Abstract
Objective
Osteonecrosis of the jaw (ONJ) is an adverse effect that is associated with bisphosphonate (BP) use. Little data are available on risk factors influencing the time of treatment until an osteonecrosis occurs.
Methods
From 1 Dec 2004 until 21 Sep 2012, the German Register collected all patients with validated diagnoses of ONJ (N = 1,229) that were reported to the national pharmaco-vigilance system or to the Register directly. We analysed 963 patients with cancerous disease and an ONJ during i.v. BP treatment. Duration of BP treatment until first diagnosis of ONJ and Kaplan–Meier curves of ONJ-free survival were analysed stratified by gender, type of BP and type of cancer.
Results
Main indications for BP treatment were breast cancer (36 %), multiple myeloma (24 %), prostate cancer (16 %) and kidney cancer (4 %). Men suffered from their ONJ earlier than women. A total of 780 patients (81 %) had their ONJ during zoledronate treatment, 93 (10 %) under pamidronate and 90 (9 %) under ibandronate treatment. ONJ-free survival in single BP users was significantly longer in pamidronate-treated patients than in zoledronate or ibandronate users. Ibandronate users had the shortest median duration of treatment (17 months), similar to that of zoledronate users (21.5 months). Sequential prescription of two different BPs prolonged the period of overall BP treatment until an ONJ occurred. Time of BP treatment was shortest in patients with kidney cancer. Age or a concomitant osteoporosis did not influence the time to event of an ONJ.
Conclusion
Systemic risk factors such as gender play a significant role in certain subgroups only. Comparative analysis of different cancer patients helps the treating oncologist/dentist to identify patients with a more imminent risk to develop an ONJ (i.e. kidney cancer, ibandronate/zoledronate use).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates (BPs) are part of an adjuvant therapy for cancer patients with bone lesions. They are readily administered because they effectively reduce the number of skeletal-related events such as progression of bone metastasis and the incidence of pathological fractures, and they reduce bone pain, thus improve the quality of life in these patients (Clines and Guise 2004). Nevertheless, that quality of life is at risk when patients under BP therapy develop an osteonecrosis of the jaw (ONJ). This adverse event has been defined as exposed bone in the maxillofacial region that has persisted for more than 8 weeks in patients with a history of BP treatment and no history of radiation in the jaw region. Accompanying local clinical signs may be wound healing disturbances, inflammation or infection, fistulas, dysaesthesia or pain (Ruggiero et al. 2009). The cumulative incidence of ONJ among cancer patients treated with intravenous (i.v.) BPs has been estimated at 0.8–12 % (Ruggiero et al. 2009), with controlled studies tending to find lower incidences around 2–3 % (Shapiro 2013), yet keeping in mind that patients with ONJ that are treated outside of studies may benefit from fewer preventive dental treatments and are less likely to be reported to the authorities (Kruger et al. 2013; Ulmner et al. 2014).
There are three generations of BPs available to treat cancerous lesions to the bone: the non-nitrogen containing first-generation clodronate, the nitrogen containing second generation including pamidronate and ibandronate, and the third generation of nitrogen containing ring structures as seen in zoledronate (Bamias et al. 2005; Ebetino et al. 2011). The relative potency of the BP increases with nitrogen substitution and even more so with the integration of a ring structure as seen in zoledronate. With regard to ONJ, many researchers have shown that the majority of patients had been administered zoledronate prior to the occurrence of an ONJ (Hoffmann et al. 2008; Wessel et al. 2008). And our previous work has brought evidence that this is not simply due to a higher rate of prescription of zoledronate but rather a reflection of zoledronate’s higher potency (Hoffmann et al. 2008; Jung et al. 2010). Furthermore, it was shown that having once taken zoledronate represents a risk factor even if the patient is currently treated with another BP (Wessel et al. 2008). Ibandronate is the second most commonly prescribed BP in Germany, and pamidronate and clodronate are commonly prescribed agents in North America and elsewhere (Ebetino et al. 2011; Hoffmann et al. 2008). Merely, a handful of researchers have compared the various BPs potencies to cause an ONJ earlier or more frequently than others (Bamias et al. 2005; Boonyapakorn et al. 2008; Durie et al. 2005).
