006). A plausible explanation is that siRNA knockdown of Jagged1 reduced both Notch1 and Notch2 expression.
The role of Notch signaling in prostate cancer cell function is controversial. Several reports suggested that overexpression of Notch1 signaling suppressed cancer proliferation (Shou et al. 2001; Wang et al. 2006), while others reported that knockdown of Notch1 suppressed proliferation (Yong et al. 2011; Yu et al. 2014; Zhang et al. 2006). A group even found that up-regulation of Notch signaling had no significant effect on cell proliferation (Whelan et al. 2009). It is worth noting that these experiments were carried out in a variety of cultured prostate cancer cell lines, which may behave differently under different culture conditions. Moreover, the responses of Notch signaling under cell culture conditions may not represent the behaviors of cancer cells in vivo. Manipulation of Notch signaling in prostate cancer organoid cultures and cancer organoid xenoplants may therefore be helpful in understanding the definitive role of Notch signaling in cell proliferation during cancer progression (Gao et al. 2014).
Notch suppresses apoptosis/anoikis in prostate cancer
Knockdown of Notch1 and Jagged1 reduces cell viability and induces apoptosis in the PC3 prostate cancer cell line (Wang et al. 2010). Further examination showed that Notch inactivation reduced Akt phosphorylation and its downstream target, mTOR. Intriguingly, PI3K/Akt inhibition by PI3 K inhibitors, LY294002 and Wortmanin, abolished Notch1 expression and mTOR phosphorylation in PC3 cells. Akt deficiency also decreased Notch1 and Jagged1 expression. These data suggested a reciprocal regulation of Notch1 and Akt pathways in prostate cancer (Wang et al. 2010).
Apart from Akt, the same group also found that down-regulation of Notch1 inhibited the FoxM1 pathway (Wang et al. 2011). Overexpression of FoxM1, a cell cycle-related transcription factor, has been associated with prostate carcinogenesis (Chandran et al. 2007; Kalin et al. 2006). LY294002 and Wortmanin, inhibitors of the PI3 K/Akt pathway, eliminated the expression of FoxM1, suggesting that the FoxM1 pathway is regulated by PI3 K/Akt. Taken together, these results suggest that down-regulation of Notch1 and Jagged1 induces apoptotic prostate cancer cell death mediated by inactivation of PI3K/Akt, FoxM1 and mTOR pathways.
Notch signaling also plays a role in modulating anoikis. Anoikis is programmed cell death induced by the loss of cell adhesion from the surrounding extracellular matrix. Anoikis is an important mechanism that may be used to prevent metastasis by triggering programmed cell death. Therefore, metastatic cancer cells would need to escape anoikis in order to colonize secondary tumors (Liotta and Kohn 2004).
Several studies have associated Notch signaling to anoikis resistance in tumor cells. For instance, in breast cancer, Notch-mediated repression of E-cadherin results in activation of β-catenin and anoikis resistance (Leong et al. 2007). In cervical cancer, activated Notch1 signaling synergizes with human papillomavirus (HPV) oncogenes in the transformation of immortalized epithelial cells and generates resistance to anoikis through activation of P13K/Akt pathway (Rangarajan et al. 2001). Although little has been reported on the correlation between Notch and anoikis in prostate cancer, a study showed that increased Notch signaling inhibited anoikis and stimulated proliferation of prostate luminal epithelial cells (Kwon et al. 2014).
As discussed earlier, the induction of ectopic elevation of Notch signaling in luminal cells preserved the luminal progenitor population by stimulation of proliferation via PI3K/Akt signaling and inhibition of differentiation to luminal cells (Kwon et al. 2014). In addition, activation of Notch signaling suppressed cell anoikis via NF-KB, and independent of Hes1, in transit amplifying luminal progenitors (Kwon et al. 2014). It is possible that aberrant Notch signaling promotes proliferation of luminal cells, inhibits anoikis and enhances prostate cancer progression and metastasis. However, additional experimental approaches to illustrating direct involvement of Notch signaling in anoikis are needed.
