Abstract
Central precocious puberty (CPP) is characterized by the same biochemical and physical features as normally timed puberty but occurs at an abnormally early age. Most cases of CPP are seen in girls, in whom it is usually idiopathic. In contrast, ~50 % of boys with CPP have an identifiable cause. The diagnosis of CPP relies on clinical, biochemical, and radiographic features. Untreated, CPP has the potential to result in early epiphyseal fusion and a significant compromise in adult height. Thus, the main goal of therapy is preservation of height potential. The gold-standard treatment for CPP is gonadotropin-releasing hormone (GnRH) analogs (GnRHa). Numerous preparations with a range of delivery systems and durations of action are commercially available. While the outcomes of patients treated for CPP have generally been favorable, more research about the psychological aspects, optimal monitoring, and long-term effects of all forms of GnRHa treatment is needed. Several potential therapeutic alternatives to GnRHa exist and await additional investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Molecular genetic etiologies of central precocious puberty (CPP) are beginning to be elucidated. |
Evaluation of CPP should be based on a combination of clinical and biochemical factors, as each parameter has specific limitations. |
The gold-standard treatment for CPP is gonadotropin-releasing hormone (GnRH) analogs (GnRHa). |
GnRHa provide sustained high concentrations of GnRH, resulting in downregulation of the hypothalamic–pituitary–gonadal axis. |
Multiple formulations of GnRHa exist. Although minor differences in gonadotropin levels are observed, all available GnRHa appear to be equally effective in terms of clinical parameters. |
Long-term outcomes of children treated with GnRHa for CPP are reassuring with regard to fertility, body mass index, and bone mineral density. |
1 Introduction
Central precocious puberty (CPP) refers to premature activation of the hypothalamic–pituitary–gonadal (HPG) axis, resulting in early development of secondary sexual characteristics. Although the exact threshold defining “normal” pubertal timing has been disputed, commonly used cutoffs to define CPP are 8 years of age for females (7.5 years for Hispanics and African Americans) and 9 years of age for males [1]. The earliest clinical manifestation of central puberty in girls is usually breast development (thelarche), followed by pubic hair (pubarche). The pubertal growth spurt typically occurs during Tanner stage II–III, with the first menstrual period, known as menarche, usually occurring at Tanner stage IV. In boys, the initial clinical sign of central puberty is testicular enlargement and the pubertal growth spurt happens later than in girls [2, 3].
Although the precise mechanisms triggering the onset of puberty are unclear, the earliest known biochemical change during puberty is increased production of kisspeptin in the hypothalamus. While kisspeptin itself has several proposed stimulatory and inhibitory signals, which have not yet been clearly elucidated, it has been shown that increased kisspeptin production results in increased gonadotropin-releasing hormone (GnRH) release. Thus, a rise in kisspeptin is widely acknowledged as the seminal event that initiates HPG axis activation during puberty [2]. Inhibition of the GnRH pulse generator decreases first during sleep, resulting in an increase of nighttime luteinizing hormone (LH) pulse amplitude during early and mid-puberty. As puberty progresses, LH pulse amplitude increases during daytime hours as well, and estrogen and testosterone levels rise accordingly.
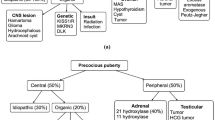
2 Etiology
CPP, for unknown reasons, is found predominantly in girls. In an observational study of the incidence of CPP in Spain, females were approximately ten times more likely to be affected than males [4], and other sources have cited a female-to-male ratio as high as 20:1 [5]. In addition, the etiology of CPP differs between the genders. While the majority of girls will have idiopathic CPP, boys are more likely to have a pathological source [1, 6]. Risk factors for CPP include a history of international adoption, as well as congenital or acquired central nervous system insults, such as hypothalamic hamartoma, septo-optic dysplasia, tumor, trauma, infection, or ischemia. Several genetic syndromes, including neurofibromatosis type 1, tuberous sclerosis, and Sturge–Weber syndrome, are associated with CPP [2]. Apart from recognized genetic syndromes, anywhere from 5.2 to 27.5 % of cases have been reported to be familial [7, 8].