Several patient-related risk factors to develop an ONJ have been discussed, for example, local risk factors such as previous dento-alveolar surgery or poor dental status or systemic factors such as age, gender or ethnicity (Hoff et al. 2011; Jadu et al. 2007). In addition, the way of BP administration and dosing intervals appear to play a key role, with i.v. application posing as a greater risk factor than oral preparations (Wessel et al. 2008; Wilkinson et al. 2007). Many researchers have published case series on the most commonly affected subgroups of patients with breast cancer, multiple myeloma and prostate cancer (Jadu et al. 2007; Rugani et al. 2014; Walter et al. 2008). Yet, the literature provides very few conclusive comparisons of the different types of cancer, so it remains unclear whether the type of cancer itself, including accompanying chemotherapeutic and other treatment regimes, could be a risk factor as well (Bamias et al. 2005). Dodson concludes that several of the above-postulated risk factors towards an ONJ were shown to have a variable if not contradictory impact, while others such as the genetic background, concomitant cancer therapies and the underlying malignant disease require further investigation (Dodson 2009). Hence, 10 years into ONJ research, we still face some uncertainties about the cause-and-effect relationship of BPs and the development of an ONJ.
The German Central Register (Register) draws from a wealth of information from more than 1,200 affected patients with clinically proven ONJ (Felsenberg et al. 2012). The Register collects all cases of ONJ nationwide and is therefore able to compare data of various subgroups and can thus provide information on: (1) systemic risk factors such as age and gender, (2) the effects of the different BPs separately from the effect of the underlying cancer on the risk to develop an ONJ, (3) the effect of sequential versus single BP use and (4) the dynamic of the disease when looking at ONJ-free survival times in cancer patients.
Methods
The German Central Register
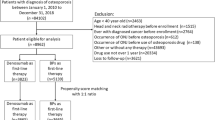
The German Central Register for ONJ under BP therapy has been implemented in the fall of 2004 at the Centre of Muscle and Bone Research (ZMK) in Berlin. The Register has been collecting cases of ONJ from across Germany since 1 December 2004. The data protection agent and the local institutional review board (Ethics committee of the Charité, protocol number EA4/102/05) approved the work of the Register. In total, 53.7 % of the cases were retrieved from the Federal Institute of Medication and Medical Products (BfArM), which corresponds to the German FDA. The remaining cases were obtained from other hospitals (17.9 %), the Charité’s own clinic of oral and maxillofacial surgery (15.7 %) and from doctor’s offices in ambulatory care (12.7 %). These latter three types of cases were directly reported by physicians to the Register via downloading a questionnaire and a patient consent form from www.charite.de/ZMK. The Register, in return, forwarded these cases to the BfArM. The Register included only those cases with a validated clinical or histological diagnosis of ONJ and excluded all cases that had either received radiation therapy to the head and neck region or had been treated for malignant lesions in that area (Ruggiero et al. 2009). Until 21 September 2012, a total of 1,229 cases of ONJ were collected in the Register.
Inclusion/exclusion criteria
For this study, we only considered cases of patients who were treated with BPs due to an underlying malignant disease. Patients that had been treated for an unknown indication (n = 29) or due to osteoporosis only (n = 96) were excluded from this analysis. Of the remaining patients, we excluded those who were treated with more than two different BPs (n = 8), those with an unknown number of different BP treatments (n = 121) and all uncertain type of BP treatments (n = 4). Due to small numbers, we also excluded the few patients that were treated with clodronate alone (n = 8). When patients were diagnosed with different types of malignancies, the current/latest was chosen as underlying disease (N = 4) or a hierarchical disease code was employed (n = 20) as follows: (1) breast cancer, (2) multiple myeloma, (3) prostate cancer, (4) kidney cancer, (5) haematologic malignancy other than multiple myeloma (6) other types of cancer and (7) unknown type of cancer.
