Abstract
The classic definition of precocious sexual maturation is the development of secondary sexual characteristics before 8 years of age in girls and before 9 years of age in boys. It is classified as central precocious puberty when premature maturation of the hypothalamic-pituitary-gonadal axis occurs, and as peripheral precocious puberty when there is excessive secretion of sex hormones, independent of gonadotropin secretion. Precocious sexual maturation is more common in girls, generally central precocious puberty of idiopathic origin. In boys, it tends to be linked to central nervous system abnormalities. Clinical evaluation should include a detailed history and physical examination, including anthropometric measurements, calculation of growth velocity, and evaluation of secondary sexual characteristics. The main sign to suspect the onset of puberty is breast tissue development (thelarche) in girls and testicular enlargement (≥4 mL) in boys. Hormonal assessment and imaging are required for diagnosis and identification of the etiology. Genetic testing should be considered if there is a family history of precocious puberty or other clinical features suggestive of a genetic syndrome. Long-acting gonadotropin-releasing hormone analogs are the standard of care for central precocious puberty management, while peripheral precocious puberty management depends on the etiology.
Conclusion: The aim of this review is to address the epidemiology, etiology, clinical assessment, and management of precocious sexual maturation.
What is Known: • The main sign to suspect the onset of puberty is breast tissue development (thelarche) in girls and testicular enlargement (≥4 mL) in boys. The classic definition of precocious sexual maturation is the development of secondary sexual characteristics before 8 years of age in girls and before 9 years of age in boys. • Long-acting gonadotropin-releasing hormone agonist (GnRHa) is the standard of care for CPP management, and adequate hormone suppression results in the stabilization of pubertal progression, a decline in growth velocity, and a decrease in bone age advancement. What is New: • Most cases of precocious sexual maturation are gonadotropin-dependent and currently assumed to be idiopathic, but mutations in genes involved in pubertal development have been identified, such as MKRN3 and DLK1. • A different preparation of long-acting GnRHa is now available: 6-month subcutaneous injection. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Puberty is a biological maturation process that represents the physical, hormonal, and psychological transition from childhood to adulthood, culminating in the development of secondary sexual characteristics and reproductive capacity. It is a complex process that involves genetic, metabolic, environmental, ethnic, geographic, and economic factors.
The exact mechanism underlying the onset of puberty remains unclear, but it is known to be influenced by factors such as adipose tissue, gastrointestinal function, adrenal androgen production, energy sensing, and physical and psychosocial stress [1]. The discovery of kisspeptin, neurokinin B, and dynorphin neuromodulators and their impact on the gonadotropin-releasing hormone (GnRH) pulse generator was very important for the understanding of the pubertal process [1]. A kisspeptin1-receptor loss-of-function mutation was first linked to idiopathic hypogonadotropic hypogonadism in 2003 [2]. Later research showed a pubertal increase in kisspeptin and kisspeptin receptor expression, accompanied by parallel changes in GnRH pulse [3]. Kisspeptin neurons of the arcuate nucleus express both dynorphin A and neurokinin B, and kisspeptin expression is highly sensitive to variations in the nutritional state or in serum leptin, which explains in part the association between overweight and early pubertal development [4].
Changes in pituitary gonadotropin secretion patterns serve as a hormonal trigger for puberty induction. Reactivation of the hypothalamic-pituitary-gonadal (HPG) axis leads to increased pulsatile secretion of GnRH, which stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn stimulate the secretion of gonadal steroids and promote gametogenesis [1, 5, 6]. The HPG axis is activated during the mid-gestation period in the fetus, turns off at the end of gestation, and is then reactivated soon after birth, with an increase in gonadotropin concentrations [7]. This transient postnatal activation of the HPG axis is called minipuberty and lasts up to about 6 months of age in boys and 3–4 years of age in girls [7].
The initial manifestation of puberty is usually breast development in girls (thelarche) and testicular enlargement in boys [8, 9]. The normal age range of onset is when 95% of children present with Tanner stage 2 (Fig. 1), which corresponds to 8–13 years of age in girls and 9–14 years of age in boys [10, 11]. Therefore, the classic definition of precocious sexual maturation is the development of secondary sexual characteristics before 8 years of age in girls and before 9 years of age in boys.
Although some studies have suggested that thelarche now occurs earlier than in the 1960s, the age of menarche has remained relatively stable over recent decades after a period of gradual decline until the mid-twentieth century in most industrialized countries [12, 13]. Therefore, the interval between thelarche and menarche appears to have increased [14]. A redefinition of the age limit for precocious sexual maturation in girls has been proposed in the USA, especially after epidemiological studies showed that clinical signs of puberty were present at 7 years of age in white girls and at 6 years of age in African-American girls [15, 16]. However, doubts have been raised about the reliability of these studies, as they estimated thelarche only by visual inspection (without palpation). In addition, a lowering of the age limit for evaluation of precocious sexual maturation may overlook some girls with true rapid progressive precocious puberty and possibly lead to the misdiagnosis of potentially treatable underlying pathology [13].
The aim of this review is to describe the clinical and laboratory features, diagnosis, advances in genetics, workup, and updated management of precocious sexual maturation.
Epidemiology
Precocious sexual maturation occurs most frequently in girls (15–20 girls for every boy), with an estimated incidence of 1 case per 5000–10,000 girls in the USA [17, 18]. A Danish study found a prevalence of 0.2% for girls and <0.05% for boys [19]. A Spanish study reported a prevalence of 37 cases per 100,000 girls and 0.46 per 100,000 boys, while in Korea the prevalence was 55.9 cases per 100,000 girls and 1.7 per 100,000 boys [20, 21].
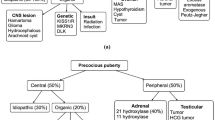
Precocious sexual maturation is classified as central precocious puberty (CPP) when premature maturation of the HPG axis occurs, and as peripheral precocious puberty (PPP) when there is excessive secretion of sex hormones from a tumoral or exogenous source, or secondary to a genetic disease, independent of gonadotropin secretion [5]. In 80% of cases, sexual precocity is central and, therefore, gonadotropin-dependent [11].
In approximately 75–90% of girls and 25–60% of boys with CPP, the cause is idiopathic [12, 22]. The identifiable causes are similar in girls and boys, as shown in Table 1 [17, 23,24,25,26,27,28,29,30,31]. The most commonly involved brain disorders are hypothalamic hamartoma, encephalitis, hydrocephalus, neurofibromatosis type 1, meningomyelocele, and neonatal encephalopathy [32]. These causes are much more frequent in boys [33]. The main risk factors for a central nervous system (CNS) etiology are younger age and male sex [21, 32].
PPP is much less frequent than CPP and can be congenital or acquired. Congenital causes include McCune-Albright syndrome (MAS), familial male-limited precocious puberty (FMPP), and congenital adrenal hyperplasia. Acquired causes include sex steroid-secreting tumors or cysts, profound primary hypothyroidism, and exogenous hormone exposure [34]. Congenital and acquired causes are summarized in Table 2, along with their prevalence in both sexes [25, 34,35,36,37,38,39,40,41,42,43,44,45,46].
Diagnosis
Clinical assessment
If precocious sexual maturation is suspected, clinical evaluation should begin with a detailed history including age of onset, rate of progression of physical changes, exposure to sex steroids, medication use, symptoms related to CNS disease (headache, visual impairment, polyuria or polydipsia, and personality change), history of traumatic brain injury or CNS infection, and family and neonatal history. Accidental ingestion of combined oral contraceptive pills, transfer of testosterone gel by skin contact, and skin exposure to substances containing estrogens or androgens are potential sources of exogenous sex steroid exposure [25]. It is also important to obtain information on puberty onset in parents and siblings (age at menarche, voice breaking, first shave, and growth spurt), as well as in other family members with precocious puberty (or height below the mid-parental height when data on puberty onset are inaccurate).