Specific genetic causes of CPP have been described relatively recently. A substitution mutation in the G-protein coupled kisspeptin receptor gene KISS1R (formerly known as GPR54) was found in a patient with CPP and was associated with delayed degradation of the ligand–receptor complex within the cell membrane. This was further linked to an extended period of downstream signaling, postulated to result in increased amplitude of GnRH pulsatility [9]. An additional KISS1R polymorphism in the promoter region has been described in Chinese girls with CPP, though a detailed knowledge of whether or how this variant impacts the expression or function of the gene is as yet unknown [10].
A mutation in KISS1, encoding the ligand kisspeptin, has also been described within an amino-terminal sequence associated with protein degradation [11]. The mutated ligand–receptor complex similarly demonstrates resistance to degradation. However, the low population frequency associated with this mutation suggests that it is a relatively uncommon cause of CPP.
More recently, ten separate heterozygous mutations in MKRN3, encoding makorin RING-finger protein 3, have been found in association with both sporadic and familial CPP [12–14]. MKRN3 is a paternally expressed imprinted gene located within the region typically affected in Prader–Willi syndrome. Although the exact function of MKRN3 in humans is as yet unknown, studies in mice have illustrated that mkrn3 mRNA is expressed in the hypothalamic arcuate nucleus, and that a decline in mkrn3 expression is temporally correlated with the rise in kiss1 expression. Other studies have postulated that downregulation of MKRN3 is permissive for increased GnRH pulses during puberty [13]. Thus, deficiency of this protein would be expected to result in a loss of inhibition of HPG axis activation. These mutations are thought to result in loss of function of the abnormal gene product. In familial cases, all affected subjects have inherited mutations from their fathers. Interestingly, there was an almost equal gender distribution of CPP among affected family members [12].
Other molecular defects have been identified with less clear or weaker associations. These include single nucleotide polymorphisms (SNPs) in the FSHB gene and the LHB gene, though the resulting molecular mechanisms that cause CPP have not been identified [15]. Mutations in the Y1 subtype receptor for neuropeptide Y (NPY) could theoretically cause precocious puberty, as NPY is thought to be an inhibitor of pulsatile GnRH secretion. However, the only currently described mutation has not correlated well with an effect on function or with CPP [16]. Additional studies have investigated genes involved in hypothalamic hamartomas and have identified several with increased expression in patients with CPP [17]. LIN28B, which is postulated to have a role in determining the timing of pubertal development, has also been proposed as a genetic target in CPP. However, its exact role in humans is not yet clear. In addition, study findings have been contradictory, and no clinically significant mutations have yet been observed that cause a functional deficit at a molecular level [18, 19].
3 Diagnosis
3.1 Clinical Features
On initial examination of the child with CPP, bilateral testicular enlargement (≥4 cc in volume) will be apparent in males, in contrast to patients with peripheral forms of precocious puberty. Girls usually present with both breast development and pubic hair, in contrast to nonpathological entities such as premature thelarche or premature adrenarche. Other signs of pathological precocious puberty include a rapid tempo of progression and linear growth acceleration. Bone age will typically be advanced, though this is certainly not exclusive to CPP and may be seen to a milder degree in numerous other conditions [2].
3.2 Biochemical Features
A GnRH stimulation test has long been considered the gold standard for the diagnosis of CPP. However, lack of availability of synthetic GnRH in the USA has led to the use of GnRH analogs (GnRHa) for this purpose instead. While precise cutoffs are difficult to establish, a peak stimulated LH of >~8 mIU/mL after GnRH and >~5 IU/L after GnRHa are considered indicative of CPP [2, 20]. An LH/FSH [luteinizing hormone/follicle-stimulating hormone] ratio of ≥2 is also consistent with CPP. However, the results should always be interpreted in light of the specific assay performed and the available sensitivity limits. An alternative diagnostic approach has been measurement of basal ultrasensitive LH, which is typically <0.3 IU/L in prepubertal children. However, basal ultrasensitive LH is often prepubertal in early CPP and thus may be falsely reassuring [1]. Measurement of basal or stimulated sex steroids, while never sufficient alone, can be helpful in evaluation of suspected CPP. This is particularly true of testosterone, whereas random estradiol levels are often unmeasurable even when advanced pubertal development is present. Even if the laboratory evaluation is unremarkable, patients should continue to be monitored over time and retested as indicated if clinical suspicion is high [1, 2, 20].