Statistical analysis
Due to the mainly non-normal distributed data, nonparametric methods were used for group comparisons. In order to fully retrace the statistical analyses, the applied statistical tests were superscripted behind each p value with superscript numbers standing for (1) binomial test for null hypothesis proportion p 0 = 0.5, (2) chi-squared goodness-of fit-test (theoretical frequencies 1/3, 1/3, 1/3), (3) Fisher’s exact test, (4) Wilcoxon rank sum test, (5) Kruskal–Wallis test and (6) Wilcoxon rank sum test with p value adjustment using step-down Sidak procedure. The Kaplan–Meier method was used to compare ‘duration of ONJ-free BP treatment’ in the three most common underlying diseases and BPs taken in both genders. (7) The logrank test was applied to compare the curves, and if applicable, (8) adjustment for multiple comparisons for the logrank test was performed. The Spearman method was used for correlation analyses. A two-sided p value of <0.05 was considered statistically significant. All statistical analyses were carried out using SAS Software Package for Windows V9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
A total of 963 patients with validated diagnoses of ONJ were analysed for this study. The baseline characteristics of all patients, stratified by gender, are shown in Table 1. Data were missing in some patients with regard to gender (n = 8), date of birth (n = 127), onset date of ONJ (n = 110), duration of treatment of the current/last BP (n = 376), duration of treatment of the previously taken BP in sequential users (n = 48), type of underlying cancer (n = 145) and existence of a concomitant osteoporosis (n = 649).
Among all patients who suffered from an ONJ in our Register, there were significantly (p 1 < 0.0001) more women than men. The subgroup of ‘other cancer’ (n = 36) consisted mainly of patients with lung cancer (n = 11), cancer of the colon/rectum (n = 7), cancer of the uterus or cervix (n = 4) and cancer of the urinary bladder (n = 3). Among those underlying diseases that affect both genders, men with kidney cancer were significantly (p 1 = 0.0308) over-represented in a 2:1 ratio when compared to women, but no such difference was seen for patients with multiple myeloma (p 1 = 0.2571). Osteoporosis occurred as a concomitant disease in 15.0 % of all patients, with the highest proportion found among patients with haematologic cancer (75 %) followed by patients with multiple myeloma (15.1 %).
With regard to BP intake, the majority of patients had a medical history of only one type of BP, and the rest had been sequentially taking two different BPs (p 2 < 0.0001). The proportion of women that were treated with two sequential BPs was significantly (p 3 = 0.0002) higher than that of men. The most commonly prescribed BP prior to the occurrence of an ONJ was zoledronate in both genders. Yet, the proportion of patients having last taken ibandronate or pamidronate was significantly higher in women than in men (each p 1 < 0.0001). The median duration of overall BP intake until the occurrence of an ONJ was significantly (p 4 = 0.0479) shorter in men than in women. Mean age at the onset of the ONJ did not correlate with the length of overall BP intake preceding the ONJ (Spearman R = 0.1520).
Bisphosphonate-specific analyses
The four most common diseases that indicated BP treatment were the same for all types of administered BPs. In descending order, these were breast cancer, multiple myeloma, prostate cancer and kidney cancer. Among all types of cancer, zoledronate was the BP that was most often administered prior to the occurrence of an ONJ, ranging from 72.4 % of all breast cancer patients to 94.9 % of all prostate cancer patients. Fewer patients had last received pamidronate, with the highest proportion found in the subgroup of patients with multiple myeloma (17.1 %). Even less people suffered their ONJ during a treatment with ibandronate, with the highest proportion found among patients with breast cancer (16.0 %). Both zoledronate and pamidronate were administered intravenously, and the majority of patients received 4 mg (zoledronate) or 90 mg (pamidronate) once per month. Ibandronate was administered both due to an underlying malignant disease (93.2 %) and due to a concomitant osteoporosis (6.8 %). Among those who suffered from their ONJ during ibandronate treatment, all were treated due to a malignant disease and the majority (90.1 %) had received an i.v. infusion, mostly at the recommended dose of 6 mg every 3–4 weeks to treat bone metastases19. As the proportion of patients with a history of sequential BP use was significantly higher (Zol vs. Ibn p 3 < 0.0001, Pam vs. Ibn p 3 < 0.0001) in the ibandronate group, a closer look was given to differences in duration of treatment in users of a single BP separately from users of two sequential BPs.