Physical examination consists of anthropometric measurements, calculation of growth velocity, and evaluation of secondary sexual characteristics according to the Tanner classification (breast development in girls, testicular volume and penile development in boys, and presence of pubic hair) (Fig. 1). The main sign to suspect the onset of puberty is thelarche in girls and testicular enlargement (≥4 mL) in boys. Testicular volume should be assessed using an orchidometer, distinguishing between unilateral vs. bilateral enlargement and checking for the presence of lumps. It may be difficult to discriminate between increased fatty tissue in breasts (lipomastia) and true glandular breast tissue in overweight and obese girls. Firm glandular tissue under the areolae is indicative of thelarche. Skin examination should also be performed to search for café-au-lait spots, which may suggest MAS or neurofibromatosis type 1 (Fig. 2).
Heights should be plotted over time on a growth chart, since a height increase crossing one or more full centile spaces supports a diagnosis of precocious puberty [12]. Moreover, the child’s height should be compared to the mid-parental height, calculated according to the following equations: for girls, ([father’s height cm − 13 cm] + mother’s height cm)/2; for boys, ([mother’s height cm + 13 cm] + father’s height cm)/2 [47].
It is important to distinguish between CPP, isolated premature thelarche (IPT), and premature adrenarche. IPT is defined as the appearance of breast tissue without other findings indicative of puberty, such as accelerated growth velocity, rapid breast development, and advanced skeletal maturation; it usually regresses over months to years [48]. IPT is a benign, self-limited condition that only rarely progresses to CPP [49]. Premature adrenarche is characterized by an increase in adrenal androgen levels independent of the HPG axis in the absence of or after ruling out other pathologic causes of androgen excess, traditionally before 8 years of age in girls and 9 years of age in boys [50]. It is generally regarded as a benign condition, but the child should be monitored for other signs of pubertal progression [51]. Premature adrenarche is clinically identified as pubarche and includes the presence of pubic and axillary hair, apocrine body odor, and acne [52].
Clinical presentation, rate of progression, and sequence of pubertal events in PPP may differ from CPP, since the onset of pubertal development may be sudden or intermittent and involve estrogens, androgens, or both [25]. Testicular volume <4 mL associated with pubic hair development and penile growth suggests PPP, whereas increased testicular volume associated with other features of puberty suggests CPP [12]. The exceptions are human chorionic gonadotropin-secreting germ-cell tumors and FMPP, which are characterized by mild testicular enlargement. FMPP, also called testotoxicosis, is a form of PPP caused by an activating mutation of the LH receptor, resulting in autonomous Leydig cell secretion of testosterone [53].
Adrenal tumors can manifest with signs of virilization due to androgen excess, such as clitoromegaly and pubic hair in girls, as well as with signs of glucocorticoid excess, such as rapid weight gain, round face, facial plethora, striae, hypertension, and hirsutism [53]. Sudden onset of vaginal bleeding along with minimal or no breast development may suggest MAS, a disease caused by a postzygotic, somatic, activating mutation of the α-subunit of the G-protein [34]. PPP in girls with MAS is caused by estrogen secreted by autonomously functioning ovarian cysts [54]. Vaginal bleeding results from withdrawal of estrogen when the cyst resolves [53]. The classic triad of MAS includes polyostotic fibrous dysplasia of bone, café-au-lait spots, and precocious sexual maturation [54] (Fig. 2).
Hormonal assessment
The initial laboratory evaluation of precocious sexual maturation includes the measurement of serum gonadotropins (LH and FSH) and sex steroids (estradiol in girls and testosterone in boys).
Suppressed FSH secretion associated with elevated sex steroid levels suggests the presence of PPP [27, 52]. LH should preferably be collected in the morning using an ultrasensitive assay with a detection limit of 0.1 IU/L [27]. The available high-sensitivity assays are immunofluorometric assay (IFMA), immunochemiluminescent assay (ICMA), and electrochemiluminescence (ECL). Second-generation immunoradiometric assays (IRMA) have sensitivities in the range of 0.1 to 0.5 lU/L [55]. Several studies have evaluated the use of basal LH to rule out CPP, with basal LH cutoffs ranging from 0.1 to 1 IU/L [55,56,57,58,59]. The sensitivity of morning basal LH for the diagnosis of CPP ranges from 56 to 100%, with a specificity of 64 to 100%, depending on the cutoff point and assay used [17]. Basal LH levels above 0.6 IU/L (IFMA) or 0.3 IU/L (ICMA, ECL) are considered pubertal, although values below these levels do not exclude CPP [27, 52, 56, 60]. Gonadotropin concentrations should be interpreted with caution in children younger than 2 years because elevated LH and FSH levels could be physiological due to minipuberty [7, 10]. Clinical monitoring of pubertal progression and growth is recommended, as it helps differentiate premature thelarche from CPP in most cases [61].
When CPP is suspected in the presence of non-diagnostic basal LH, a GnRH stimulation test is indicated, with LH measurement in a single blood sample at 30–40 min after intravenous injection of a short-acting GnRH (gonadorelin 100 μg) [62]. A stimulated peak LH of at least 5 IU/L suggests that puberty is activated [10, 56]. It is important to emphasize that, in early puberty, LH pulses initially occur only during certain sleep stages [63]. For example, an association has been found between slow-wave sleep and nocturnal increase in LH pulses during puberty [64]. In the unavailability of a short-acting GnRH, a long-acting GnRH agonist (GnRHa), leuprorelin 3.75 mg, can be used, with LH measurement in a single blood sample at 30–180 min (or at more time points, depending on the protocol used) after intramuscular injection. We perform a single LH measurement 2 h after leuprorelin injection due to its simplicity and high accuracy. A response >5 IU/L is suggestive of puberty [5, 22, 55, 65, 66], but other cutoff points, ranging from 4 to 8 IU/L, have also been suggested [17]. A GnRHa-stimulated peak LH-to-FSH ratio of 0.6 to 1.0 has been proposed as an indicator of pubertal activation, but its sensitivity and specificity are not greater than those of GnRHa-stimulated peak LH alone [11]. Girls with IPT may have a FSH-predominant response [67]. The main disadvantages of the GnRHa stimulation test are the high cost and risk of injection-site reaction.
In boys, morning testosterone concentration is a useful marker of sexual precocity, since prepubertal concentrations rule out precocious puberty [52]. On the other hand, low estradiol levels do not exclude the diagnosis of precocious sexual maturation in girls [17, 68]. Nevertheless, high estradiol concentrations in the presence of suppressed gonadotropins strongly suggest PPP [69].
Thyroid function should also be assessed, since precocious pseudopuberty due to long-standing hypothyroidism, although rare, is possible [44]. In addition, insulin-like growth factor-1 (IGF-1) levels usually increase in early puberty, more commonly in girls, which may be helpful in diagnosing CPP [63]. IGF-1 levels might also be correlated with higher insulin levels [70].
Imaging assessment
Bone age assessment with a radiograph of the left hand and wrist is indicated. The most commonly used methods are the Greulich and Pyle atlas and the Tanner-Whitehouse 3 (TW3) method [71, 72]. The use of automated measurement systems with artificial intelligence has increased [73]. Bone age is often advanced in patients with precocious sexual maturation; when the advancement exceeds either 1 year or 2 standard deviations the chronological age, it is considered a significant advance in maturation [74]. However, in the early stages of CPP, bone age advancement may not be remarkable [14]. Intra- and inter-observer variability is another limitation. Bone age can be used to predict adult height, but its reliability is low and tends toward overestimation [75,76,77,78]. It is also worth noting that, in the presence of glucocorticoid excess or hypothyroidism, there may be no bone age advancement.
