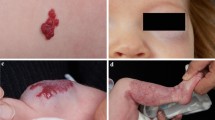
Abstract
The purpose of the study is to describe the experience of a multidisciplinary team in a tertiary hospital regarding the management of Infantile Hemangiomas (IH). The method employed is a retrospective analysis of patients with IH followed in a tertiary pediatric hospital between January 2010 and May 2022. A total of 393 IH were diagnosed (56.7% female), with a median age of 5 months (interquartile range (IQR), 3–10). Imaging investigation was necessary for diagnosis and for exclusion of other IH in 9.2% and 14.3%, respectively. Focal (74.0%) and superficial (59.7%) lesions were more frequent as was facial location (35.9%). Pre-treatment ulceration or hemorrhage occurred in 6.6%. At follow-up, 87.4% regressed partially and 12.6% completely; 2.7% relapsed. Propranolol was started in 30.0% of cases for a median period of 9 months (IQR, 6–12), mainly due to esthetic concerns (41.9%). Side effects occurred in 8.3% (sleep disturbance in 5.1%). Only 1.7% were refractory and 5.9% had a rebound effect. Eleven patients were treated with topical timolol and 41 underwent surgery. Patients that were treated with propranolol had more risk factors (p = 0.016) and presented deeper lesions (p < 0.001) with a larger diameter (p < 0.001); total IH regression was less frequent (p < 0.001). Since 2020, twice-daily dosage was more frequently prescribed than three times daily (p = 0.007) and inpatient initiation of propranolol decreased (p = 0.750), without significant difference in the incidence of adverse reactions, duration of treatment, and lesion evolution.
Conclusions: Our protocol proved to be safe and feasible in an outpatient setting and twice daily administration of propranolol was effective. The majority of IH showed at least partial regression. Early detection of high-risk IH is paramount and a multidisciplinary assessment by a specialized team is essential for adequate management.
What is Known: • IH are the most common vascular tumors in childhood. Although the majority evolves favorably, treatment may be warranted in selected cases. • Early detection of high-risk IH is paramount, and a multidisciplinary assessment by a specialized team is essential for adequate management. | |
What is New: • One-third of our sample was treated with propranolol. These patients had more risk factors and presented deeper lesions with a larger diameter, and tumor total regression was less frequent. • Our results reinforce safety and feasibility of propranolol initiation in an outpatient setting, including twice daily dosage. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infantile hemangiomas (IH) are the most common benign vascular tumor in children, with an estimated prevalence of 4–5%, being more frequent among Caucasian infants and in females [1,2,3,4,5]. Prematurity, low birth weight, multiple gestation, older maternal age, pre-eclampsia/placenta previa, and family history are known risk factors [3, 6]. Most IH are solitary and superficial and can occur anywhere on the skin, mucous membranes, and viscera despite being more common in the head and cervical region [4], [7,8,9]. Pathophysiology is yet to be completely understood but IH appear to be the result of a dysregulation of both vasculogenesis, and angiogenesis and multiple studies suggest that hypoxia may have a key role [10,11,12,13].
The natural history is distinctive from other congenital vascular malformations. IH generally appear within the first days to weeks of life and then grow continuously during the first year. However, precursor lesions may be present at birth or manifest during the early neonatal period as a pale area or a telangiectatic red macula [10, 14, 15]. The proliferative phase is followed by a spontaneous involution phase that typically begins after 1 year and lasts a variable number of years [10, 16]. In the absence of treatment, 50–70% of children will have residual lesions, such as scarring, atrophy, redundant skin, discoloration, and telangiectasias [10, 16,17,18].
The author’s aim was to characterize a sample of patients with IH followed in a tertiary hospital and to describe our experience regarding their management. We aimed to assess the prevalence of children that underwent treatment with oral propranolol, as well as its’ efficacy and safety profile, and the proportion of patients submitted to surgery. Finally, we tried to evaluate the application of our hospital protocol, which was revised and updated in 2020 and simplified initiation of treatment and posology.
Management approach should be individualized, based upon the IH characteristics and phase of evolution, the presence or possibility of complications, and the age of the patient [3], [8]. The diagnosis is mainly clinical [10]. A watchful waiting approach is recommended in the majority of cases that evolve favorably, with periodic follow-up complemented by serial photography. However, an infant with actual or potential risk for complications should be referred to a specialized tertiary center for evaluation by a multidisciplinary team for specific diagnostic work-up and possible treatment initiation [8]. Oral propranolol has become the treatment of choice for problematic IH, as it is both effective and safe [19,20,21]. In selected cases, other therapeutic options may be considered.