Notch and prostate cancer stem cells
The presence of prostate cancer stem cells is associated with chemotherapy resistance, tumor aggressiveness and poor prognosis (Domingo-Domenech et al. 2012). In gene profiling of DU145 prostate cancer cells, a subpopulation with CD133high/CD44high was isolated and found to express high levels of Notch1, Jagged1, Dll1 and Dll3 (Oktem et al. 2014). CD133 and CD44 were both markers for cancer stem cells; therefore, it is believed that this subpopulation could be the putative prostate cancer stem cell (Oktem et al. 2014). In addition, it was reported that in human prostate cancer tissue samples, a subpopulation conferring docetaxel resistance, characterized by loss of epithelial differentiation markers and HLA class 1 (HLA1) antigens and marked up-regulation of Notch and Hedgehog signaling, was detected (Domingo-Domenech et al. 2012). As these cells with docetaxel-resistant phenotype exhibited tumor-initiating capacity and were more abundantly observed in metastatic tumors, it could be speculated that this subpopulation might be a cancer stem cell capable of initiating or contributing to prostate cancer progression (Domingo-Domenech et al. 2012). Another report by Kwon et al. demonstrated the presence of a cancer stem cell-like subpopulation that could proliferate but could not differentiate and prevent apoptosis (Kwon et al. 2014). Furthermore, overexpression of Notch1 led to proliferation, anoikis resistance and rescue of this subpopulation, while Domingo-Domenech et al. proceeded to demonstrate that combined targeted inhibition of Notch and Hedgehog signaling pathways significantly depleted this subpopulation, implicating a promising therapeutic strategy toward prostate cancer (Domingo-Domenech et al. 2012; Kwon et al. 2014).
Association of Notch and hypoxia in prostate cancer progression
Although extreme hypoxia conditions tend to hamper tumor cell survival, milder hypoxia conditions lead to pre-conditioning of tumor cells, increasing their ability to adapt to low-oxygen conditions for survival. A study reported that induction of chronic hypoxia in LNCaP human prostate cancer cells activated Notch3, but not Notch1 or Notch2, signaling to sustain cell proliferation (Danza et al. 2013). Although initially the expression of Notch3 transmembrane full-length (FL) protein in LNCaP cell was almost undetectable under hypoxia, treatment with DAPT, a γ-secretase inhibitor, increased Notch3 FL and intracellular domain expression, indicating activation of Notch3 receptor activity under low-oxygen conditions. An increase in the frequency of Notch3 immunostaining was also observed in tumor biopsies with higher Gleason score, further implicating a role for Notch3 in prostate cancer progression (Danza et al. 2013).
The same group also reported that Notch signaling modulated hypoxia-induced neuroendocrine differentiation (NED) in LNCaP cells (Danza et al. 2012). The paracrine function of neuroendocrine-differentiated prostate cancer cells enhanced proliferation, invasion and metastasis, leading to a poorer prognosis and androgen deprivation therapy resistance. Exposure of LNCaP cells to low oxygen promoted NED, and while under hypoxic conditions, Notch1 and Notch2 mRNA and protein expression, as well as Hey1 and Hes1 expression, were all down-regulated. Conversely, overexpression of dominant-negative Hes1 in LNCaP cells promoted NED, which further validates that hypoxia-induced NED is regulated by inhibition of Notch receptor activity through down-regulation of Hes1 transcription (Danza et al. 2012).
In conclusion, these data suggest that hypoxia triggers the activation of Notch3 to sustain proliferation of prostate cancer cells, while inhibiting Notch1 and Notch2 signaling, to induce NED to promote prostate cancer progression. Notably, tumor hypoxia has also been associated with enhanced epithelial–mesenchymal transition (EMT), a process which favors cancer metastasis, and thus, Notch signaling seems to be involved in converting a hypoxic stimulus into EMT (Sahlgren et al. 2008), although no experimental evidence in a prostate cancer model has yet been provided.
Notch and cell migration and invasion in prostate cancer
When Notch1 was overexpressed in both human tissue samples and cultured prostate cancer cell lines, Notch1 promoted tumor invasion (Bin Hafeez et al. 2009). Bin Hafeez et al. demonstrated that knockdown of Notch1 in PC3 and 22Rv1 cells significantly attenuated cell invasion (Bin Hafeez et al. 2009). Through the microarray analysis, knockdown of Notch1 further revealed a significant decrease in the expression of extracellular proteins involved in cell invasion, including matrix metalloproteinase-9 (MMP9) and urokinase plasminogen activator (uPA) (Bin Hafeez et al. 2009), which are NF-KB downstream genes. Another study also demonstrated that Notch1 and/or Jagged1 silencing inhibited NF-KB DNA-binding activity (Wang et al. 2010). Together, these results suggest that Notch1 deficiency causes a decrease in NF-KB activity and its downstream targets, including MMP and uPA, thus decreasing cancer cell invasiveness (Bin Hafeez et al. 2009; Wang et al. 2010). Furthermore, Notch signaling was reported to be associated with metastatic cell behavior (cell motility). Treatment with DAPT, a Notch inhibitor, down-regulated Hes1 and decreased cell motility in LNCaP and PC3 cells after 48 h (Scorey et al. 2006). These findings suggest that Notch expression promotes metastasis and invasion in prostate cancer.
Another study showed that Notch signaling inhibited cell invasiveness in prostate cancer; however, this anti-invasive role of Notch was repressed by prostate tumor overexpressed-1 (PTOV1) (Alana et al. 2014), an adaptor protein whose expression is significantly increased in prostate cancer (Benedit et al. 2001; Santamaria et al. 2003). In PTOV1 knockdown prostate epithelial cells, Notch-dependent expression of Hey1 and Hes1 was up-regulated and constitutively active Notch1 receptor attenuated the invasion and anchorage-independent growth of prostate cancer cells (Alana et al. 2014).