Magnetic resonance imaging (MRI) of the brain is recommended for all boys with CPP and for girls under 6 years of age. Performing MRI in girls aged 6–8 years without symptoms of CNS disease is controversial, since the prevalence of CNS lesions is lower in this age group (25% in girls <6 years of age vs. 3% in 6–8 years of age in a meta-analysis); also, the test is expensive, requiring intravenous administration of contrast agents and possibly sedation [14, 22, 25, 79]. Therefore, it has been suggested that, in otherwise asymptomatic girls, the pros and cons of imaging should be discussed with the parents [22]. Nevertheless, most authors recommend performing MRI in all girls younger than 8 years with CPP [5, 14, 17, 80].
Pelvic ultrasound is considered a rapid, noninvasive, and low-cost method to assess uterine development and ovarian volume and to detect ovarian cysts and tumors (Fig. 3). The size and morphology of the uterus and ovaries are relatively stable during childhood: the volume of each ovary is less than 2 cm3, with follicles less than 9 mm; uterine length is less than 4 cm, with a diameter less than 1.5 cm [81]. The uterine fundus and cervix have a similar width, arranged in a tubular configuration with a fundus-to-cervix ratio of approximately 1. During puberty, the uterus progressively increases in size, becoming wider than the cervix and assuming the typical pear shape found in adults [81, 82]. Many studies have shown increased ovarian and uterine volume in girls with CPP compared with prepubertal controls [83,84,85]. Although uterine volume cutoff points range from 1.8 to 4 cm3 [82], they show low sensitivity for differential diagnosis due to the large overlap of values between groups [86,87,88]. Doppler ultrasound facilitates the assessment of utero-ovarian blood flow and flow impedance measurement in this vascular tree (Fig. 4). The pulsatility index, defined as the difference between peak systolic flow and end-diastolic flow divided by the mean maximum flow velocity, reflects the impedance to blood flow in the vessel distal to the sampling point [89]. Two studies assessing flow velocity in the uterine artery of healthy women in different age groups identified an increase in the pulsatility index in the prepubertal stage and a decrease during puberty, which could reflect a progressive increase in blood flow to the uterus [90, 91]. A further increase in the pulsatility index occurs in adulthood, when the uterus is fully developed and angiogenesis is complete. It has been determined that a low pulsatility index at the uterine artery level has a high diagnostic value for precocious sexual maturation [92, 93].
Pelvic ultrasound. a Uterine volume calculated according to the formula for ellipsoid volume: longitudinal diameter × anteroposterior diameter × transverse diameter × 0.5233. b Diameters of the uterine fundus and cervix, to calculate the fundus-to-cervix ratio. c Endometrial thickness. d Ovarian volume in a prepubertal girl. e Normal pubertal ovary with 22 follicles with a diameter smaller than 0.6 cm. Courtesy of Iara Lucena, MD
Genetic assessment
Familial precocious puberty is typically defined as the occurrence of more than one affected family member [94]. The identification of MKRN3 and DLK1 mutations in familial CPP highlights the important role of genetic factors in the pathophysiology of precocious sexual maturation [95]. Currently, MKRN3 mutations are the most common genetic etiology of monogenic CPP, with a prevalence ranging from 33 to 46% in familial cases and from 0.4 to 5% in sporadic cases [96,97,98,99,100,101]. The MKRN3 gene is located in the critical region of Prader-Willi and Angelman syndromes (locus 15q11-q13) and has maternal imprinting (silencing); therefore, patients develop CPP only when they inherit the mutated allele from their fathers [95, 102]. The clinical features of CPP due to mutations that inactivate MKRN3 are similar to those of idiopathic CPP [82]. Comparable to MKRN3, carriers of DLK1 defects only manifest CPP if the defect is inherited from their fathers [103]. In addition, CPP is part of the clinical manifestations of some genetic syndromes, such as Temple syndrome, Silver-Russell syndrome, and Williams-Beuren syndrome. Therefore, genetic testing should be considered if there is a family history of precocious puberty or other clinical features [94].
Management
CPP
Long-acting GnRHa is the standard of care for CPP management. The action of GnRHa relies on sustained high concentrations of GnRH that result in a paradoxical downregulation and subsequent suppression of the HPG axis, thus inhibiting gonadotropin secretion [104].
Management with adequate hormone suppression results in the stabilization of pubertal progression, a decline in growth velocity, and a decrease in bone age advancement [14]. As shown in Table 3, different preparations are available: intramuscular 4-week, 12-week, or 6-month depot forms; 6-month subcutaneous injection; and subcutaneous implant [105,106,107,108,109]. Although marketed to be replaced annually, the implant can suppress puberty for at least 2 years [110].
The main goals of treatment are the preservation of adult height, synchronization of puberty with peers, alleviation of psychosocial distress, and, in girls, avoiding menarche at an early age [27]. No randomized controlled trials have examined the effects of GnRHa treatment on height or other outcomes. Height outcomes have been mainly assessed by using historical controls and differences between achieved and predicted adult height based on bone age before treatment, which are highly imprecise. For girls, the increase in final height ranges from 2 to 10 cm [111]. Girls who begin GnRHa treatment before 6 years of age obtain the greatest benefit, while those who begin treatment between 6 and 8 years of age have variable outcomes [75, 112]. On the other hand, girls treated after 8 years of age show no increase in adult height [113, 114]. Factors associated with increased adult height are earlier age at the start of puberty, younger bone age at diagnosis, prompt treatment, greater height at diagnosis, and greater target height [14]. For boys, a recent study reported that the predicted adult height significantly increased by approximately 4.1 cm after GnRHa treatment [115]. There is currently insufficient evidence to establish an age parameter for treatment in boys.
The most common side effects of GnRHa treatment are headaches, hot flashes, and injection-site reactions, which are usually mild [105]. Rarely, a sterile abscess develops with intramuscular injection or implant and may result in loss of efficacy [21, 116,117,118]. Vaginal bleeding can occur after the first injection, usually in girls with advanced pubertal development and possibly due to a transient increase in estradiol secretion [17]. A negative influence on long-term reproductive potential or bone mineral density has not been observed [108]. Weight gain can also be a side effect of GnRHa treatment in patients with normal body mass index; however, it is unclear whether overweight or obese patients experience significant weight gain after treatment [119,120,121,122]. The implant is typically inserted into the upper inner arm under local anesthesia and promotes a rapid and profound suppression of the HPG axis for 1 year after insertion [123]. One concern is the risk of device fracture during extraction, which may rarely require ultrasound-guided removal of the remaining fragments [93].
Treatment monitoring is based on clinical parameters such as linear growth, Tanner staging, and skeletal maturation. Breast or testicular development, bone age advancement, and high growth velocity are suggestive of treatment failure [27, 124]. When treatment fails, after confirming adherence to GnRHa administration, the dose can be increased or the dosing interval can be reduced as an option [124].
There is no agreement about the need for biochemical measurements to assess treatment effectiveness. In children receiving GnRHa therapy, random ultrasensitive LH levels can remain in the pubertal range, despite apparent puberty suppression [125]. Stimulated LH levels using GnRH, aqueous GnRHa, or the free GnRHa contained in depot preparations can be used to evaluate treatment if the clinical results are poor [22, 27, 105]. Suppression of LH secretion to less than 2.5–4.5 IU/L (the cutoff point varies in the literature) according to IFMA, ICMA, or ECL is an adequate target in patients on monthly or trimonthly GnRHa therapy [126,127,128]. A recent study found first-void urinary LH measured with ECL useful in assessing puberty suppression during GnRHa treatment, with a cutoff of 1.01 IU/L for the highest sensitivity (92%) and specificity (100%) [129].