4 Imaging
Pelvic ultrasound has been found to be a useful adjunct to support the diagnosis of CPP over other forms of puberty in girls, especially in equivocal situations. Uterine and ovarian dimensions have a stronger association with bone age than with chronological age and are correlated with CPP up to the age of 8 years [21]. While proposed cutoffs for uterine and ovarian volumes exist, these have been somewhat variable, and other studies have suggested a considerable overlap between patients with and without CPP, making reliable parameters difficult to establish. For those who present for evaluation after the age of 8 years, ultrasound parameters become even more difficult to interpret, as there is an even greater overlap in uterine and ovarian dimensions between prepubertal and early pubertal girls [21–24]. The finding of small ovarian follicles on a pelvic ultrasound is normal even in prepubertal girls [25]. Clinicians should further keep in mind that ultrasound results may be technician dependent.
The role of brain magnetic resonance imaging (MRI) in the evaluation of patients with CPP has been debated. Boys are more likely to have a pathological cause, making diagnostic imaging for intracranial pathology an essential tool in their evaluation [6]. However, controversy exists regarding recommendations in girls. When female CPP patients without neurological symptoms are screened with MRI, the incidence of positive findings is approximately 15 % [26, 27]. However, some of the abnormalities that are found may be incidental and unrelated to the CPP. In one study, 86 % of 182 girls had normal MRIs, 11 % had mild abnormalities believed to be unrelated to CPP, and 3 % had hamartomas, leading the authors to conclude that routine screening was not indicated in this population, particularly in girls older than 6 years [26]. This is in contrast to a prior study of 67 girls, in whom six of ten with MRI findings had hamartomas, while the remainder were diagnosed with an astrocytoma, teratoma, arachnoid cyst, and pineal cyst. Three of the ten had lesions requiring surgical intervention, leading the authors to conclude that MRI should be part of routine evaluation in CPP regardless of age [27]. Investigations into clinical and biochemical features of patients with intracranial pathology have suggested that younger age at onset, more rapid tempo, and higher levels of sex steroids or gonadotropins are predictive features. However, these overlap to such an extent that no specific cutoff has been identified that can be used to determine whether or not to obtain an MRI in any individual patient. For this reason, many institutions include a brain MRI as a universal part of the evaluation in all children diagnosed with CPP [26–29].
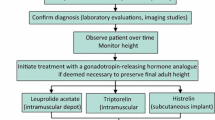
5 Treatment
The primary goal of CPP treatment is to preserve final adult height. However, it should be recognized that some patients will have a nonprogressive or slowly progressive form of CPP, and these patients can achieve normal adult height without any intervention [20]. Therefore, a period of observation is usually appropriate prior to starting treatment. In patients who do show progression of CPP, there is significant variability in the degree of height gained after discontinuation of treatment, even among patients with the same bone age [30–32]. Numerous studies have demonstrated that the greatest gain in final height is achieved in girls with onset of puberty before 6 years of age, although girls with onset between 6 and 8 years of age may still reap some benefit from treatment. In contrast, girls aged ≥8 years have not been found to benefit from intervention in terms of height. Thus, treatment in this age group is usually not indicated. An additional issue is that outcomes of treatment are typically defined as the difference between predicted adult height at baseline and the actual height achieved. Unfortunately, height prediction methods are notoriously flawed [33] and have often been found to overpredict height in the setting of early puberty. Therefore, it is impossible to predict the precise amount of additional height that will be gained by an individual patient as a result of putting puberty on hold. While preliminary evidence suggests that electronic methods of bone age assessment may be more accurate, there is minimal information available thus far about their use in precocious puberty [34]. Evidence regarding treatment benefit in males is more limited, as they comprise a relatively small proportion of patients with CPP. The existing data suggest a significant improvement in final height after treatment of CPP in boys [35], though the same measurement and prediction limitations exist.