Single bisphosphonate use
Table 2 documents patients treated with only one BP. The duration of treatment was significantly different (p 5 < 0.0001) for the various BPs. Zoledronate and ibandronate users had significantly shorter treatment times before diagnosis of an ONJ than pamidronate users (Zol vs. Pam p 6 < 0.0001, Ibn vs. Pam p 6 < 0.0001). There was no significant difference between zoledronate and ibandronate users (Zol vs. Ibn p 6 = 0.2020) among single BP users.
Sequential bisphosphonate use
We analysed the effect of a previous BP medication history on the duration of treatment of the current BP. Table 2 shows that in sequential users that had last taken zoledronate or ibandronate, the overall (added) duration of BP intake was significantly longer than in single BP users of the same last medication (single vs. consecutive: \(p_{\text{Zol}}^{4}\) < 0.0001 and \(p_{\text{Ibn}}^{4}\) < 0.0001). When patients were treated with two sequential BPs, there was no statistical difference in the total duration of intake between the current users of zoledronate and ibandronate (p 4 = 0.1721). Patients with ONJ under zoledronate had been previously treated with another BP for 26.5 months and ibandronate users for 24.5 months. Among patients who had suffered from an ONJ during an ibandronate treatment, the previous intake of zoledronate resulted in a significantly shorter duration (median of 10.0 vs. 31.0 months) of sequential ibandronate intake than a previous intake of pamidronate did (Ibn_Zol vs. Ibn_Pam p 4 = 0.0211). No such effect was seen for current zoledronate users with a medication history of either pamidronate or ibandronate intake (p 4 = 0.3295).
Disease-specific analyses
When looking at all patients, the proportion that suffered their ONJ during the various BP treatments was significantly different in all subcategories of underlying diseases (each p 2 < 0.0001), with zoledronate being the most commonly administered BP prior to the occurrence of an ONJ for each disease. In detail, the two most commonly prescribed last BPs were zoledronate (72.4 %) and ibandronate (16.0 %) for breast cancer, zoledronate (73.7 %) and pamidronate (17.1 %) for multiple myeloma, zoledronate (94.9 %) and pamidronate or ibandronate (each 2.6 %) for prostate cancer, and zoledronate (90.5 %) and pamidronate (7.1 %) for kidney cancer.
Table 3 shows cancer-specific analyses of patients with a medication history of only one BP. In patients with breast cancer, the duration of BP treatment varied significantly among the various types of BPs (p 5 = 0.0005) with the shortest duration in ibandronate users and the longest in pamidronate users. Detailed pairwise analysis underlined this (Zol vs. Pam p 6 = 0.0015, Ibn vs. Pam p 6 = 0.0048), but the difference between zoledronate and ibandronate users was less apparent (Zol vs. Ibn p 6 = 0.0917). When looking at the single BP users of Zoledronate, we found a disease-specific difference (p 5 = 0.0050) in duration of BP treatment. In that subcategory, patients with kidney cancer (KC) suffered from their ONJ the earliest, whereas patients with breast cancer (BC) and those with multiple myeloma (MM) got ill significantly later (pairwise comparison BC vs. KC p 6 = 0.0066, MM vs. KC p 6 = 0.0066). Inside the subgroup of patients with kidney cancer under single zoledronate treatment, men suffered from their ONJ significantly earlier than women (median of 9.0 vs. 14.0 months, p 4 = 0.0173). No such gender-specific difference was seen in patients with multiple myeloma (males vs. females: median of 27.0 vs. 25.0 months, p 4 = 0.2271). The presence of a concomitant disease of osteoporosis did not influence the duration of BP treatment until an ONJ was diagnosed in users of a single BP (p 4 = 0.9756).