Notch and angiogenesis
Angiogenesis has been associated with tumor growth. It has been suggested that Notch signaling may be crucial for homeostasis of the normal vasculature, as well as tumor angiogenesis. Experiments targeting Dll4, the main Notch ligand in angiogenesis, in solid tumors revealed that blocking of Notch signaling increases nonproductive angiogenesis and decelerates tumor growth, but facilitates metastasis (Hu et al. 2012; Ridgway et al. 2006; Sainson and Harris 2007; Thurston et al. 2007).
Although limited studies have correlated Notch with angiogenesis in prostate cancer, it has become clear that Notch signaling plays a role in vascular development and tumor angiogenesis. Bin Hafeez et al. detected a higher level of Notch1 protein in tumor areas that surround the vasculature, suggesting that Notch may facilitate angiogenesis in prostate cancer (Bin Hafeez et al. 2009). Intriguingly, Li et al. demonstrated that overexpression of Dll4 in PC3 cells reduced angiogenesis but improved vessel perfusion and tumor oxygenation by improving vasculature structure and function, resulting in enhanced tumor progression in cancer xenografts (Li et al. 2007).
Therefore, it seems that the function of Notch signaling in angiogenesis in prostate cancer might be the same as compared to other solid tumors (Hu et al. 2012). Dll4-activated Notch signaling in vessel endothelial cells would be crucial for tumor progression, as overexpression improved tumor vasculature (Li et al. 2007). However, the impacts of other Notch ligands on prostate cancer angiogenesis remain unknown and require further investigation.
Notch and prostate cancer metastasis
Cancer metastasis is the leading cause of cancer mortality (Hu et al. 2012). Studies have also confirmed that prostate cancers frequently metastasize to the bone (Cooper et al. 2003), lymph nodes (Swanson et al. 2006) and brain (Salvati et al. 2005). Various prostate cancer cell lines derived from these sites are used as experimental models, for example, PC3 and C4-2B (derived from bone), DU145 (derived from brain) and LNCaP (derived from lymph node) (Bin Hafeez et al. 2009; Shou et al. 2001; Zayzafoon et al. 2004). However, the roles of Notch signaling in prostate cancer metastasis have not been well elucidated. From the discussions in this review, it seems that the Notch signaling pathway may facilitate prostate cancer metastasis, as Notch plays roles in cell migration and invasion, anoikis and apoptosis resistance, and regulating angiogenesis (Fig. 3). Immunohistochemical analysis from 154 samples of benign, localized and metastatic prostate cancer samples showed significantly higher Jagged1 expression in metastatic samples (Santagata et al. 2004). Furthermore, higher Jagged1 expression in localized tumors is associated with higher incidence of reoccurrence after radical prostatectomy (Santagata et al. 2004). Similarly, in transgenic prostates from TRAMP mice, higher Notch1 mRNA levels were expressed upon metastasis to the lymph nodes (Shou et al. 2001). Moreover, when examining the aberrant expressed EMT markers in prostate cancer, Sethi et al. showed that only Notch1 was significantly overexpressed in prostate cancer bone metastasis compared to primary prostate cancer (Sethi et al. 2010). This result showed that Notch1 might be directly involved in prostate cancer bone metastasis.
Notch signaling in prostate cancer hallmarks. Contradicting results have suggested Notch signaling to either promote or inhibit prostate cell proliferation, as well as cell migration and invasion. Reports have also suggested that activation of Notch suppresses cell apoptosis, anoikis and angiogenesis, while promoting metastasis. Under hypoxic conditions, Notch signaling indirectly promotes hypoxia endurance in prostate cancer cells by sustaining cell proliferation
In the progression of bone metastasis, Zayzafoon et al. demonstrated a possible mechanism involving Notch signaling, in which interaction of prostate metastatic cells with osteoblasts transformed cancer cells into osteoblast-like phenotype, inducing osteoblastic lesions (Zayzafoon et al. 2004). Accordingly, Notch1 expression was significantly increased in the cancer cell line C4-2B (derived from bone) and in bone samples from patients who developed bone metastases from a primary prostate tumor (Zayzafoon et al. 2004). Presence of Hes1 expression in C4-2B cell further confirmed the activation of Notch signaling in osteogenic induction. In addition, osteogenic induction also showed up-regulation of ERK phosphorylation. However, Notch and ERK signaling pathways were regulated independent of one another. An increase in the mRNA and nuclear localization of Runx2, an essential transcription factor for differentiation of osteoblasts, was also observed. As the expression and activation of Runx2 was attenuated by U0126 and not L-685,458, therefore, Runx2 was ERK-dependent. The presence of Hes1 and Runx2 was required for OSE-2 DNA binding, activating the expression of osteoblast specific gene, and thus transforming and producing osteoblast-like cell. This finding proposed a mechanism on how prostate cancer is capable of metastasizing to bone in vivo specifically expressing the Notch1 receptor (Zayzafoon et al. 2004).