Concerns about psychosocial functioning are often used as a justification for treatment of CPP. However, the existing data regarding the psychological aspects of CPP are limited and inconsistent. Insufficient controls and methodological problems render many studies difficult to interpret, compounded by the use of several different assessments, which make comparisons difficult. The current data do not consistently support problems in regard to body image, self-esteem, or sexual behavior in patients with CPP. Differences, where found, tend to be modest and suggest that patients with CPP may engage in psychosexual behaviors only slightly earlier than children with on-time puberty. The prevalence of psychopathology does not seem to differ from that in the general population [36]. Similarly, one study of girls with CPP and their mothers at the time of diagnosis found no difference in psychological distress as compared with girls who had early normal puberty, even prior to treatment [37]. At this point, there is no consensus regarding whether CPP is associated with psychological distress and/or whether treatment ameliorates these problems, and more data in this area are needed [20].
GnRHa are well established as a standard of care for the treatment of CPP worldwide. While numerous delivery systems and routes of administration exist, depot intramuscular injections or sustained-release preparations have been most widely used. These drugs are believed to work by providing a steady concentration of GnRH activity instead of the pulsatile variation in levels characteristic of native GnRH release, which results in paradoxical downregulation and suppression of the HPG axis. Monthly depot leuprolide acetate has been the most common form of injection therapy used in the USA. Although extended-release 3-monthly depot leuprolide preparations have been available in Europe and elsewhere for many years, they have been approved by the US Food and Drug Administration (FDA) only recently and are available in 11.25 and 30 mg dosage forms. Although patients on 11.25 mg 3-monthly injections have consistently been shown to have higher stimulated LH and FSH levels than patients receiving 7.5 mg monthly injections or 22.5 mg 3-monthly injections, this has not been accompanied by significant differences in sex steroid levels or clinical parameters [38, 39]. Additional information about these preparations has been derived from a phase III open-label study involving patients receiving 3-monthly depot leuprolide acetate at 11.25 and 30 mg doses for 36 months [40]. Of 72 patients, only two discontinued therapy prior to 36 months because of treatment failure, while 20 discontinued therapy to undergo age-appropriate puberty and 24 continued to receive 3-monthly depot leuprolide for the full study period. As in previous studies, LH escape was seen in a minority of patients on stimulation testing, but this did not correlate with clinical features suggesting lack of suppression. Thus, 3-monthly depot leuprolide seems to be both safe and effective for long-term use [38–40].
Adverse effects are similar for 1- and 3-monthly depot injections and include local reactions and pain at the injection site. Sterile abscess formation has been reported after administration of long-acting injection formulations. Although children who experience sterile abscess formation from long-acting preparations have subsequently been treated successfully with daily leuprolide, there are reported cases in the adult literature of resistance to GnRHa following sterile abscesses [41].
A popular alternative approach to depot GnRHa injections is the histrelin implant, which was approved by the FDA in 2007. This nonbiodegradable implant is made of a flexible hydrogel containing 50 mg of the potent GnRHa histrelin and is placed subcutaneously, usually in the inner aspect of the upper arm. The initial histrelin implant was first developed for treatment of metastatic prostate cancer, where it was found to successfully suppress LH and testosterone levels for up to 1 year. The implant was later reformulated to release histrelin at a higher dose of 65 μg/day for use in children with CPP. An initial pilot study in 11 girls previously treated with depot triptorelin showed satisfactory maintenance of LH and FSH suppression on stimulation tests. This was accompanied by clinical evidence of pubertal suppression, including regression of breast development, a decrease in growth velocity, and a decline in bone age advancement over 15 months. In addition to satisfactory clinical benefit, parents reported less discomfort and lifestyle interference overall than with monthly injections [42]. Following this initial report, a phase III study in 36 patients with CPP demonstrated profound suppression of the HPG axis within 1 month of implantation whether subjects were naïve or previously treated with a GnRHa [43]. The long-term extension phase of this study has now been completed and demonstrated significant improvements in predicted adult height after up to 6 years of sequential annual histrelin implants [44]. Reassuringly, body mass index (BMI) z-scores remained normal throughout the treatment interval.