Patients with familial precocious puberty show adequate clinical and laboratory responses to long-acting GnRH analogs, similar to patients with idiopathic CPP [95]. There is no consensus on the optimal age to discontinue GnRHa therapy. The decision should be individualized and consider factors such as actual and predicted height, synchronization of puberty with peers, and psychological distress [27]. Treatment withdrawal should be evaluated at 12 to 12.5 years of bone age in girls and at 13 to 13.5 years of bone age in boys [17, 105]. Spontaneous menses occur approximately 12 months after interruption of GnRH treatment [107].
PPP
PPP management depends on the etiology, as shown in Table 2 [36, 41, 53, 54, 130,131,132,133,134,135,136,137,138,139]. Management of congenital adrenal hyperplasia is aimed at suppressing adrenal androgen production with glucocorticoids [140].
In MAS, girls with rapidly progressive puberty, frequent menses, accelerated growth, and bone age advancement may benefit from treatment [141]. Aromatase inhibitors have been used in the management of PPP in girls with MAS as they bind to the cytochrome P450 portion of aromatase, thus inhibiting the conversion of androgens to estrogens [28]. Third-generation aromatase inhibitors, such as anastrozole and letrozole, are more potent and better tolerated than earlier-generation agents, but only letrozole has been shown to be effective in the management of MAS [45]. Tamoxifen and fulvestrant are used as second-line or adjuvant therapy [141]. Treatment may include letrozole at a single daily dose of 2.5 mg for the entire treatment period or in escalating doses, starting with 0.5 mg/m2/day for 7 days, then 1 mg/m2/day on days 8–14, and then 1.5 mg/m2/day thereafter. However, if there is any progression of PPP, a dose of 2 mg/m2/day is suggested [139]. Ovarian surgery for cysts should be avoided, as disease is usually bilateral [141]. In boys with MAS, however, PPP has not been extensively studied because of its very low frequency, and all available treatment information is derived from case reports. Bisphosphonates (pamidronate and zoledronate) are proposed for persistent, moderate-to-severe bone pain. It remains unclear whether bisphosphonates can reduce fibrous dysplasia lesion size or progression [141]. Evidence for the efficacy and safety of denosumab is currently scarce, and its use is not recommended outside the context of a clinical trial [141].
For boys with FMPP, the most frequent approach involves the combination of an antiandrogen with a third-generation aromatase inhibitor [53]. The most common combination is spironolactone (5.7 mg/kg/day, up to 450–500 mg/day) plus anastrozole (1 mg/day), which has been shown to improve final adult height and to reduce the rate of bone maturation [132, 142, 143]. Ketoconazole and cyproterone acetate have also been suggested as alternative therapies [131].
Surgical resection is the first-line therapy for sex steroid-secreting tumors, with the exception of functioning follicular ovarian cysts, because they tend to regress spontaneously [53, 144]. It should be noted that CPP and PPP can occur concomitantly in children due to early activation of the HPG axis, especially in those with significant bone age advancement; in this case, additional GnRHa treatment may be necessary [25].
Conclusion and perspectives
Puberty is a complex process that marks the transition from childhood to adulthood, and its mechanisms are not fully understood. Most cases of CPP are currently assumed to be idiopathic, but the recent identification of genes involved in pubertal development shows that genetic factors play a role in its pathophysiology. PPP is a heterogeneous disorder resulting from a wide range of gonadal and extragonadal conditions that involve diverse clinical manifestations, which requires accurate etiological investigation for correct management. Some questions remain unanswered, such as whether CNS imaging should be considered in all girls with CPP, the best method for monitoring hormone suppression, and optimal timing of treatment discontinuation. Future clinical trials should compare different drugs and dosages in GnRHa therapy, particularly in boys.
Data Availability
N/A
Abbreviations
- CNS:
-
Central nervous system
- CPP:
-
Central precocious puberty
- ECL:
-
Electrochemiluminescence
- FMPP:
-
Familial male-limited precocious puberty
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- GnRHa:
-
Gonadotropin-releasing hormone agonist
- HPG:
-
Hypothalamic-pituitary-gonadal
- ICMA:
-
Immunochemiluminescent assay
- IFMA:
-
Immunofluorometric assay
- IGF-1:
-
Insulin-like growth factor-1
- IPT:
-
Isolated premature thelarche
- LH:
-
Luteinizing hormone
- MRI:
-
Magnetic resonance imaging
- MAS:
-
McCune-Albright syndrome
- PPP:
-
Peripheral precocious puberty
- TW3:
-
Tanner-Whitehouse 3
References
Livadas S, Chrousos GP (2019) Molecular and environmental mechanisms regulating puberty initiation: an integrated approach. Front Endocrinol (Lausanne) 10:828. https://doi.org/10.3389/fendo.2019.00828
de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E (2003) Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 100:10972–10976. https://doi.org/10.1073/pnas.1834399100
Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM (2005) Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A 102:2129–2134. https://doi.org/10.1073/pnas.0409822102
Bianco SD (2012) A potential mechanism for the sexual dimorphism in the onset of puberty and incidence of idiopathic central precocious puberty in children: sex-specific kisspeptin as an integrator of puberty signals. Front Endocrinol (Lausanne) 3:149. https://doi.org/10.3389/fendo.2012.00149
Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Júnior G (2016) Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab 60:163–172. https://doi.org/10.1590/2359-3997000000144
Abreu AP, Kaiser UB (2016) Pubertal development and regulation. Lancet Diabetes Endocrinol 4:254–264. https://doi.org/10.1016/S2213-8587(15)00418-0
Lanciotti L, Cofini M, Leonardi A, Penta L, Esposito S (2018) Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front Endocrinol (Lausanne) 9:410. https://doi.org/10.3389/fendo.2018.00410
Marshall WA, Tanner JM (1969) Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303. https://doi.org/10.1136/adc.44.235.291
Marshall WA, Tanner JM (1970) Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23. https://doi.org/10.1136/adc.45.239.13
Carel JC, Léger J (2008) Clinical practice. Precocious puberty. N Engl J Med 358:2366–2377. https://doi.org/10.1056/NEJMcp0800459
Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP (2003) The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 24:668–693. https://doi.org/10.1210/er.2002-0019
Bradley SH, Lawrence N, Steele C, Mohamed Z (2020) Precocious puberty. BMJ 368:l6597. https://doi.org/10.1136/bmj.l6597
Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A (2012) Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 77:137–145. https://doi.org/10.1159/000336325
Soriano-Guillén L, Argente J (2019) Central precocious puberty, functional and tumor-related. Best Pract Res Clin Endocrinol Metab 33:101262. https://doi.org/10.1016/j.beem.2019.01.003
Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM (1997) Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99:505–512. https://doi.org/10.1542/peds.99.4.505
Kaplowitz PB, Oberfield SE (1999) Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 104:936–941. https://doi.org/10.1542/peds.104.4.936
Latronico AC, Brito VN, Carel JC (2016) Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol 4:265–274. https://doi.org/10.1016/S2213-8587(15)00380-0
Partsch CJ, Sippell WG (2001) Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update 7:292–302. https://doi.org/10.1093/humupd/7.3.292
Teilmann G, Pedersen CB, Jensen TK, Skakkebaek NE, Juul A (2005) Prevalence and incidence of precocious pubertal development in Denmark: an epidemiologic study based on national registries. Pediatrics 116:1323–1328. https://doi.org/10.1542/peds.2005-0012
Kim SH, Huh K, Won S, Lee KW, Park MJ (2015) A significant increase in the incidence of central precocious puberty among Korean girls from 2004 to 2010. Gonzalez-Bulnes A, editor. PLoS One 10:e0141844. https://doi.org/10.1371/journal.pone.0141844
Soriano-Guillén L, Corripio R, Labarta JI, Cañete R, Castro-Feijóo L, Espino R, Argente J (2010) Central precocious puberty in children living in Spain: incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab 95:4305–4313. https://doi.org/10.1210/jc.2010-1025
Aguirre RS, Eugster EA (2018) Central precocious puberty: from genetics to treatment. Best Pract Res Clin Endocrinol Metab 32:343–354. https://doi.org/10.1016/j.beem.2018.05.008
Chalumeau M, Chemaitilly W, Trivin C, Adan L, Bréart G, Brauner R (2002) Central precocious puberty in girls: an evidence-based diagnosis tree to predict central nervous system abnormalities. Pediatrics 109:61–67. https://doi.org/10.1542/peds.109.1.61
Yoon JS, So CH, Lee HS, Lim JS, Hwang JS (2018) Prevalence of pathological brain lesions in girls with central precocious puberty: possible overestimation? J Korean Med Sci 33:e329. https://doi.org/10.3346/jkms.2018.33.e329
Eugster EA (2019) Update on precocious puberty in girls. J Pediatr Adolesc Gynecol 32:455–459. https://doi.org/10.1016/j.jpag.2019.05.011
Kerrigan JF, Ng Y, Chung S, Rekate HL (2005) The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 12:119–131. https://doi.org/10.1016/j.spen.2005.04.002
Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, ESPE-LWPES GnRH Analogs Consensus Conference Group (2009) Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 123:e752–e762. https://doi.org/10.1542/peds.2008-1783
Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, Kim DH, Ha S, Seo GH, Kim HS (2019) Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr 208:221–228. https://doi.org/10.1016/j.jpeds.2018.12.022
Boyce AM, Florenzano P, de Castro LF, Collins MT (1993) Fibrous dysplasia/McCune-Albright syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews Seattle (WA): University of Washington, Seattle [updated 2019 Jun 27].