Notch as a potential diagnostic and therapeutic target in prostate cancer
Given the profound involvement of Notch signaling in the pathogenesis of prostate cancer despite its disputable role as an oncogene or a tumor suppressor, the Notch pathway has become the ideal target for diagnostic and pharmacological intervention.
With the expression of Jagged1 and Notch1 evidently higher in high-grade prostate cancer and metastatic cells than that of low-grade and benign prostatic cells, Notch and Notch ligands can be highly correlated with prostate cancer progression and recurrence (Santagata et al. 2004; Zhu et al. 2013). Moreover, genomic profiling results have identified Jagged1 expression as one of the markers of stem cell-like prostate cancer cells (Duhagon et al. 2010). Therefore, Jagged1 and Notch1 may be useful markers in cancer grading. Interestingly, proteomic analysis of DU145 exosomes has also indicated an enrichment of Notch3 in prostate cancer exosomes, which may provide a practical biomarker for prostate cancer detection by noninvasive methods (Webber et al. 2014).
Among the prostate cancer hallmarks discussed, Notch signaling clearly suppresses cell apoptosis, anoikis and angiogenesis. This evidence strongly proposed that the usage of Notch inhibitors in prostate cancer treatment might promote cell death and attenuate tumor growth. Further investigation by Domingo-Domenech et al. suggested excitingly that, in combination with a Hedgehog signaling inhibitor, Notch inhibition depleted chemotherapy-resistant cancer cells in hormone-refractory prostate cancer (Domingo-Domenech et al. 2012). Therefore, Notch inhibitors can be used as a supplement in addition to chemotherapy as it can potentiate cell death and deplete chemo-resistant cancer stem cell population.
γ-secretase inhibitors (GSIs) are often used to target Notch pathway activity, with the aim to prevent the generation of NICD. GSIs treatment has successful suppressed cancer progression in T-ALL, prostate, breast and lung cancer models (Carvalho et al. 2014). However, GSIs treatment causes gastrointestinal toxicity due to their lack of specificity (van Es et al. 2005). Selective Notch inhibitors, for example antibodies targeting specific Notch receptors, or even synthetic peptides targeting Notch downstream, RBPJ, could offer more precise inhibitory effects, and thus lowering toxicity (Carvalho et al. 2014; Yong et al. 2011). It is also an interesting idea to restrain tumor growth through the up-regulation of non-canonical Notch ligand Dlk1 or SCUBE1 in cancer-associated fibroblasts (Orr et al. 2013). Moreover, NUMB and several microRNAs including miR-30a and miR-132 have been suggested to regulate Notch signaling, which may serve as targets for suppressing Notch in prostate cancer (Flores et al. 2014; Ortega et al. 2014; Salta et al. 2014).
As the roles of Notch in cancer cell proliferation and invasion remain unclear, it is not surprising that clinical trials with Notch inhibitors generate little encouraging outcomes in prostate cancer patients (Carvalho et al. 2014). Moreover, as suggested by Masuda et al., Notch signaling can serve in both autonomous and non-autonomous manner (Masuda and Izpisua Belmonte 2014). Autonomous Notch signaling modulates cancer cells themselves for proliferation and cell survival, while non-autonomous signaling acts on vascular endothelial cells, regulating angiogenesis. Therefore, inhibition of Notch signaling may induce nonfunctional angiogenesis, leading to hypoxia, which may favor cancer stem cell growth and promote metastasis (Hu et al. 2012; Masuda and Izpisua Belmonte 2014). This might be a plausible explanation to the limited clinical trial success with Notch inhibitors in prostate cancer patients.
Taken together, Notch inhibitors could potentially be used to target therapy-resistant cancer stem cell population, improving chemotherapy efficiency. However, the therapeutic potentials of Notch inhibitors require further examination, as Notch signaling has diverted effects on various aspects of prostate cancer hallmarks.
Conclusions
While Notch signaling has been established to be important in regulating normal prostate development, its function in prostate cancer etiology and progression remains debatable. Although Notch is overexpressed in prostate cancer, we do not know for sure whether Notch up-regulation would promote prostate cancer progression, or whether Notch signaling is elevated as a compensatory effect of prostate cancer progression. In fact, the conflicting data of Notch’s role either as an oncogene or as a tumor suppressor are not surprising at all. These seemingly different functions of Notch are highly context-dependent. Notch signaling, being highly conserved and present in various tissues, can be activated by different stimuli and respond differently in different microenvironments. In addition, different types of Notch receptors, ligands and targeted genes can also initiate different Notch mechanisms. Therefore, Notch signaling is complicated in its function. As discussed, Notch signaling could exhibit diverse functions not only in basal and luminal cells, but also in different stages of prostate development and in different stages of cancer progression.