ONJ-free survival of cancer patients
When comparing the duration of BP treatment until the diagnosis of an ONJ by employing Kaplan–Meier curves, we found that after 24 months of single BP therapy, 52.6 % of females and 52.8 % of males had already suffered from an ONJ (p 7 = 0.9978). Kaplan–Meier curves displaying a graphical analysis of ONJ-free survival during BP treatment are shown for both genders in Figs. 1 and 2 (stratified by BP) and in Figs. 3 and 4 (stratified by underlying disease).
When stratifying treatment curves for the various BP treatments in single BP users, statistically significant differences were found in females (p 7 < 0.0001) and in males (p 7 = 0.0056). Pairwise comparison in females revealed significant differences in ONJ-free duration of BP treatments in zoledronate versus pamidronate users (p 8 = 0.0001) and pamidronate vs. ibandronate users (p 8 < 0.0001) but only as a trend for zoledronate versus ibandronate users (p 8 = 0.0651). Figure 1 shows that among females, ONJ had already occurred at 24 months of treatment in 75.0 % of ibandronate users, in 55.4 % of zoledronate users and in 16.1 % of pamidronate users. Figure 2 shows that among males, ONJ had already occurred at 24 months of treatment in 54.8 % of zoledronate users and in 28.6 % of pamidronate users.
After stratifying treatment curves for the three most common indications for BP treatment (Figs. 3, 4), statistically significant differences were observed for males (p 7 = 0.0005) but not for females (p 7 = 0.8589). Among females, ONJ had already occurred at 24 months of zoledronate treatment in 72.7 % of patients with kidney cancer, in 58.2 % of patients with breast cancer and in 46.2 % of patients with multiple myeloma. Among males, ONJ had already occurred at 24 months of zoledronate treatment in 82.4 % of patients with kidney cancer, in 58.2 % of patients with prostate cancer and in 36.8 % of patients with multiple myeloma. The difference between males with multiple myeloma versus those with kidney cancer was statistically significant (p 8 = 0.0018). The gender-specific difference of ONJ-free treatment among patients with kidney cancer was statistically significant (p 7 = 0.0455).
Discussion
In order to gain new insights into the etio-pathophysiology behind an ONJ, it is necessary to look at large populations of patients. The German Central Register consists of more than 1,200 patients with validated diagnoses of ONJ. The Register is unique in that it is independent of the pharmaceutical industry or studies sponsored thereby, and the Register therefore represents all types of affected patients from across a country of 80 million inhabitants (Federal Statistical Office). The Register can be used to compare the different BPs’ potencies to cause an ONJ and to draw conclusions on individual risk factors such as the patients underlying disease, co-morbidities, co-medication, gender or age. Many research groups have published descriptive data of case series or small studies, or tried to establish risk factors that raise the cumulative hazard to develop an ONJ, often utilising medical claims data (Cartsos et al. 2008; Wilkinson et al. 2007). Yet, to our knowledge, only very few small case series have offered comparative analyses of the time-to-event dynamic towards the development of an osteonecrosis (Bamias et al. 2005; Boonyapakorn et al. 2008; Durie et al. 2005). Now that treating physicians have been sensitised to the entity of an ONJ and the most important associated risk factors, detailed information is needed to establish treatment guidelines for individual subgroups of cancer patients.