Ghizzoni L, Gasco V (2010) Premature pubarche. Horm Res Paediatr 73:420–422. https://doi.org/10.1159/000308178
Boyce AM, Chong WH, Shawker TH, Pinto PA, Linehan WM, Bhattacharryya N, Merino MJ, Singer FR, Collins MT (2012) Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab 97:E1782–E1790. https://doi.org/10.1210/jc.2012-1791
Stephen M, Zage P, Waguespack S (2011) Gonadotropin-dependent precocious puberty: neoplastic causes and endocrine considerations. Int J Pediatr Endocrinol 2011:184502. https://doi.org/10.1155/2011/184502
Brunner HG, Otten BJ (1999) Precocious puberty in boys. N Engl J Med 341:1763–1765. https://doi.org/10.1056/NEJM199912023412311
Schoelwer M, Eugster EA (2016) Treatment of peripheral precocious puberty. Endocr Dev 29:230–239. https://doi.org/10.1159/000438895
Urban MD, Lee PA, Plotnick LP, Migeon CJ (1978) The diagnosis of Leydig cell tumors in childhood. Am J Dis Child 132:494–497. https://doi.org/10.1001/archpedi.1978.02120300054011
Englund AT, Geffner ME, Nagel RA, Lippe BM, Braunstein GD (1991) Pediatric germ cell and human chorionic gonadotropin-producing tumors. Clinical and laboratory features. Am J Dis Child 145:1294–1297. https://doi.org/10.1001/archpedi.1991.02160110086026
Hemady ZS, Siler-Khodr TM, Najjar S (1978) Precocious puberty in juvenile hypothyroidism. J Pediatr 92:55–59. https://doi.org/10.1016/s0022-3476(78)80070-5
Chrousos GP, Detera-Wadleigh SD, Karl M (1993) Syndromes of glucocorticoid resistance. Ann Intern Med 119:1113–1124. https://doi.org/10.7326/0003-4819-119-11-199312010-00009
Charmandari E, Kino T, Chrousos GP (2004) Familial/sporadic glucocorticoid resistance. Ann N Y Acad Sci 1024:168–181. https://doi.org/10.1196/annals.1321.014
Metwalley K, Farghaly H (2013) Aromatase excess syndrome presenting with prepubertal gynecomastia in an Egyptian child with type 1 neurofibromatosis. Indian J Hum Genet 19:472. https://doi.org/10.4103/0971-6866.124379
Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, Yue W, Mitsiades CW, Flor AW, Chrousos GP (1998) The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription 1. J Clin Endocrinol Metab 83:1348–1357. https://doi.org/10.1210/jcem.83.4.4697
Starzyk J, Starzyk B, Bartnik-Mikuta A, Urbanowicz W, Dziatkowiak H (2001) Gonadotropin releasing hormone-independent precocious puberty in a 5 year-old girl with suprasellar germ cell tumor secreting β-hCG and α-fetoprotein. J Pediatr Endocrinol Metab 14:789–796. https://doi.org/10.1515/jpem.2001.14.6.789
Cornacchia MA, Bhushan S, Arguello R (2018) A case of familial male-limited precocious puberty in a child with Klinefelter syndrome. J Endocr Soc 2:1131–1136. https://doi.org/10.1210/js.2018-00192
Cabrera SM, DiMeglio LA, Eugster EA (2013) Incidence and characteristics of pseudoprecocious puberty because of severe primary hypothyroidism. J Pediatr 162:637–639. https://doi.org/10.1016/j.jpeds.2012.10.043
Zou CC, Liang L, Dong GP, Zhao ZY (2008) Peripheral precocious puberty: a retrospective study for six years in Hangzhou, China. J Paediatr Child Health 44:415–418. https://doi.org/10.1111/j.1440-1754.2008.01320.x
Ringel MD, Schwindinger WF, Levine MA (1996) Clinical implications of genetic defects in G proteins: the molecular basis of McCune-Albright syndrome and Albright hereditary osteodystrophy. Med (Baltimore) 75:171–184. https://doi.org/10.1097/00005792-199607000-00001
Tanner JM, Goldstein H, Whitehouse RH (1970) Standards for children’s height at ages 2-9 years allowing for height of parents. Arch Dis Child 45:755–762. https://doi.org/10.1136/adc.45.244.755
Khokhar A, Mojica A (2018) Premature thelarche. Pediatr Ann 47:e12–e15. https://doi.org/10.3928/19382359-20171214-01
Bizzarri C, Spadoni GL, Bottaro G, Montanari G, Giannone G, Cappa M, Cianfarani S (2014) The response to gonadotropin releasing hormone (GnRH) stimulation test does not predict the progression to true precocious puberty in girls with onset of premature thelarche in the first three years of life. J Clin Endocrinol Metab 99:433–439. https://doi.org/10.1210/jc.2013-3292
Novello L, Speiser PW (2018) Premature adrenarche. Pediatr Ann 47:e7–e11. https://doi.org/10.3928/19382359-20171214-04
DeSalvo DJ, Mehra R, Vaidyanathan P, Kaplowitz PB (2013) In children with premature adrenarche, bone age advancement by 2 or more years is common and generally benign. J Pediatr Endocrinol Metab 26:215–221. https://doi.org/10.1515/jpem-2012-0283
Brito VN, Latronico AC, Arnhold IJP, Mendonça BB (2008) Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq Bras Endocrinol Metabol 52:18–31. https://doi.org/10.1590/s0004-27302008000100005
Haddad NG, Eugster EA (2019) Peripheral precocious puberty including congenital adrenal hyperplasia: causes, consequences, management and outcomes. Best Pract Res Clin Endocrinol Metab 33:101273. https://doi.org/10.1016/j.beem.2019.04.007
Corica D, Aversa T, Pepe G, De Luca F, Wasniewska M (2018) Peculiarities of precocious puberty in boys and girls with McCune-Albright syndrome. Front Endocrinol (Lausanne) 9:337. https://doi.org/10.3389/fendo.2018.00337
Neely EK, Hintz RL, Wilson DM, Lee PA, Gautier T, Argente J, Stene M (1995) Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr 127:40–46. https://doi.org/10.1016/s0022-3476(95)70254-7
Resende EAMR, Lara BHJ, Reis JD, Ferreira BP, Pereira GA, Borges MF (2007) Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab 92:1424–1429. https://doi.org/10.1210/jc.2006-1569
Houk CP, Kunselman AR, Lee PA (2009) Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics 123:e1059–e1063. https://doi.org/10.1542/peds.2008-1180
Pasternak Y, Friger M, Loewenthal N, Haim A, Hershkovitz E (2012) The utility of basal serum LH in prediction of central precocious puberty in girls. Eur J Endocrinol 166:295–299. https://doi.org/10.1530/EJE-11-0720
Lee DS, Ryoo NY, Lee SH, Kim S, Kim JH (2013) Basal luteinizing hormone and follicular stimulating hormone: is it sufficient for the diagnosis of precocious puberty in girls? Ann Pediatr Endocrinol Metab 18:196–201. https://doi.org/10.6065/apem.2013.18.4.196
Brito VN, Batista MC, Borges MF, Latronico AC, Kohek MB, Thirone AC, Jorge BH, Arnhold IJ, Mendonca BB (1999) Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab 84:3539–3544. https://doi.org/10.1210/jcem.84.10.6024
Kaplowitz PB (2020) For premature thelarche and premature adrenarche, the case for waiting before testing. Horm Res Paediatr:1–4. https://doi.org/10.1159/000512764
Kaplowitz P, Bloch C (2016) Evaluation and referral of children with signs of early puberty. Pediatrics 137(1):e20153732. https://doi.org/10.1542/peds.2015-3732