A significant refinement of the histrelin implant as a therapeutic option has been the recognition that a single implant lasts at least 2 years. Given the known rate of release of 65 mcg of histrelin per day, a 50 mg implant should theoretically last 2 years. That this is indeed the case was demonstrated in a prospective study in 33 children with CPP in whom a single implant was left in place for 2 years. Peak stimulated LH levels at 12 and 24 months were equivalent, and clinical indices of CPP improved progressively. Use of a single implant for 2 years has the potential to significantly decrease costs and numbers of surgical procedures in children treated with this modality [45].
The most common adverse event associated with the histrelin implant is breakage and/or difficulty with localization of the device. These events occur only during explantation and have been noted to take place in 15–39 % of procedures, with a higher likelihood of breakage when the implant is left in place for 2 years [44–47]. Additional reported adverse events include local reactions, which are, for the most part, minor and self-limited. Sterile abscess formation [41], keloids [44], site infection [45], and implant extrusion [42] have rarely been reported. Placement and removal of the implant requires a minor surgical procedure. This is typically accomplished in an outpatient setting, using local anesthesia with the addition of conscious sedation if necessary [47]. In rare cases of difficulty with implant localization, ultrasound has proved to be a useful modality. Characteristics of the most frequently used GnRHa are summarized in Table 1.
6 Adjunctive Treatments
6.1 Nonaromatizable Anabolic Steroids
Oxandrolone has been used to improve growth in patients for other indications. The exact mechanism has not been elucidated, but a stimulatory effect on the growth plate has been postulated. A small nonrandomized study of ten patients receiving oxandrolone in addition to GnRHa for severe deceleration of growth velocity during treatment for CPP suggested that this combination might improve adult height, compared with GnRHa alone. However, larger randomized studies have not been performed [48].
6.2 Growth Hormone
Small and nonrandomized studies have demonstrated a significant improvement in final adult height over pretreated predicted adult height in patients treated with GnRHa and growth hormone (GH) as compared with patients treated with GnRHa alone. However, larger randomized studies are currently lacking, and routine use of GH in this setting is not recommended [49].
6.3 Aromatase Inhibitors
Aromatase inhibitors have the potential to attenuate estrogenic effects on skeletal maturation and to delay epiphyseal fusion. A small randomized study suggested slower bone age advancement and improved adult height in Chinese boys with CPP receiving letrozole [50]. However, in general, the experience with these compounds in CPP has been very limited.
7 Monitoring
Children who are being treated for CPP should receive regular follow-up during which pubertal progression or suppression can be followed and documented. Tanner staging, determination of growth velocity, and assessment of skeletal maturation via bone age radiographs are all important indices of suppression. Whether laboratory testing should be routinely included during follow-up is controversial. While several different strategies for biochemical testing exist, no gold standard for how best to monitor children undergoing treatment for CPP has been established. A GnRH- or leuprolide-stimulated peak LH should be <4 IU/L in adequately suppressed children, and random serum gonadotropin levels should theoretically be in the prepubertal range (ultrasensitive LH <0.3 IU/L). However, random ultrasensitive LH levels have been noted to be elevated above prepubertal levels in children who are well suppressed on GnRHa therapy across all forms of treatment, including the histrelin implant [51, 52]. Therefore, the utility of measuring random LH levels in children undergoing treatment for CPP is highly questionable.
8 Resumption of the Native Hypothalamic–Pituitary–Gonadal Axis
In studies following patients beyond discontinuation of treatment, the mean time from cessation of injectable depot GnRHa to menses has been found to be 1.5 ± 0.5 years. Some studies have found a slightly shorter time to menses in girls who experienced menarche before treatment than in those who did not. Although less is known about boys, the existing data suggest that clinicians can expect advancement of the Tanner stage within 6 months of discontinuation of treatment [52].
Because use of the histrelin implant is more recent, the data are somewhat more limited but thus far seem to indicate similar results, with the average time from explantation to menarche being 12.75 (95 % confidence interval 9.6–15.9) months, with a range of 2–36 months. Likewise, in males, resumption of pubertal progression was seen on examination within 1 year. A negative trend has been noted between the total duration of GnRHa therapy and the time to menarche, whereas the age at explantation and the time to menarche were significantly inversely correlated [53].