Concerning demographic and systemic risk factors, our data have shown that women are more often affected by an ONJ than men are. Our previous work has shown that women are indeed over-represented in our Register of ONJ patients when compared to prescription data of the German population (Jung et al. 2010). This disproportion likely reflects better survival rates of women and might therefore be simply due to more women receiving BP treatments and living longer while under BP treatment than being a true personal risk factor for the female gender (Hoffmann et al. 2008). In patients with kidney cancer, we found that men are over-represented. This only mirrors the higher prevalence (approximate 2:1 ratio) of this malignancy in men (Scher and Motzer 2005). Nevertheless, gender-specific differences must exist as our data showed that overall, men developed their ONJ slightly earlier than women. This effect was seen strongest in the subgroup of kidney cancer under single zoledronate treatment. Older age and possible associated co-morbidities have been variably discussed as risk factor towards an ONJ (Dodson 2009), but according to our data, older age at the start of BP treatment does not promote an earlier occurrence of an ONJ. In light of our previous research, we have already established that it is rather the cancer-related survival after the start of BP treatment that strongly influences whether there will be time to develop an ONJ or not (Hoffmann et al. 2008; Jung et al. 2010).
As expected, zoledronate was the most often administered BP prior to the occurrence of an ONJ among our patients (Dodson 2009; Hoffmann et al. 2008). We have shown before that, at least among women, zoledronate users are affected by an ONJ more often than prescription data of all available BPs would suggest (Jung et al. 2010). Surprisingly though, ibandronate appears to be as potent as zoledronate when looking at the duration of BP treatment until an ONJ is diagnosed. Ibandronate users actually had the shortest duration of treatment, similar to those of Zoledronate users. Ibandronate has been less studied in previous research on ONJ as it less commonly prescribed in Europe and not approved for adjuvant cancer therapy in the USA (FDA 2014). The effects of zoledronate have been widely studied, and our data support findings by Wessel and others that the use of zoledronate is a risk factor towards an ONJ (Bamias et al. 2005; Durie et al. 2005; Wessel et al. 2008). According to our data, even the mere history of a previous intake of zoledronate can significantly reduce the time of treatment of the sequentially prescribed BP until an ONJ occurs, especially when oncologists switch to ibandronate treatment.
Interestingly, the switching of the type of BP treatment during the course of adjuvant cancer therapy seems to be a protective factor against the development of an ONJ (Bamias et al. 2005; Corso et al. 2007; Hoff et al. 2008). Our data show that sequential versus single BP use nearly doubles the overall duration of BP treatment until the ONJ occurs. On a cellular level, switching BPs in the course of treatment might make sense, as the BPs act at different points in the mevalonate pathway and have various potential to cause other biochemical effects, not only on osteoclast/osteoblast interaction but also on inhibiting angiogenesis or tissue regeneration (Sharma et al. 2013). In light of our results on the similar potential of ibandronate and zoledronate to promote an ONJ within the same time frame, it might make sense to start off with or switch to a BP of an earlier generation such as pamidronate if other aspects of the patient’s quality of life allow for it. Alternatively, dosing intervals could be altered, but only a few researchers have started to publish their results on that (Corso et al. 2007; Hadji et al. 2011; Shapiro 2013).
Obviously, the underlying malignancy plays a role as potential risk factor towards ONJ. Our baseline data in large agree with others that breast cancer (~36 %), multiple myeloma (~24 %) and prostate cancer (~16 %) make up most of the patients affected by ONJ (Hoff et al. 2011). The most interesting result from our data is that kidney cancer is the fourth largest (4 %) subgroup in Germany and appears to be a major risk factor. So far, only a few case reports of kidney cancer with ONJ have made it to publication and some question the use of BPs in this subgroup due to the high incidence of adverse events including ONJ (McKay et al. 2014). Nevertheless, treating urologists and oral surgeons should be aware that patients with kidney cancer, especially male patients, will suffer from an ONJ on average much earlier than all other cancer patients under BP treatment.