In summary, Notch signaling is not a simple pathway but a signaling network, modulating different hallmarks of prostate cancer. The understanding of Notch signaling will help us to explain the physical and pathological phenomenon and provide the basis for disease therapeutics. The effects of Notch pathway intervention in clinical practice are not clear at the moment and continued in-depth research is required.
References
Abate-Shen C, Shen MM (2000) Molecular genetics of prostate cancer. Genes Dev 14:2410–2434
Ables JL, Breunig JJ, Eisch AJ, Rakic P (2011) Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12:269–283. doi:10.1038/nrn3024
Alana L et al (2014) Prostate tumor OVerexpressed-1 (PTOV1) down-regulates HES1 and HEY1 Notch targets genes and promotes prostate cancer progression. Mol Cancer 13:74. doi:10.1186/1476-4598-13-74
Allenspach EJ, Maillard I, Aster JC, Pear WS (2002) Notch signaling in cancer. Cancer Biol Ther 1:466–476
Andersen P, Uosaki H, Shenje LT, Kwon C (2012) Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol 22:257–265. doi:10.1016/j.tcb.2012.02.003
Andersson ER, Lendahl U (2014) Therapeutic modulation of Notch signalling—Are we there yet? Nat Rev Drug Discov 13:357–378. doi:10.1038/nrd4252
Aoyagi K, Shima I, Wang M, Hu Y, Garcia FU, Stearns ME (1998) Specific transcription factors prognostic for prostate cancer progression. Clin Cancer Res 4:2153–2160
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Baladron V et al (2005) dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res 303:343–359. doi:10.1016/j.yexcr.2004.10.001
Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, Parker MG (2005) Hey1, a mediator of Notch signaling, is an androgen receptor corepressor. Mol Cell Biol 25:1425–1436. doi:10.1128/MCB.25.4.1425-1436.2005
Benedit P et al (2001) PTOV1, a novel protein overexpressed in prostate cancer containing a new class of protein homology blocks. Oncogene 20:1455–1464. doi:10.1038/sj.onc.1204233
Berman DM et al (2004) Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol 267:387–398. doi:10.1016/j.ydbio.2003.11.018
Bin Hafeez B et al (2009) Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin Cancer Res 15:452–459. doi:10.1158/1078-0432.CCR-08-1631
Blackwood JK et al (2011) In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J Pathol 225:181–188. doi:10.1002/path.2965
Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S (1997) Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90:281–291
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689. doi:10.1038/nrm2009
Bray SJ, Takada S, Harrison E, Shen SC, Ferguson-Smith AC (2008) The atypical mammalian ligand delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev Biol 8:11. doi:10.1186/1471-213X-8-11
Brou C et al (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease. TACE Mol Cell 5:207–216
Bush G, diSibio G, Miyamoto A, Denault JB, Leduc R, Weinmaster G (2001) Ligand-induced signaling in the absence of furin processing of Notch1. Dev Biol 229:494–502. doi:10.1006/dbio.2000.9992
Carvalho FL, Simons BW, Eberhart CG, Berman DM (2014) Notch signaling in prostate cancer: a moving target. Prostate 74:933–945. doi:10.1002/pros.22811
Ceder JA, Jansson L, Helczynski L, Abrahamsson PA (2008) Delta-like 1 (Dlk-1), a novel marker of prostate basal and candidate epithelial stem cells, is downregulated by Notch signalling in intermediate/transit amplifying cells of the human prostate. Eur Urol 54:1344–1353. doi:10.1016/j.eururo.2008.03.006
Chandran UR et al (2007) Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 7:64. doi:10.1186/1471-2407-7-64
Chang C, Lee SO, Yeh S, Chang TM (2014) Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 33:3225–3234. doi:10.1038/onc.2013.274
Choi JH, Park JT, Davidson B, Morin PJ, Shih Ie M, Wang TL (2008) Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res 68:5716–5723. doi:10.1158/0008-5472.CAN-08-0001
Choi N, Zhang B, Zhang L, Ittmann M, Xin L (2012) Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21:253–265. doi:10.1016/j.ccr.2012.01.005
Cooper CR et al (2003) Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 97:739–747. doi:10.1002/cncr.11181
Cunha GR, Hayward SW, Wang YZ (2002) Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485. doi:10.1046/j.1432-0436.2002.700902.x
Danza G et al (2012) Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol Cancer Res 10:230–238. doi:10.1158/1541-7786.MCR-11-0296
Danza G et al (2013) Notch3 is activated by chronic hypoxia and contributes to the progression of human prostate cancer. Int J Cancer 133:2577–2586. doi:10.1002/ijc.28293
Domingo-Domenech J et al (2012) Suppression of acquired docetaxel resistance in prostate cancer through depletion of Notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 22:373–388. doi:10.1016/j.ccr.2012.07.016
Donjacour AA, Thomson AA, Cunha GR (2003) FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol 261:39–54
D’Souza B, Meloty-Kapella L, Weinmaster G (2010) Canonical and non-canonical Notch ligands. Curr Top Dev Biol 92:73–129. doi:10.1016/S0070-2153(10)92003-6
Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL (2010) Genomic profiling of tumor initiating prostatospheres. BMC Genom 11:324. doi:10.1186/1471-2164-11-324
Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J (1991) TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66:649–661
Fischer A, Gessler M (2007) Delta-Notch–and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res 35:4583–4596. doi:10.1093/nar/gkm477
Fleming RJ (1998) Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol 9:599–607. doi:10.1006/scdb.1998.0260
Flores AN, McDermott N, Meunier A, Marignol L (2014) NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat Rev Urol 11:499–507. doi:10.1038/nrurol.2014.195
Fraering PC et al (2004) Purification and characterization of the human gamma-secretase complex. Biochemistry 43:9774–9789. doi:10.1021/bi0494976
Frank SB, Miranti CK (2013) Disruption of prostate epithelial differentiation pathways and prostate cancer development. Front Oncol 3:273. doi:10.3389/fonc.2013.00273
Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA (2003) Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol 264:352–362
Gaiano N, Fishell G (2002) The role of Notch in promoting glial and neural stem cell fates. Annu Rev Neurosci 25:471–490. doi:10.1146/annurev.neuro.25.030702.130823
Gaisa NT et al (2011) Clonal architecture of human prostatic epithelium in benign and malignant conditions. J Pathol 225:172–180. doi:10.1002/path.2959
Gao D et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159:176–187. doi:10.1016/j.cell.2014.08.016
Gelmann EP (2002) Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015
Grego-Bessa J et al (2007) Notch signaling is essential for ventricular chamber development. Dev Cell 12:415–429. doi:10.1016/j.devcel.2006.12.011
Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD (2005) BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol 288:334–347. doi:10.1016/j.ydbio.2005.08.018
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A (2005) Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development 132:1819–1830. doi:10.1242/dev.01724
Heer R, Douglas D, Mathers ME, Robson CN, Leung HY (2004) Fibroblast growth factor 17 is over-expressed in human prostate cancer. J Pathol 204:578–586. doi:10.1002/path.1668
Hu YY, Zheng MH, Zhang R, Liang YM, Han H (2012) Notch signaling pathway and cancer metastasis. Adv Exp Med Biol 727:186–198. doi:10.1007/978-1-4614-0899-4_14
Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R (2010) Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 30:3489–3498. doi:10.1523/JNEUROSCI.4987-09.2010
Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255. doi:10.1002/jcp.10208
Joutel A et al (1996) Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710. doi:10.1038/383707a0
Kalin TV et al (2006) Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res 66:1712–1720. doi:10.1158/0008-5472.CAN-05-3138
Kannan S et al (2013) Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med 210:321–337. doi:10.1084/jem.20121527
Kao HY et al (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev 12:2269–2277
Karantanos T, Corn PG, Thompson TC (2013) Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32:5501–5511. doi:10.1038/onc.2013.206
Kidd S, Lieber T (2002) Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev 115:41–51
Kidd S, Kelley MR, Young MW (1986) Sequence of the Notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol 6:3094–3108
Kwon OJ et al (2014) Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 5:4416. doi:10.1038/ncomms5416
Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W (2001) Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol 232:301–314. doi:10.1006/dbio.2001.0187
Lavery DN, Villaronga MA, Walker MM, Patel A, Belandia B, Bevan CL (2011) Repression of androgen receptor activity by HEYL, a third member of the Hairy/Enhancer-of-split-related family of Notch effectors. J Biol Chem 286:17796–17808. doi:10.1074/jbc.M110.198655
Lawson DA, Witte ON (2007) Stem cells in prostate cancer initiation and progression. J Clin Invest 117:2044–2050. doi:10.1172/JCI32810
Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A (2007) Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through slug-induced repression of E-cadherin. J Exp Med 204:2935–2948. doi:10.1084/jem.20071082
Li L et al (1997) Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16:243–251. doi:10.1038/ng0797-243
Li JL et al (2007) Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67:11244–11253. doi:10.1158/0008-5472.CAN-07-0969
Liotta LA, Kohn E (2004) Anoikis: cancer and the homeless cell. Nature 430:973–974. doi:10.1038/430973a
Lobry C et al (2013) Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med 210:301–319. doi:10.1084/jem.20121484
Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A (1998) The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA 95:8108–8112
Long RM, Morrissey C, Fitzpatrick JM, Watson RW (2005) Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clin Sci (Lond) 108:1–11. doi:10.1042/CS20040241