9 Outcomes
Girls with CPP have been found to have a higher BMI than their peers at diagnosis. However, this observation is confounded by the natural increase in BMI during puberty. Indeed, some authors have found that while BMI increases in general during treatment, the overall BMI standard deviation score (SDS) does not change. When BMI is evaluated after GnRHa treatment has been completed, there does not appear to be an adverse effect of treatment on BMI in girls with CPP, nor does there appear to be a large impact of CPP itself on BMI at adult height [31, 54, 55].
Results regarding the incidence of polycystic ovary syndrome (PCOS) in GnRHa-treated patients have been quite variable and contradictory, with some authors finding markedly increased rates of PCOS and other authors finding little or no difference. These results are even more difficult to interpret, as multiple criteria for the diagnosis of PCOS exist. Currently, no consensus exists on whether CPP or treatment with GnRHa results in an increased risk of PCOS [2, 54, 56].
Although bone mineral density (BMD) has been seen to be slightly reduced during treatment in girls, these changes do not appear to be sustained. This decrease is thought to be secondary to suppression of ovarian function. However, after treatment is discontinued and ovarian activity resumes, BMD is regained, and so girls are not significantly different from their peers without CPP, according to evaluation at adult height [31].
10 Reproductive Function and Fertility
Limited information exists regarding the long-term effects of treatment for CPP on endocrine and reproductive function. In one study of 49 females receiving monthly depot leuprolide, 20 were followed to adulthood (age 18–26 years). Of these, 80 % reported regular menstrual cycles. Seven of 20 women reported a total of 12 pregnancies, with six live births, five spontaneous or elective terminations, one ongoing pregnancy, and no reports of stillbirth [52]. Though achievement of short-term treatment goals and resumption of puberty seem to be similar in girls treated with 3-monthly leuprolide and the histrelin implant, it remains to be seen whether similar long-term results can be expected. In addition, long-term data are notably lacking for all forms of treatment with regard to fertility and endocrine function beyond the third decade, as well as the timing of menopause.
11 Future Directions
Although multiple preparations now exist for GnRHa treatment of CPP, further options are under investigation or may be considered. A 6-month formulation of triptorelin, for example, is currently under investigation, providing the potential for even less frequent dosing for those who do not wish to undergo a procedure for the histrelin implant [57].
Other targets for therapy could also be considered. Because GnRH agonists work by stimulation of receptors, leading to desensitization, there is an initial period of increased stimulation, leading to an LH flare, which sometimes precipitates vaginal bleeding in girls with advanced pubertal development before suppression takes place. A GnRH antagonist, however, would theoretically forego this initial phase by disrupting LH pulsatility without an initial flare. Kisspeptin agonists and antagonists, by acting upstream of GnRH, would be expected to have effects similar to those of GnRH agonist and antagonist therapies, respectively. However, since kisspeptin analogs would not work directly at the gonadotropin receptor, they would have the additional theoretical benefit of interrupting pulsatile GnRH and gonadotropin secretion without lowering gonadotropin release below basal secretory levels. Therefore, sex steroid levels under treatment with these agents could be expected to more closely mimic normal physiology [58]. Though use of kisspeptin antagonists in humans has not yet been studied, animal studies have suggested that kisspeptin analogs are able to cross the blood–brain barrier and suppress puberty [58].
Because existing biochemical markers can be unreliable, monitoring of treatment is also a worthwhile area of research. Markers under investigation include free alpha-subunit (FAS), which rises with suppression of the HPG axis. Though GnRHa levels decrease gradually with discontinuation of depot intramuscular injections, FAS levels are seen to acutely decrease within days of histrelin implant removal, preceding LH, FSH, and estradiol rises by weeks. In one case, elevated FAS levels beyond the expected time period were attributable to retained histrelin implant fragments and fell only after all fragments had been removed, highlighting the utility of FAS as a target for relatively rapid assessment of HPG axis recovery. A short “rebound” elevation in FAS can be seen in patients 3–8 weeks after histrelin implant removal. This effect is short lived and self limited, although the reasons for it are unclear [59].