Bisphosphonates and ONJ are often discussed in patients with osteoporosis as well since these patients receive BPs on a widespread basis, even though mostly in oral applications or once yearly i.v. doses (Rote Liste). ONJ is very rare in these patients as has been previously reported from Sweden and from the data of our German Register (Felsenberg et al. 2012; Ulmner et al. 2014). In cancer patients of our Register, the comorbidity of an osteoporosis had no influence on the time until first diagnosis of an ONJ. Knowing the extremely low incidence of ONJ in patients with osteoporosis, this finding is not surprising and it may help calm cancer patients to know that their concomitant osteoporosis does not promote the earlier development of a potential ONJ.
Despite drawing conclusions from a wealth of >1,200 patients with ONJ, a weakness of our research is the high number of incomplete data sets. Missing data is a common problem in case series that were retrieved from adverse event reporting systems/authorities as hundreds of physicians and several bureaucratic agencies are involved in the reporting process (EU Law). And even though the Register collects its cases nationwide, it is still unknown how many of the total number of ONJs have truly been reported. We assume that, due to active recruitment in Germany, the fraction of reported cases would be higher than the suggested 25 % from a Scandinavian pharmaco-vigilance study on ONJ (Ulmner et al. 2014).
The strength of this research is that, due to the unique nature of the Register, we were able to identify risk factors of ONJ from a broad basis of cancer patients and are therefore able to provide treating oncologists with some hands-on information on personal risk factors and time-to-event data. Our cases were not retrieved from medical claims data but represent clinically proven cases of ONJ. To sum up, our current research indicates that risk factors towards an earlier development of an ONJ can be zoledronate and ibandronate use, kidney cancer and being a male patient. Protective factors against an ONJ can be sequential prescription of different BPs and female gender. Concomitant diagnoses of osteoporosis or older age appear to be neither harmful nor protective. Unique is the information on ONJ-free survival in the various subgroups. Knowing what time frame to deal with will facilitate the implementation of treatment guidelines (Hadji et al. 2011). Last but not least, the main focus for all physicians should lie in prevention, hence, the education of the oncological patient and full access to dental procedures prior to and throughout any BP treatment (Ruggiero et al. 2009).
References
Bamias A et al (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23:8580–8587. doi:10.1200/JCO.2005.02.8670
Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G (2008) Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol 44:857–869
Cartsos VM, Zhu S, Zavras AI (2008) Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Assoc 139:23–30
Clines GA, Guise TA (2004) Mechanisms and treatment for bone metastases. Clin Adv Hematol Oncol 2:295–302
Corso A, Varettoni M, Zappasodi P, Klersy C, Mangiacavalli S, Pica G, Lazzarino M (2007) A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia 21:1545–1548
EU Law Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010. http://ec.europa.eu/health/files/eudralex/vol-1/dir_2010_84/dir_2010_84_en.pdf. http://ec.europa.eu/health/files/eudralex/vol-1/dir_2010_84/dir_2010_84_en.pdf. Accessed 27 June 2014
Dodson TB (2009) Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 67:44–52. doi:10.1016/j.joms.2008.12.004
Durie BG, Katz M, Crowley J (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353:99–102 discussion 199–102
Ebetino FH et al (2011) The relationship between the chemistry and biological activity of the bisphosphonates. Bone 49:20–33. doi:10.1016/j.bone.2011.03.774
FDA (2014) US Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 09 September 2014
Federal Statistical Office. German Statistical Yearbook 2014. https://www.destatis.de. Accessed 03 July 2014