Materials and methods
Study design and sample
We conducted an observational retrospective, descriptive, and analytical study. All pediatric patients with the diagnosis of IH followed in the outpatient department of our tertiary hospital between January 2010 and May 2022 were included. All patients submitted to IH’s surgical removal in whom the anatomopathological examination revealed an alternative diagnosis were excluded.
Data collection and variable definition
Data was collected by assessing the patients’ electronic clinical files. Demographic variables including sex and age at diagnosis and at treatment were collected. Delay to first evaluation was defined as the period of time between the date when the lesion was first noticed or the date of referral and the date of first appointment. Delay to diagnosis was defined as time between the first hospital visit (including pediatric, pediatric cardiology, and pediatric surgery appointments) and diagnosis.
Data on IH characterization and natural history was also collected (location and size of the lesion, risk factors, associated syndromes or comorbidities, and complications). IH were classified according to tissue penetration (superficial, deep, and combined) and distribution (focal, two to five lesions, segmental, multiple, and undetermined). The work-up for confirmation of diagnosis and/or exclusion of other IH was reviewed, as well as the need for evaluation by specialists. Treatment approach is classified as pharmacological (propranolol and other drugs), laser, or surgery. Partial or total regression considered color, diameter, and, when applicable, depth improvement. Whenever possible, the lesions were photographed (with the caregiver’s consent). Refractory IH was considered when there was no clinical improvement (color or size) following treatment. Relapse was defined as a lesion’s regrowth after an initial partial or total regression [22].
Regarding propranolol treatment, the following data was collected: place of initiation (inpatient versus outpatient), dosage, duration, and adverse reactions. Rebound effect was defined as a darkening of the lesion and/or a size increase after propranolol suspension.
Hospital protocol for oral propranolol administration
Treatment with a manipulated solution of propranolol chloride (1 mg/mL or 5 mg/mL) was considered in infants with high-risk criteria (Fig. 1). Since the revision of the protocol in January 2020, a twice-daily dosage was adopted (rather than thrice daily). Verbal and written information about IH natural history, potential adverse reactions, and their management was carefully explained to caregivers.
Ethics
This research complies with all the relevant national regulations and institutional policies and is in accordance to the tenets of the Helsinki Declaration. The study was approved by the Ethics Committee of Centro Hospitalar Universitário do Porto and Institute of Biomedical Sciences Abel Salazar.
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics 27.0. Continuous variables are expressed as median and interquartile range (IQR). Differences between groups in continuous variables were evaluated with independent sample t test. Differences in the distribution of categorical variables were evaluated with chi-square tests and ANOVA. All p values are two-sided and were considered statistically significant if < 0.05.
Results
The sample baseline characterization is described in Table 1. A total of 393 patients were diagnosed with IH. Most were referred by general family clinicians (n = 150; 38.7%) or other hospitals (n = 124; 32%). Delay to first appointment and to diagnosis was 1 (IQR, 1–4) and 0 months (IQR 0–0), respectively. Fifty-nine (15.0%) patients had risk factors: prematurity (n = 54; 13.7%) and low birth weight (n = 42; 10.7%). Significant comorbidities such as cardiopathy (n = 11; 2.8%) and other malformations (n = 7; 1.8%) were present in 5.9%. Tumor locations are listed in Table 1; there were no IH exclusive of the airway or liver in our sample. IH were more frequently superficial (n = 227; 59.7%) and focal (n = 289; 74.1%). Imaging investigation with lesion ultrasound with Doppler was necessary for diagnosis in 36 (9.2%) patients; abdominal ultrasound with Doppler or resonance angiography was performed in 63 (16.0%) patients for exclusion of other IH. Thirty (7.7%) patients exhibited 32 complications prior to treatment initiation, the more common being ulceration (n = 25; 6.3%). One hundred and eighteen patients were treated with oral propranolol, 11 (2.8%) were treated with topical timolol, 41 (10.4%) underwent surgery, and 3 (0.8%) were treated with laser therapy. Cases without indication for treatment were only reevaluated once, and those treated with propranolol had a median follow-up of 20 months (IQR, 12–42). During follow-up, 160 (87.4%) tumors regressed partially and 23 (12.6%) completely and 2 (2.7%) relapsed, one after propranolol and the other after surgery.