12 Conclusion
CPP is seen most often in girls and is associated with a multitude of conditions. A substantial proportion (over a quarter) of cases are familial, and genetic causes have begun to be elucidated. The diagnosis is based on a combination of clinical and biochemical factors. Treatment with a GnRHa provides the greatest potential benefit for patients who are younger at the time of onset of CPP. Multiple treatment options are available, and more recent options have the benefit of less frequent dosing, with potential for improved compliance. Though several adjunctive treatments have been proposed, evidence supporting these treatments is generally sparse in CPP, and thus they cannot be recommended for routine use. Biochemical markers, bone age, and growth velocity should be followed during treatment to ensure efficacy. The available evidence shows that GnRHa are safe and effective, and long-term data suggest that reproductive function is satisfactory after discontinuation of treatment. However, long-term data, particularly regarding the newer formulations, are still lacking. Continued pharmacological and molecular genetic investigation and rigorously conducted prospective studies will continue to enhance knowledge and optimize treatment in children with CPP.
References
Nebesio TD, Eugster EA. Current concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc Health Care. 2007;37(2):50–72. doi:10.1016/j.cppeds.2006.10.005.
Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013;98(6):2198–207. doi:10.1210/jc.2013-1024.
Tanner JM, Davies PSW. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–29. doi:10.1016/s0022-3476(85)80501-1.
Soriano-Guillen L, Corripio R, Labarta JI, Canete R, Castro-Feijoo L, Espino R, et al. Central precocious puberty in children living in Spain: incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab. 2010;95(9):4305–13. doi:10.1210/jc.2010-1025.
Lee PA, Neely EK, Fuqua J, Yang D, Larsen LM, Mattia-Goldberg C, et al. Efficacy of leuprolide acetate 1-month depot for central precocious puberty (CPP): growth outcomes during a prospective, longitudinal study. Int J Pediatr Endocrinol. 2011;2011(1):7. doi:10.1186/1687-9856-2011-7.
Choi KH, Chung SJ, Kang MJ, Yoon JY, Lee JE, Lee YA, et al. Boys with precocious or early puberty: incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann Pediatr Endocrinol Metab. 2013;18(4):183–90. doi:10.6065/apem.2013.18.4.183.
de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89(4):1794–800. doi:10.1210/jc.2003-030361.
Rohn R, Rousonelos G. Familial sexual precocity. Am J Dis Child. 1986;140(8):741–2. doi:10.1001/archpedi.1986.02140220023017.
Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–15. doi:10.1056/NEJMoa073443.
Luan X, Yu H, Wei X, Zhou Y, Wang W, Li P, et al. GPR54 polymorphisms in Chinese girls with central precocious puberty. Neuroendocrinology. 2007;86(2):77–83. doi:10.1159/000107511.
Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276–80. doi:10.1210/jc.2009-2421.
Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–75. doi:10.1056/NEJMoa1302160.
Macedo DB, Abreu AP, Reis AC, Montenegro LR, Dauber A, Beneduzzi D, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99(6):E1097–103. doi:10.1210/jc.2013-3126.
Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab. 2014;99(4):E647–51. doi:10.1210/jc.2013-4084.
Zhao Y, Chen T, Zhou Y, Li K, Xiao J. An association study between the genetic polymorphisms within GnRHI, LHbeta and FSHbeta genes and central precocious puberty in Chinese girls. Neurosci Lett. 2010;486(3):188–92. doi:10.1016/j.neulet.2010.09.049.
Freitas KC, Ryan G, Brito VN, Tao YX, Costa EM, Mendonca BB, et al. Molecular analysis of the neuropeptide Y1 receptor gene in human idiopathic gonadotropin-dependent precocious puberty and isolated hypogonadotropic hypogonadism. Fertil Steril. 2007;87(3):627–34. doi:10.1016/j.fertnstert.2006.07.1519.
Parent AS, Matagne V, Westphal M, Heger S, Ojeda S, Jung H. Gene expression profiling of hypothalamic hamartomas: a search for genes associated with central precocious puberty. Horm Res. 2008;69(2):114–23. doi:10.1159/000111815.
Park SW, Lee ST, Sohn YB, Cho SY, Kim SH, Kim SJ, et al. LIN28B polymorphisms are associated with central precocious puberty and early puberty in girls. Korean J Pediatr. 2012;55(10):388–92. doi:10.3345/kjp.2012.55.10.388.