Felsenberg D, Lopez S, Gabbert T, Hoffmeister B (2012) Osteonecrosis of the jaw in patients with osteoporosis. Osteologie 21:207–212
Hadji P, Gnant M, Aapro M, Lipton A, Coleman R (2011) Dosing of zoledronic acid throughout the treatment continuum in breast cancer. Crit Rev Oncol Hematol 79:175–188. doi:10.1016/j.critrevonc.2010.07.017
Hoff AO et al (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23:826–836. doi:10.1359/jbmr.080205
Hoff AO, Toth B, Hu M, Hortobagyi GN, Gagel RF (2011) Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann NY Acad Sci 1218:47–54. doi:10.1111/j.1749-6632.2010.05771.x
Hoffmann F, Jung TI, Felsenberg D, Glaeske G (2008) Pattern of intravenous bisphosphonate use in outpatient care in Germany. Pharmacoepidemiol Drug Saf 17:896–903. doi:10.1002/pds.1634
Jadu F, Lee L, Pharoah M, Reece D, Wang L (2007) A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol 18:2015–2019. doi:10.1093/annonc/mdm370
Jung TI, Hoffmann F, Glaeske G, Felsenberg D (2010) Disease-specific risk for an osteonecrosis of the jaw under bisphosphonate therapy. J Cancer Res Clin Oncol 136:363–370. doi:10.1007/s00432-009-0662-9
Kruger TB, Sharikabad MN, Herlofson BB (2013) Bisphosphonate-related osteonecrosis of the jaw in four Nordic countries and an indication of under-reporting. Acta Odontol Scand 71:1386–1390. doi:10.3109/00016357.2013.764007
McKay RR, Lin X, Perkins JJ, Heng DY, Simantov R, Choueiri TK (2014) Prognostic Significance of Bone Metastases and Bisphosphonate Therapy in Patients with Renal Cell Carcinoma. Eur Urol. doi:10.1016/j.eururo.2014.02.040
Rote Liste Rote Liste Service GmbH. www.rote-liste.de. Accessed 27 June 2014
Rugani P, Luschin G, Jakse N, Kirnbauer B, Lang U, Acham S (2014) Prevalence of bisphosphonate-associated osteonecrosis of the jaw after intravenous zoledronate infusions in patients with early breast cancer. Clin Oral Invest 18:401–407. doi:10.1007/s00784-013-1012-5
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12. doi:10.1016/j.joms.2009.01.009
Scher HI, Motzer RJ (2005) Bladder and renal cell carcinomas. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL (eds) Harrison’s principles of internal medicine, vol I, 16th edn. McGraw-Hill Companies, New York, pp 541–543
Shapiro CL (2013) Bisphosphonate-related osteonecrosis of jaw in the adjuvant breast cancer setting: risks and perspective. J Clin Oncol 31:2648–2650. doi:10.1200/JCO.2013.48.6837
Sharma D et al. (2013) Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect Vascular cell 5:1 doi:10.1186/2045-824X-5-1
Ulmner M, Jarnbring F, Torring O (2014) Osteonecrosis of the jaw in Sweden associated with the oral use of bisphosphonate. J Oral Maxillofac Surg 72:76–82. doi:10.1016/j.joms.2013.06.221
Walter C et al (2008) Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol 54:1066–1072. doi:10.1016/j.eururo.2008.06.070
Wessel JH, Dodson TB, Zavras AI (2008) Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg 66:625–631. doi:10.1016/j.joms.2007.11.032
Wilkinson GS, Kuo YF, Freeman JL, Goodwin JS (2007) Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis. J Natl Cancer Inst 99:1016–1024
Acknowledgments
We thank Stefanie Lopez-Schueler for help with data entry and file organisation and Juliane Luettke (Dipl. Biomathematician) for help with statistical analysis and interpretation. This work was partially supported by the non-profit ‘Elsbeth Bonhoff Foundation’ and by the ‘Centre of Muscle and Bone Research (ZMK)’.
Conflict of interest
The Centre of Muscle and Bone Research (ZMK) received funding to finance clinical studies from several pharmaceutical companies in the past (Bayer, Amgen, Anwerina, Novartis, Procter and Gamble, Roche, MSD, Sanofi-Aventis, TEVA, Chugai, Lilly, Servier).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabbert, T.I., Hoffmeister, B. & Felsenberg, D. Risk factors influencing the duration of treatment with bisphosphonates until occurrence of an osteonecrosis of the jaw in 963 cancer patients. J Cancer Res Clin Oncol 141, 749–758 (2015). https://doi.org/10.1007/s00432-014-1853-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1853-6