Table 2 shows the characteristics of the patients selected for oral propranolol treatment. The main indications for treatment were actual or risk for esthetic (n = 49; 41.9%) or functional (n = 46; 40.3%) compromise and ulceration (n = 18; 15.8%); one patient had PHACE syndrome (posterior fossa anomalies, hemangioma, arterial anomalies, cardiac anomalies, and eye anomalies). Treatment initiation before the age of 6 months was not significantly associated with better outcomes regarding color (p = 0.608), diameter (p = 1.000), deepness (p = 0.180), lesion regression (p = 0.357), and recurrence (p = 0.729). Significant adverse reactions occurred in 8.3% patients, more commonly sleep disturbance (n = 5; 4.6%) and bradycardia (n = 3; 1.8%). Only 1 (0.8%) was refractory to propranolol for which he underwent surgery. A rebound effect was observed in 6 (5.1%) patients despite gradual suspension.
Tables 3 shows a comparative analysis between patients that underwent treatment with only oral propranolol (group 1) and those in whom no medical treatment was performed (group 0). Patients from group 1 had more risk factors (p = 0.016) and presented deeper lesions (p < 0.001) with a larger diameter (p < 0.001). Total IH’s regression was less frequent in group 1 (p < 0.001).
Since 2020, twice-daily dosage was more frequently prescribed (p = 0.007) and inpatient initiation of propranolol decreased (p = 0.750), without significant difference in the incidence of adverse reactions, treatment duration, and lesion evolution. The results of the comparative analysis between twice- and thrice-daily dosage are shown in Table 4.
Discussion
Consistent with what is reported in the literature, IH were more prevalent in females, mostly located in the face region and the majority were superficial solitary lesions [3, 8]. In our sample, the incidence of complications was lower than reported, with ulceration being the most prevalent. Previous studies stated that up to 12% of IH referred to pediatric centers were complex and prone to complications and that ulceration occurred in 10–25% [23]. Size, location, and type are determinants for ulceration, with large, superficial, and segmental tumors being substantially more likely to ulcerate [10].
Median delay to first appointment was 1 month, a relatively short period of time, not exceeding the window of opportunity during which evaluation and possible treatment would be of maximum benefit in most patients. Disease heterogeneity can make differential diagnosis challenging, especially for IH with limited or minimal growth and those with a deep component [8, 24, 25]. For this reason, in the case of diagnostic doubt, infants should be referred early for specialized consultation for evaluation and possible imaging investigation. A recent study advocated that the optimal time for referral or initiation of treatment was 1 month of age, which is much earlier than most infants are typically referred to or evaluated by IH specialists, as seen in our sample. The reason behind this indication for such early referral was that IH growth was found to be nonlinear, with an accelerated period of rapid growth between 5 and 7 weeks of age [14, 15].
Most IH are diagnosed clinically. Imaging studies and other investigations should only be performed in selected cases, such as diagnostic uncertainty (atypical appearance or growth), presence of five or more cutaneous IH (need to screen for hepatic IH), or if associated anatomic abnormalities are suspected [8, 10]. Doppler ultrasound should be the initial imaging test for diagnostic work-up and for hepatic HI screening and eventually to monitor progression and response to treatment [26, 27]. Magnetic resonance imaging (MRI) should be performed to address possible underlying structural abnormalities, namely, in infants at risk for PHACE or LUMBAR (lower body IH, urogenital anomalies/ulceration, myelopathy, bony deformities, arterial anomalies, renal anomalies) syndromes. These should be suspected in the presence of large segmental IH of the face/scalp and lumbosacral/perineal area, respectively [27,28,29]. As expected, imaging investigation was necessary in a minority of cases: Doppler ultrasound of the lesion was performed in 9.1%, abdominal ultrasound in 11.7% and MRI in 4.3%. In this last group, one infant had PHACE syndrome and was treated with both oral propranolol and laser, with partial regression of the segmental IH. Twenty (5.1%) infants had periorbital IH and were referred to ophthalmologic consultation to rule out amblyopia. Thyroid stimulating hormone screening was performed in two patients who had multifocal IH to rule out secondary hypothyroidism, which was not confirmed.