Marker PC, Donjacour AA, Dahiya R, Cunha GR (2003) Hormonal, cellular, and molecular control of prostatic development. Dev Biol 253:165–174
Masuda S, Izpisua Belmonte JC (2014) A recipe for targeted therapy in prostate cancer. Nat Rev Urol 11:419. doi:10.1038/nrurol.2013.110-c1
McDonnell TJ et al (1992) Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52:6940–6944
Miele L (2006) Notch signaling. Clin Cancer Res 12:1074–1079. doi:10.1158/1078-0432.CCR-05-2570
Miki J, Rhim JS (2008) Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer Prostatic Dis 11:32–39. doi:10.1038/sj.pcan.4501018
Morgan TH (1917) The theory of the gene. Am Nat 51:513–544
Mulligan P et al (2011) A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Mol Cell 42:689–699. doi:10.1016/j.molcel.2011.04.020
Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev Biol 228:151–165. doi:10.1006/dbio.2000.9960
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 100:14920–14925. doi:10.1073/pnas.2436557100
Nagel AC, Krejci A, Tenin G, Bravo-Patino A, Bray S, Maier D, Preiss A (2005) Hairless-mediated repression of Notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol Cell Biol 25:10433–10441. doi:10.1128/MCB.25.23.10433-10441.2005
Nantermet PV et al (2004) Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J Biol Chem 279:1310–1322. doi:10.1074/jbc.M310206200
Nicolas M et al (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33:416–421. doi:10.1038/ng1099
Oda T et al (1997) Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 16:235–242. doi:10.1038/ng0797-235
Oktem G et al (2014) Expression profiling of stem cell signaling alters with spheroid formation in CD133/CD44 prostate cancer stem cells. Oncol Lett 7:2103–2109. doi:10.3892/ol.2014.1992
Orr B, Grace OC, Vanpoucke G, Ashley GR, Thomson AA (2009) A role for Notch signaling in stromal survival and differentiation during prostate development. Endocrinology 150:463–472. doi:10.1210/en.2008-0383
Orr B et al (2013) Reduction of pro-tumorigenic activity of human prostate cancer-associated fibroblasts using Dlk1 or SCUBE1. Dis Model Mech 6:530–536. doi:10.1242/dmm.010355
Ortega M, Bhatnagar H, Lin AP, Wang L, Aster JC, Sill H, Aguiar RC (2014) A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia. doi:10.1038/leu.2014.302
Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C (2012) Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 14:1131–1138. doi:10.1038/ncb2600
Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D (2013) Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med 19:1054–1060. doi:10.1038/nm.3259
Park JT et al (2006) Notch3 gene amplification in ovarian cancer. Cancer Res 66:6312–6318. doi:10.1158/0008-5472.CAN-05-3610
Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W (1999) Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol 209:28–39. doi:10.1006/dbio.1999.9229
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Rangarajan A et al (2001) Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20:3427–3436. doi:10.1093/emboj/20.13.3427
Raouf A et al (2008) Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3:109–118. doi:10.1016/j.stem.2008.05.018
Reiter RE et al (1998) Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA 95:1735–1740
Ridgway J et al (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–1087. doi:10.1038/nature05313
Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L (2008) Rational targeting of Notch signaling in cancer. Oncogene 27:5124–5131. doi:10.1038/onc.2008.226
Robbins J, Blondel BJ, Gallahan D, Callahan R (1992) Mouse mammary tumor gene int-3: a member of the Notch gene family transforms mammary epithelial cells. J Virol 66:2594–2599
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105:6392–6397. doi:10.1073/pnas.0802047105
Sainson RC, Harris AL (2007) Anti-Dll4 therapy: Can we block tumour growth by increasing angiogenesis? Trends Mol Med 13:389–395. doi:10.1016/j.molmed.2007.07.002
Salta E, Lau P, Sala Frigerio C, Coolen M, Bally-Cuif L, De Strooper B (2014) A self-organizing miR-132/Ctbp2 circuit regulates bimodal Notch signals and glial progenitor fate choice during spinal cord maturation. Dev Cell 30:423–436. doi:10.1016/j.devcel.2014.07.006
Salvati M et al (2005) Brain metastasis from prostate cancer. Report of 13 cases and critical analysis of the literature. J Exp Clin Cancer Res 24:203–207
Santagata S et al (2004) JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res 64:6854–6857. doi:10.1158/0008-5472.CAN-04-2500
Santamaria A et al (2003) PTOV-1, a novel protein overexpressed in prostate cancer, shuttles between the cytoplasm and the nucleus and promotes entry into the S phase of the cell division cycle. Am J Pathol 162:897–905. doi:10.1016/S0002-9440(10)63885-0
Scorey N, Fraser SP, Patel P, Pridgeon C, Dallman MJ, Djamgoz MB (2006) Notch signalling and voltage-gated Na + channel activity in human prostate cancer cells: independent modulation of in vitro motility. Prostate Cancer Prostatic Dis 9:399–406. doi:10.1038/sj.pcan.4500894