Potau N, Ibáñez L, Sentis M, Carrascosa A (1999) Sexual dimorphism in the maturation of the pituitary-gonadal axis, assessed by GnRH agonist challenge. Eur J Endocrinol 141:27–34. https://doi.org/10.1530/eje.0.1410027
Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE (2012) Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab 97:1–8. https://doi.org/10.1210/jc.2012-2692
Fuqua JS (2013) Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab 98:2198–2207. https://doi.org/10.1210/jc.2013-1024
Sathasivam A, Garibaldi L, Shapiro S, Godbold J, Rapaport R (2010) Leuprolide stimulation testing for the evaluation of early female sexual maturation. Clin Endocrinol (Oxf) 73:375–381. https://doi.org/10.1111/j.1365-2265.2010.03796.x
Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB Jr (1988) Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 67:474–479. https://doi.org/10.1210/jcem-67-3-474
Soriano-Guillén L, Argente J (2011) Pubertad precoz central: aspectos epidemiológicos, etiológicos y diagnóstico-terapéuticos. An Pediatría 74:336.e1–336.e13. https://doi.org/10.1016/j.anpedi.2010.11.003
Partsch CJ, Heger S, Sippell WG (2002) Management and outcome of central precocious puberty. Clin Endocrinol (Oxf) 56:129–148. https://doi.org/10.1046/j.0300-0664.2001.01490.x
Sørensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A (2012) Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol 166:903–910. https://doi.org/10.1530/EJE-12-0106
Greulich WW, Pyle SI (1959) Radiographic atlas of skeletal development of the hand and wrist, 2nd edn. Stanford University Press, Stanford, CA, USA
Tanner JM, Healy MJR, Cameron N, Goldstein H (2001) Assessment of skeletal maturity and prediction of adult height (TW3 method). London: W.B. Saunders.
Prokop-Piotrkowska M, Marszałek-Dziuba K, Moszczyńska E, Szalecki M, Jurkiewicz E (2020) Traditional and new methods of bone age assessment - an overview. J Clin Res Pediatr Endocrinol. 0:0. https://doi.org/10.4274/jcrpe.galenos.2020.2020.0091
Melmed S, Koenig R, Rosen C, Auchus R, Goldfine A (2016). Williams textbook of endocrinology, 14th ed. Elselvier.
Bar A, Linder B, Sobel EH, Saenger P, DiMartino-Nardi J (1995) Bayley-Pinneau method of height prediction in girls with central precocious puberty: correlation with adult height. J Pediatr 126:955–958. https://doi.org/10.1016/s0022-3476(95)70221-0
Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SDC, Kuohung W, Xu S, Gryngarten M, Escobar ME, Arnhold IJP, Mendonca BB, Kaiser UB, Latronico AC (2010) Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab 95:2276–2280. https://doi.org/10.1210/jc.2009-2421
Carel JC, Lahlou N, Roger M, Chaussain JL (2004) Precocious puberty and statural growth. Hum Reprod Update 10:135–147. https://doi.org/10.1093/humupd/dmh012
Brito VN, Latronico AC, Cukier P, Cukier P, Teles MG, Silveira LFG, Arnhold IJP, Mendonca BB (2008) Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J Clin Endocrinol Metab 93:2662–2669. https://doi.org/10.1210/jc.2007-2183
Cantas-Orsdemir S, Garb JL, Allen HF (2018) Prevalence of cranial MRI findings in girls with central precocious puberty: a systematic review and meta-analysis. J Pediatr Endocrinol Metab 31:701–710. https://doi.org/10.1515/jpem-2018-0052
Mogensen SS, Aksglaede L, Mouritsen A, Sørensen K, Main KM, Gideon P, Juul A (2012) Pathological and incidental findings on brain MRI in a single-center study of 229 consecutive girls with early or precocious puberty. PLoS One 7:e29829. https://doi.org/10.1371/journal.pone.0029829
Ziereisen F, Guissard G, Damry N, Avni EF (2005) Sonographic imaging of the paediatric female pelvis. Eur Radiol 15:1296–1309. https://doi.org/10.1007/s00330-005-2648-6
de Vries L, Phillip M (2011) Role of pelvic ultrasound in girls with precocious puberty. Horm Res Paediatr 75:148–152. https://doi.org/10.1159/000323361
Wen X, Wen D, Zhang H, Zhang H, Yang Y (2018) Observational study pelvic ultrasound a useful tool in the diagnosis and differentiation of precocious puberty in Chinese girls. Med (Baltimore) 97:e0092. https://doi.org/10.1097/MD.0000000000010092
Binay C, Simsek E, Bal C (2014) The correlation between GnRH stimulation testing and obstetric ultrasonographic parameters in precocious puberty. J Pediatr Endocrinol Metab 27:1193–1199. https://doi.org/10.1515/jpem-2013-0363
Kılıç A, Durmuş MS, Ünüvar E, Yıldız I, Aydın BK, Uçar A, Bundak R, Baş F, Darendeliler F, Oğuz F et al (2012) Clinical and laboratory characteristics of children referred for early puberty: preponderance in 7-8 years of age. J Clin Res Pediatr Endocrinol 4:208–212. https://doi.org/10.4274/jcrpe.736
Lee SH, Joo EY, Lee JE, Jun YH, Kim MY (2016) The diagnostic value of pelvic ultrasound in girls with central precocious puberty. Chonnam Med J 52:70–74. https://doi.org/10.4068/cmj.2016.52.1.70
Yu J, Shin HY, Lee SH, Kim YS, Kim JH (2015) Usefulness of pelvic ultrasonography for the diagnosis of central precocious puberty in girls. Korean J Pediatr 58:294–300. https://doi.org/10.3345/kjp.2015.58.8.294
Eksioglu AS, Yilmaz S, Cetinkaya S, Cinar G, Yildiz YT, Aycan Z (2013) Value of pelvic sonography in the diagnosis of various forms of precocious puberty in girls. J Clin Ultrasound 41:84–93. https://doi.org/10.1002/jcu.22004
Long MG, Boultbee JE, Hanson ME, Begent RHJ (1989) Doppler time velocity waveform studies of the uterine artery and uterus. Br J Obstet Gynaecol 96:588–593. https://doi.org/10.1111/j.1471-0528.1989.tb03261.x
Laursen EM, Holm K, Brocks V, Jarden M, Müller J (1996) Doppler assessment of flow velocity in the uterine artery during pubertal maturation. Ultrasound Obstet Gynecol 8:341–345. https://doi.org/10.1046/j.1469-0705.1996.08050341.x
Ziereisen F, Heinrichs C, Dufour D, Saerens M, Avni EF (2001) The role of Doppler evaluation of the uterine artery in girls around puberty. Pediatr Radiol 31:712–719. https://doi.org/10.1007/s002470100463
Battaglia C, Mancini F, Regnani G, Persico N, Iughetti L, De Aloysio D (2003) Pelvic ultrasound and color Doppler findings in different isosexual precocities. Ultrasound Obstet Gynecol 22:277–283. https://doi.org/10.1002/uog.154
Paesano PL, Colantoni C, Mora S, di Lascio A, Ferrario M, Esposito A, Ambrosi A, Maschio AD, Russo G (2019) Validation of an accurate and noninvasive tool to exclude female precocious puberty: pelvic ultrasound with uterine artery pulsatility index. AJR Am J Roentgenol 213:451–457. https://doi.org/10.2214/AJR.18.19875
de Vries L, Kauschansky A, Shohat M, Phillip M (2004) Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab 89:1794–1800. https://doi.org/10.1210/jc.2003-030361