Decisions regarding intervention should be considered by a multidisciplinary team and based not only on risk stratification but also on the age and comorbidities of the child, location, classification and stage of evolution of the tumor, any actual or potential complications, and parental preferences [8, 10]. Approximately one-third of our sample underwent treatment with propranolol. Although there are no studies to date on the proportion of IH that are treated with propranolol, this percentage is probably overrated as a selection bias might have occurred since this study was carried out in a specialized tertiary center with inclusion of more complex and severe cases. Also, referral is more likely with larger or more visible IH; minor tumors are not usually referred. Despite this, it is known that the majority of infants who actually receive treatment do so to prevent uncontrolled growth leading to permanent disfigurement [8, 30]. This is in line with our results as the main indication for treatment was risk for esthetic compromise, present in almost half of our sample. Median age for propranolol initiation was four months, which shows that our hospital protocol follows the current literature that recommends initiation of treatment ideally between 5 weeks and 5 months of age, before the completion of the proliferative phase, in order to prevent poor outcomes [8, 15]. Patients that underwent treatment with propranolol before the age of 6 months tendentially had better outcomes, but this difference was not statistically significant. Therefore, we believe that treatment initiation after the recommended timings could still be beneficial in some patients and should be considered individually. The median duration of therapy was 9 months, which is also in agreement with the recommendations that therapy must be maintained for at least 6 months or until the end of the proliferative phase, which generally occurs by 1 year of age [8, 30]. The rationale for this recommendation is based on both efficacy and the risk of rebound after discontinuation. A large randomized controlled trial conducted in 2015 showed that a duration of 6 months of therapy was superior to 3 months [31]. Other study revealed that rebound growth may occur even after 6 months of therapy and that the greatest risk was in those in whom treatment had been discontinued prior to nine to 12 months of age [32]. Lack of response to treatment is rare and occurred in 0.8% of our patients, a percentage similar to what has been previously reported (0.9%) [33]. In turn, rebound growth after propranolol discontinuation is more common, being noted in 14–25% of children [22, 32, 34]. Segmental distribution and depth of the IH have been associated with an increased risk of relapse. Some children may need a second course of propranolol or topical timolol, but most cases do not require treatment [22]. Notably, in our sample, a rebound effect occurred in only six (5.1%) patients, three of which underwent a second cycle with propranolol and one underwent surgery. Our strategy, namely, treatment duration and gradual reduction, was probably a protective effect of rebound [35]. Among our patients, 8.3% experienced adverse effects. Similar percentages have been previously described in the literature (7–16%) [36,37,38]. Children treated with propranolol should be closely monitored and clinicians should educate caregivers about potential adverse effects. The most common are sleep disorders, somnolence, and irritability. Others less frequent but more worrisome include bronchospasm, hypotension, bradycardia, and hypoglycemia. To reduce the risk of hypoglycemia, propranolol should be given with or shortly after meals and the doses should be held at times of feeding refusal or vomiting. However, routine glucose screening is not indicated [8, 10]. Pretreatment electrocardiography (ECG) is controversial, once both hypotension and bradycardia tend to be mild and asymptomatic in children with no preexisting cardiac comorbidities. Although initially advocated by some, the most recent guidelines do not include routine ECG [8, 20]. However, in our current practice, all patients are evaluated by a pediatric cardiology and both ECG and echocardiogram are performed before starting propranolol to rule out the main contraindications to this therapy.
The results from the comparative analysis have shown that the group of patients that underwent treatment with propranolol had more risk factors and presented deeper and larger lesions. Total IH’s regression was less frequent in this group, probably because the rate and extent of regression appear to be proportional to size, with larger lesions exhibiting longer periods of growth and involution [14, 39]. Initiation of propranolol as outpatient versus inpatient is changing as more evidence accumulates that cardiovascular and other acute toxicities rarely occur [8, 20]. Similarly, there are still no evidence-based recommendations on dosing frequency (twice daily versus thrice times daily), but both the Food and Drug Administration labeling and the European Medicine Evaluation Agency labeling are for twice-daily dosing [8]. Although we analyzed a short period of time after the application of our revised protocol (approximately 2 years), twice-daily dosage as well as inpatient initiation of propranolol had no significant differences in the incidence of adverse effects, duration of treatment, and lesion evolution. We consider that propranolol can be safely started on an outpatient basis for most infants and that twice daily frequency is equally safe and effective, in addition to being more comfortable for caregivers and increasing compliance.