Sethi S, Macoska J, Chen W, Sarkar FH (2010) Molecular signature of epithelial–mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 3:90–99
Shen MM, Abate-Shen C (2010) Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 24:1967–2000. doi:10.1101/gad.1965810
Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ (2001) Dynamics of Notch expression during murine prostate development and tumorigenesis. Cancer Res 61:7291–7297
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. doi:10.3322/caac.21166
Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, Ball DW (2001) Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 61:3200–3205
Swanson GP, Thompson IM, Basler J (2006) Current status of lymph node-positive prostate cancer: incidence and predictors of outcome. Cancer 107:439–450. doi:10.1002/cncr.22034
Thurston G, Noguera-Troise I, Yancopoulos GD (2007) The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 7:327–331. doi:10.1038/nrc2130
Valdez JM et al (2012) Notch and TGFbeta form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell Stem Cell 11:676–688. doi:10.1016/j.stem.2012.07.003
Valve EM, Nevalainen MT, Nurmi MJ, Laato MK, Martikainen PM, Harkonen PL (2001) Increased expression of FGF-8 isoforms and FGF receptors in human premalignant prostatic intraepithelial neoplasia lesions and prostate cancer. Lab Invest 81:815–826
van Es JH et al (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963. doi:10.1038/nature03659
Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA (1992) Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 52:6182–6187
Wang Y, Hayward S, Cao M, Thayer K, Cunha G (2001) Cell differentiation lineage in the prostate. Differentiation 68:270–279
Wang XD, Shou J, Wong P, French DM, Gao WQ (2004) Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem 279:24733–24744. doi:10.1074/jbc.M401602200
Wang XD et al (2006) Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol 290:66–80. doi:10.1016/j.ydbio.2005.11.009
Wang Z, Li Y, Banerjee S, Sarkar FH (2008) Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res 28:3621–3630
Wang X et al (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500. doi:10.1038/nature08361
Wang Z et al (2010) Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem 109:726–736. doi:10.1002/jcb.22451
Wang Z et al (2011) Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J Cell Biochem 112:78–88. doi:10.1002/jcb.22770
Webber J et al (2014) Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol Cell Proteomics 13:1050–1064. doi:10.1074/mcp.M113.032136
Weinmaster G, Roberts VJ, Lemke G (1991) A homolog of Drosophila Notch expressed during mammalian development. Development 113:199–205
Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S (1985) Nucleotide sequence from the neurogenic locus Notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43:567–581
Whelan JT, Kellogg A, Shewchuk BM, Hewan-Lowe K, Bertrand FE (2009) Notch-1 signaling is lost in prostate adenocarcinoma and promotes PTEN gene expression. J Cell Biochem 107:992–1001. doi:10.1002/jcb.22199
Wu CT et al (2007) Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA 104:12679–12684. doi:10.1073/pnas.0704940104
Wu X et al (2011) Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism. Dev Biol 356:337–349. doi:10.1016/j.ydbio.2011.05.659
Yatim A et al (2012) NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol Cell 48:445–458. doi:10.1016/j.molcel.2012.08.022
Yong T, Sun A, Henry MD, Meyers S, Davis JN (2011) Down regulation of CSL activity inhibits cell proliferation in prostate and breast cancer cells. J Cell Biochem 112:2340–2351. doi:10.1002/jcb.23157
Yu Y, Zhang Y, Guan W, Huang T, Kang J, Sheng X, Qi J (2014) Androgen receptor promotes the oncogenic function of overexpressed Jagged1 in prostate cancer by enhancing cyclin B1 expression via Akt phosphorylation. Mol Cancer Res 12:830–842. doi:10.1158/1541-7786.MCR-13-0545
Zayzafoon M, Abdulkadir SA, McDonald JM (2004) Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem 279:3662–3670. doi:10.1074/jbc.M308158200
Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH (2006) Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer 119:2071–2077. doi:10.1002/ijc.22077
Zhu H, Zhou X, Redfield S, Lewin J, Miele L (2013) Elevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancers. Am J Transl Res 5:368–378
Acknowledgments
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation (Y2111329), Zhejiang Provincial Medicines Health Technology Project (2011KYB066), Zhejiang Provincial Traditional Medicine Technology Project (2011ZB099), Hangzhou Health Technology Project (20110833B05) and the Zhejiang Provincial Medical Association Clinical Research Funding Project (2012ZYC-A35).
Conflict of interest
The authors declare that they have no known conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, G., Ma, L., Meng, Q. et al. Notch signaling in the prostate: critical roles during development and in the hallmarks of prostate cancer biology. J Cancer Res Clin Oncol 142, 531–547 (2016). https://doi.org/10.1007/s00432-015-1946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1946-x