Canton APM, Seraphim CE, Brito VN, Latronico AC (2019) Pioneering studies on monogenic central precocious puberty. Arch. Endocrinol Metab 63:438–444. https://doi.org/10.20945/2359-3997000000164
Macedo DB, Abreu AP, Reis ACS, Montenegro LR, Daber A, Beneduzzi D, Cukier P, Silveira LFG, Teles MG, Carroll RS et al (2014) Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene Makorin Ring Finger 3. J Clin Endocrinol Metab 99:E1097–E1103. https://doi.org/10.1210/jc.2013-3126
Bessa DS, Macedo DB, Brito VN, França MM, Montenegro LR, Cunha-Silva M, Silveira LG, Hummel T, Bergadá I, Braslavskyet D et al (2017) High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology 105:17–25. https://doi.org/10.1159/000446963
Simon D, Ba I, Mekhail N, Ecosse E, Paulsen A, Zenaty D, Houang M, Perelroizen MJ, de Filippo GP, Salerno M et al (2016) Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol 174:1–8. https://doi.org/10.1530/EJE-15-0488
Lee H, Jin HS, Shim Y, Jeong HR, Kwon E, Choi V, Kim MC, Chung IS, Jeong SY, Hwang JS (2016) Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res 48:118–122. https://doi.org/10.1055/s-0035-1548938
Ortiz-Cabrera NV, Riveiro-Álvarez R, López-Martínez MA, Pérez-Segura P, Aragón-Gómez I, Trujillo-Tiebas MJ, Soriano-Guillén L (2017) Clinical exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Horm Res Paediatr 87:88–94. https://doi.org/10.1159/000453262
Valadares LP, Meireles CG, De Toledo IP, Oliveira RS, Castro LCG, Abreu AP, Carroll RS, Latronico AC, Kaiser UB, Guerra ENS et al (2019) MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc 3:979–995. https://doi.org/10.1210/js.2019-00041
Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB (2013) Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med 368:2467–2475. https://doi.org/10.1056/NEJMoa1302160
Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman S (2000) The DLK1 and GTL2 genes are linked and reciprocally imprinted. Genes Dev 14:1997–2002
Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E (1978) Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633. https://doi.org/10.1126/science.100883
Eugster EA (2019) Treatment of central precocious puberty. J Endocr Soc 3:965–972. https://doi.org/10.1210/js.2019-00036
Cook JS, Doty KL, Conn PM, Hansen JR (1992) Assessment of depot leuprolide acetate dose-adequacy for central precocious puberty. J Clin Endocrinol Metab 74:1206–1209. https://doi.org/10.1210/jcem.74.5.1569169
Guaraldi F, Beccuti G, Gori D, Ghizzoni L (2016) Management of endocrine disease: long-term outcomes of the treatment of central precocious puberty. Eur J Endocrinol 174(3):R79–R87. https://doi.org/10.1530/EJE-15-0590
Oostdijk W, Rikken B, Schreuder S, Otten B, Odink R, Rouwé C, Jansen M, Gerver WJ, Waelkens J, Drop S (1996) Final height in central precocious puberty after long term treatment with a slow release GnRH agonist. Arch Dis Child 75:292–297. https://doi.org/10.1136/adc.75.4.292
Klein KO, Freire A, Gryngarten MG, Kletter GB, Benson M, Miller BS, Dajani TS, Eugster EA, Mauras N (2020) Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J Clin Endocrinol Metab 105:e3660–e3671. https://doi.org/10.1210/clinem/dgaa479
Lewis KA, Goldyn AK, West KW, Eugster EA (2013) A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr 163:1214–1216. https://doi.org/10.1016/j.jpeds.2013.05.033
Bereket A (2017) A critical appraisal of the effect of gonadotropin-releasing hormon analog treatment on adult height of girls with central precocious puberty. J Clin Res Pediatr Endocrinol 9:33–48. https://doi.org/10.4274/jcrpe.2017.S004
Lazar L, Padoa A, Phillip M (2007) Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab 92:3483–3489. https://doi.org/10.1210/jc.2007-0321
Kaplowitz PB, Backeljauw PF, Allen DB (2018) Toward more targeted and cost-effective gonadotropin-releasing hormone analog treatment in girls with central precocious puberty. Horm Res Paediatr 90:1–7. https://doi.org/10.1159/000491103
Bertelloni S, Massart F, Miccoli M, Baroncelli GI (2017) Adult height after spontaneous pubertal growth or GnRH analog treatment in girls with early puberty: a meta-analysis. Eur J Pediatr 176:697–704. https://doi.org/10.1007/s00431-017-2898-8
Shim YS, Lim KI, Lee HS, Hwang JS (2020) Long-term outcomes after gonadotropin-releasing hormone agonist treatment in boys with central precocious puberty. PLoS One 15:e0243212. https://doi.org/10.1371/journal.pone.0243212
Toro CA, Aylwin CF, Lomniczi A (2018) Hypothalamic epigenetics driving female puberty. J Neuroendocrinol 30:e12589. https://doi.org/10.1111/jne.12589
Carel JC, Lahlou N, Jaramillo O, Montauban V, Teinturier C, Colle M, Lucas C, Chaussain JL (2002) Treatment of central precocious puberty by subcutaneous injections of leuprorelin 3-month depot (11.25 mg). J Clin Endocrinol Metab 87:4111–4116. https://doi.org/10.1210/jc.2001-020243
Johnson SR, Nolan RC, Grant MT, Price GJ, Siafarikas A, Bint L, Choong CS (2012) Sterile abscess formation associated with depot leuprorelin acetate therapy for central precocious puberty. J Paediatr Child Health 48:E136–E139. https://doi.org/10.1111/j.1440-1754.2011.02083.x
Park J, Kim JH (2017) Change in body mass index and insulin resistance after 1-year treatment with gonadotropin-releasing hormone agonists in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 22:27–35. https://doi.org/10.6065/apem.2017.22.1.27
Yang WJ, Ko KH, Lee KH, Hwang IT, Oh YJ (2017) The different effects of gonadotropin-releasing hormone agonist therapy on body mass index and growth between normal-weight and overweight girls with central precocious puberty. Ann Pediatr Endocrinol Metab 22:49–54. https://doi.org/10.6065/apem.2017.22.1.49
Censani M, Feuer A, Orton S, Askin G, Vogiatzi M (2019) Changes in body mass index in children on gonadotropin-releasing hormone agonist therapy with precocious puberty, early puberty or short stature. J Pediatr Endocrinol Metab 32:1065–1070. https://doi.org/10.1515/jpem-2019-0105
Lee SJ, Yang EM, Seo JY, Kim CJ (2012) Effects of gonadotropin-releasing hormone agonist therapy on body mass index and height in girls with central precocious puberty. Chonnam Med J 48:27–31. https://doi.org/10.4068/cmj.2012.48.1.27
Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, Saenger P, Shulman D, Silverman L, Flood L, Gray W, Tierney D (2007) Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab 92:1697–1704. https://doi.org/10.1210/jc.2006-2479
Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, Charmandari E, Lee PA, Freire AV, Ropelato MG, Yazid Jalaludin M, Mbogo J, Kanaka-Gantenbein C, Luo X, Eugster EA, Klein KO, Vogiatzi MG, Reifschneider K, Bamba V, Garcia Rudaz C, Kaplowitz P, Backeljauw P, Allen DB, Palmert MR, Harrington J, Guerra-Junior G, Stanley T, Torres Tamayo M, Miranda Lora AL, Bajpai A, Silverman LA, Miller BS, Dayal A, Horikawa R, Oberfield S, Rogol AD, Tajima T, Popovic J, Witchel SF, Rosenthal SM, Finlayson C, Hannema SE, Castilla-Peon MF, Mericq V, Medina Bravo PG (2019) Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr 91:357–372. https://doi.org/10.1159/000501336
Neely EK, Silverman LA, Geffner ME, Danoff TM, Gould E, Thornton PS (2013) Random unstimulated pediatric luteinizing hormone levels are not reliable in the assessment of pubertal suppression during histrelin implant therapy. Int J Pediatr Endocrinol 2013:20. https://doi.org/10.1186/1687-9856-2013-20
Badaru A, Wilson DM, Bachrach LK, Fechner P, Gandrud LM, Durham E, Wintergerst K, Chi C, Klein KO, Neely EK (2006) Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab 91:1862–1867. https://doi.org/10.1210/jc.2005-1500
Acharya SV, Gopal RA, George J, Bandgar TR, Menon PS, Shah NS (2009) Utility of single luteinizing hormone determination 3 h after depot leuprolide in monitoring therapy of gonadotropin-dependent precocious puberty. Pituitary 12:335–338. https://doi.org/10.1007/s11102-009-0184-0
Demirbilek H, Alikasifoglu A, Gonc NE, Ozon A, Kandemir N (2012) Assessment of gonadotrophin suppression in girls treated with GnRH analogue for central precocious puberty; validity of single luteinizing hormone measurement after leuprolide acetate injection. Clin Endocrinol (Oxf) 76:126–130. https://doi.org/10.1111/j.1365-2265.2011.04185.x
Yüce Ö, Bideci A, Çelik N, Çamurdan O, Cinaz P (2020) Diagnostic value of urinary luteinizing hormone levels in the monitoring of precocious puberty treatment. Arch. Endocrinol Metab 64:121–127. https://doi.org/10.20945/2359-3997000000212
Lim YY, Chan RM, Loke KY, Ho CW, Lee YS (2014) Familial male-limited precocious puberty in neurofibromatosis type I. Eur J Pediatr 173:219–222. https://doi.org/10.1007/s00431-013-2141-1
Almeida MQ, Brito VN, Lins TSS, Guerra-Junior G, Castro M, Antonini SR, Arnhold IJP, Mendonca BB, Latronico AC (2008) Long-term treatment of familial male-limited precocious puberty (testotoxicosis) with cyproterone acetate or ketoconazole. Clin Endocrinol (Oxf) 69:93–98. https://doi.org/10.1111/j.1365-2265.2007.03160.x
Agopiantz M, Journeau P, Lebon-Labich B, Sorlin A, Cuny T, Weryha G, Leheup B (2016) McCune-Albright syndrome, natural history and multidisciplinary management in a series of 14 pediatric cases. Ann Endocrinol (Paris) 77:7–13. https://doi.org/10.1016/j.ando.2016.01.002
Estrada A, Boyce AM, Brillante BA, Guthrie LC, Gafni RI, Collins MT (2016) Long-term outcomes of letrozole treatment for precocious puberty in girls with McCune–Albright syndrome. Eur J Endocrinol 75:477–483. https://doi.org/10.1530/EJE-16-0526
Livadas S, Bothou C (2019) Management of the female with non-classical congenital adrenal hyperplasia (NCCAH): a patient-oriented approach. Front Endocrinol (Lausanne) 10:366. https://doi.org/10.3389/fendo.2019.00366
Sharma B, Singh H, Saran S, Mathur S (2017) Primary hypothyroidism presenting as a pituitary macroadenoma and precocious puberty. Thyroid Res Pract 14:81–85. https://doi.org/10.4103/trp.trp_6_17
Ramos CO, Macedo DB, Bachega TASS, Nascimento ML, Madureira G, Latronico AC, Brito VN, Mendonca BB (2019) Premature pubarche due to exogenous testosterone gel or intense diaper rash prevention cream use: a case series. Horm Res Paediatr 91:411–415. https://doi.org/10.1159/000495664
Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH (2003) Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr 143:60–66. https://doi.org/10.1016/S0022-3476(03)00128-8
Alonso LC, Rosenfield RL (2002) Oestrogens and puberty. Best Pract Res Clin Endocrinol Metab 16:13–30. https://doi.org/10.1053/beem.2002.0177
Feuillan P, Calis K, Hill S, Shawker T, Robey PG (2007) Collins MT (2007) Letrozole treatment of precocious puberty in girls with the McCune-Albright syndrome: a pilot study. J Clin Endocrinol Metab 92:2100–2106. https://doi.org/10.1210/jc.2006-2350
White PC (2018) Update on diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Curr Opin Endocrinol Diabetes Obes 25:178–184. https://doi.org/10.1097/MED.0000000000000402
Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, Arundel P, Shaw N, Pos VD, Underhil A, Portero D, Heral L, Heegaard AM, Masi L, Monsell F, Stanton R, Dijkstra PDS, Brandi ML, Chapurlat R, Hamdy NAT, Collins MT (2019) Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis 14:139. https://doi.org/10.1186/s13023-019-1102-9
Leschek EW, Flor AC, Bryant JC, Jones JV, Barnes KM, Cutler GB Jr (2017) Effect of antiandrogen, aromatase inhibitor, and gonadotropin-releasing hormone analog on adult height in familial male precocious puberty. J Pediatr 190:229–235. https://doi.org/10.1016/j.jpeds.2017.07.047
Leschek EW, Jones J, Barnes KM, Hill SC, Cutler GB Jr (1999) Six-year results of spironolactone and testolactone treatment of familial male-limited precocious puberty with addition of deslorelin after central puberty onset. J Clin Endocrinol Metab 84:175–178. https://doi.org/10.1210/jcem.84.1.5413
Papanikolaou A, Michala L (2015) Autonomous ovarian cysts in prepubertal girls. How aggressive should we be? A review of the literature. J Pediatr Adolesc Gynecol 28:292–296. https://doi.org/10.1016/j.jpag.2015.05.004
Code availability
N/A
Funding
Financial support was provided by the Hospital de Clínicas de Porto Alegre Research and Events Support Fund (FIPE-HCPA) and Medical Science Program: Endocrinology, Universidade Federal do Rio Grande do Sul, Brazil.
Author information
Authors and Affiliations
Contributions
Sandra Pinho Silveiro had the idea for the article. Amanda Veiga Cheuiche and Leticia Guimarães da Silveira performed the literature search and data analysis. Amanda Veiga Cheuiche wrote the first draft of the manuscript. Leila Cristina Pedroso de Paula, Iara Regina Siqueira Lucena, and SandraPinho Silveiro critically revised the work.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
N/A
Consent for publication
The participant’s mother has consented to publishing the photographs.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheuiche, A.V., da Silveira, L.G., de Paula, L.C.P. et al. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr 180, 3073–3087 (2021). https://doi.org/10.1007/s00431-021-04022-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04022-1