Topic beta-blockers may be used in thin superficial IH and may also have a role in small ulcerated lesions and in preventing rebound growth after propranolol [40, 41]. Surgery and laser therapy are being used less frequently. Surgery could be indicated in IH that ulcerate, obstruct, or deform vital structures or involve esthetically sensitive areas and for residual skin changes, but only if medical therapy is thought to pose a greater risk and the resultant scar is likely to be acceptable [8, 10, 42]. In turn, the most accepted use of pulsed dye laser is the treatment of ulceration, post-involution erythema, and/or telangiectasias [43].
We acknowledge some important limitations to our study, including its retrospective nature, which limits data collection and the drawing of inferences from our results. Despite these limitations, to our knowledge, this is the largest European study assessing the management of IH and we reviewed information regarding a 12-year experience. Since it was carried out in a tertiary center, a selection bias is likely, probably neglecting smaller IH. Nevertheless, the vast majority of patients showed at least partial regression even in the absence of treatment.
Early detection of high-risk IH is paramount, requiring an increased awareness by pediatricians and general practitioners to identify potentially problematic lesions while in the early proliferation stage. Approximately one-third of the patients was treated with propranolol, a percentage that was probably overestimated due to the selection bias of more severe cases. In this series, no serious adverse effects were detected. Only 10.4% of patients underwent surgery and no complications were reported. Our hospital protocol, which was revised in 2020 based on the updated international literature, proved to be safe and feasible in an outpatient setting, reenforcing safety and efficacy of twice-daily dosage of propranolol.
Abbreviations
- ECG:
-
Electrocardiography
- IH:
-
Infantile hemangiomas (IH)
- LUMBAR:
-
Lower body IH, urogenital anomalies/ulceration, myelopathy, bony deformities, arterial anomalies, renal anomalies
- MRI:
-
Magnetic resonance imaging
- PHACE:
-
Posterior fossa anomalies, hemangioma, arterial anomalies, cardiac anomalies, and eye anomalies
References
Munden A et al (2015) Prospective study of infantile hemangiomas: incidence, clinical characteristics, and association with placental anomalies. HHS Public Access 170(4):907–913
Kilcline C, Frieden IJ (2008) Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol 25(2):168–173
Haggstrom AN et al (2007) Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 150(3):291–294
Drolet BA, Esterly NB, Frieden IJ (1999) “Hemangiomas in children,” Prim Care 173–181
Cordisco MR (2009) “Hemangiomas of infancy: epidemiology”, Hemangiomas Vasc. Malformations An Atlas Diagnosis Treat 138:17–21
Blei F, Walter J, Orlow SJ, Marchuk DA (1998) Familial segregation of hemangiomas and vascular malformations as an autosomal dominant trait. Arch Dermatol 134(6):718–722
Frieden IJ et al (1997) Guidelines of care for hemangiomas of infancy. J Am Acad Dermatol 37(4):631–637
Krowchuk DP et al (2019) Clinical practice guideline for the management of infantile hemangiomas. Pediatr 143(1):1–28
Hand JL, Frieden IJ (2002) Vascular birthmarks of infancy: resolving nosologic confusion. Am J Med Genet 108(4):257–264
Léauté-Labrèze C, Harper JI, Hoeger PH (2017) Infantile haemangioma. Lancet 390(10089):85–94
Colonna V, Resta L, Napoli A, Bonifazi E (2010) Placental hypoxia and neonatal haemangioma: clinical and histological observations. Br J Dermatol 162(1):208–209
de Jong S, Itinteang T, Withers AHJ, Davis PF, Tan ST (2016) Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res 308(4):219–227
Leon-Villapalos J, Wolfe K, Kangesu L (2005) GLUT-1: An extra diagnostic tool to differentiate between haemangiomas and vascular malformations. Br J Plast Surg 58(3):348–352
Chang LC et al (2008) Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 122(2):360–367
Tollefson MM, Frieden IJ (2012) “Early growth of infantile hemangiomas: what parents’photographs tell us,” Pediatr 130(2)
Bauland CG, Lüning TH, Smit JM, Zeebregts CJ, Spauwen PHM (2011) Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg 127(4):1643–1648
Couto RA, MacLellan RA, Zurakowski D, Greene AK (2012) Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg 130(3):619–624
Baselga E et al (2016) Risk factors for degree and type of sequelae after involutionof untreated hemangiomas of infancy. JAMA Dermatol 152(11):1239–1243
Sánchez-Carpintero I, Ruiz-Rodriguez R, López-Gutiérrez JC (2011) Propranolol in the treatment of infantile hemangioma: clinical effectiveness, risks, and recommendations. Actas Dermosifiliogr 102(10):766–779
Drolet B (2015) Initiation and use of propranolol for infantile hemangioma : report of a consensus conference abstract
Bauman NM et al (2014) Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol - Head Neck Surg 140(4):323–330
Ahogo CK et al (2013) Factors associated with the relapse of infantile haemangiomas in children treated with oral propranolol. Br J Dermatol 169(6):1252–1256
Chamlin SL et al (2007) “Multicenter prospective study of ulcerated hemangiomas,” J Pediatr 151(6)
Martin JM, Sanchez S, González V, Cordero P, Ramon D (2019) Infantile hemangiomas with minimal or arrested growth: a retrospective case series. Pediatr Dermatol 36(1):125–131
Ma EH, Robertson SJ, Chow CW, Bekhor PS (2017) Infantile hemangioma with minimal or arrested growth: further observations on clinical and histopathologic findings of this unique but underrecognized entity. Pediatr Dermatol 34(1):64–71
Rotter A et al (2017) Ultrasonography as an objective tool for assessment of infantile hemangioma treatment with propranolol. Int J Dermatol 56(2):190–194
Menapace D, Mitkov M, Towbin R, Hogeling M (2016) The changing face of complicated infantile hemangioma treatment. Pediatr Radiol 46(11):1494–1506
Garzon MC et al (2016) PHACE syndrome: consensus-derived diagnosis and care recommendations. J Pediatr 178:24–33.e2
Iacobas I et al (2010) LUMBAR: Association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr 157(5):795–801.e7
Hoeger PH et al (2015) Treatment of infantile haemangiomas: recommendations of a European expert group. Eur J Pediatr 174(7):855–865
Léauté-Labrèze C et al (2015) A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 372(8):735–746
Shah SD et al (2016) “Rebound growth of infantile hemangiomas after propranolol therapy,” Pediatr 137(4)
Caussé S et al (2013) Propranolol-resistant infantile haemangiomas. Br J Dermatol 169(1):125–129
Wedgeworth E et al (2016) Propranolol in the treatment of infantile haemangiomas: lessons from the European Propranolol in the Treatment of Complicated Haemangiomas (PITCH) Taskforce survey. Br J Dermatol 174(3):594–601
Chang L et al (2017) “When to stop propranolol for infantile hemangioma,” Sci Rep 7:1–7
Thai T, Wang CY, Chang CY, Brown JD (2019) “Central nervous system effects of oral propranolol for infantile hemangioma: a systematic review and meta-analysis,” J Clin Med 8(2)
Prey S et al (2016) Safety of propranolol therapy for severe infantile hemangioma. JAMA - J Am Med Assoc 315(4):413–415
Li X, Yang K, Li H, Huo R (2019) Propranolol treatment for infantile hemangiomas: short-term adverse effects and follow-up to age two. Biomed Res Int 2016:2019
Metry DW, Hebert AA (2000) Benign cutaneous vascular tumors of infancy: when to worry, what to do. Arch Dermatol 136(7):905–914
Boos MD, Castelo-Soccio L (2016) Experience with topical timolol maleate for the treatment of ulcerated infantile hemangiomas (IH). J Am Acad Dermatol 74(3):567–570
Mannschreck DB, Huang AH, Lie E, Psoter K, Puttgen K (2019) Topical timolol as adjunct therapy to shorten oral propranolol therapy for infantile hemangiomas. Pediatr Dermatol 36(3):283–289
Mcheik JN, Renauld V, Duport G, Vergnes P, Levard G (2005) Surgical treatment of haemangioma in infants. Br J Plast Surg 58(8):1067–1072
Chinnadurai S, Sathe NA, Surawicz T (2016) Laser treatment of infantile hemangioma: a systematic review. Lasers Surg Med 48(3):221–233
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by Rita Gomes, Luís Salazar, Carolina Fraga, Mário Rui Correia, and Joana Barbosa-Sequeira. Data analysis was performed by Rita Gomes and Luís Salazar. The first draft of the manuscript was written by Rita Gomes and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This research complies with all the relevant national regulations and institutional policies and is in accordance to the tenets of the Helsinki Declaration. The study was approved by the Ethics Committee of Centro Hospitalar Universitário do Porto and Institute of Biomedical Sciences Abel Salazar.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomes, R., Salazar, L., Fraga, C. et al. Management of infantile hemangiomas—experience of a tertiary hospital. Eur J Pediatr 182, 1611–1618 (2023). https://doi.org/10.1007/s00431-023-04827-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04827-2