Silveira-Neto AP, Leal LF, Emerman AB, Henderson KD, Piskounova E, Henderson BE, et al. Absence of functional LIN28B mutations in a large cohort of patients with idiopathic central precocious puberty. Horm Res Paediatr. 2012;78(3):144–50. doi:10.1159/000342212.
Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Group E-LGACC, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123(4):e752–62. doi:10.1542/peds.2008-1783.
Eksioglu AS, Yilmaz S, Cetinkaya S, Cinar G, Yildiz YT, Aycan Z. Value of pelvic sonography in the diagnosis of various forms of precocious puberty in girls. J Clin Ultrasound. 2013;41(2):84–93. doi:10.1002/jcu.22004.
Badouraki M, Christoforidis A, Economou I, Dimitriadis AS, Katzos G. Evaluation of pelvic ultrasonography in the diagnosis and differentiation of various forms of sexual precocity in girls. Ultrasound Obstetr Gynecol. 2008;32(6):819–27. doi:10.1002/uog.6148.
de Vries L, Horev G, Schwartz M, Phillip M. Ultrasonographic and clinical parameters for early differentiation between precocious puberty and premature thelarche. Eur J Endocrinol. 2006;154(6):891–8. doi:10.1530/eje.1.02151.
Herter LD, Golendziner E, Flores JAM, Moretto M, Di Domenico K, Becker E, et al. Ovarian and uterine findings in pelvic sonography: comparison between prepubertal girls, girls with isolated thelarche, and girls with central precocious puberty. J Ultrasound Med. 2002;21(11):1237–46.
Pienkowski C, Cartault A, Carfagna L, Ernoult P, Vial J, Lemasson F, et al. Ovarian cysts in prepubertal girls. Endocr Dev. 2012;22:101–11. doi:10.1159/000326627.
Pedicelli S, Alessio P, Scire G, Cappa M, Cianfarani S. Routine screening by brain magnetic resonance imaging is not indicated in every girl with onset of puberty between the ages of 6 and 8 years. J Clin Endocrinol Metab. 2014;99(12):4455–61. doi:10.1210/jc.2014-2702.
Ng SM. Cranial MRI scans are indicated in all girls with central precocious puberty. Arch Dis Child. 2003;88(5):414–8. doi:10.1136/adc.88.5.414.
Chalumeau M, Hadjiathanasiou CG, Ng SM, Cassio A, Mul D, Cisternino M, et al. Selecting girls with precocious puberty for brain imaging: validation of European evidence-based diagnosis rule. J Pediatr. 2003;143(4):445–50. doi:10.1067/s0022-3476(03)00328-7.
Mogensen SS, Aksglaede L, Mouritsen A, Sorensen K, Main KM, Gideon P, et al. Pathological and incidental findings on brain MRI in a single-center study of 229 consecutive girls with early or precocious puberty. PloS One. 2012;7(1):e29829. doi:10.1371/journal.pone.0029829.
Carel JC, Roger M, Ispas S, Tondu F, Lahlou N, Blumberg J, et al. Final height after long-term treatment with triptorelin slow release for central precocious puberty: importance of statural growth after interruption of treatment. French Study Group of Decapeptyl in Precocious Puberty. J Clin Endocrinol Metab. 1999;84(6):1973–8. doi:10.1210/jcem.84.6.5647.
Pasquino AM, Pucarelli I, Accardo F, Demiraj V, Segni M, Di Nardo R. Long-term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogs: impact on adult height, body mass index, bone mineral content, and reproductive function. J Clin Endocrinol Metab. 2008;93(1):190–5. doi:10.1210/jc.2007-1216.
Lazar L, Padoa A, Phillip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007;92(9):3483–9. doi:10.1210/jc.2007-0321.
Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004;10(2):135–47. doi:10.1093/humupd/dmh012.
Thodberg HH. Clinical review: an automated method for determination of bone age. J Clin Endocrinol Metab. 2009;94(7):2239–44. doi:10.1210/jc.2008-2474.
Mul D, Bertelloni S, Carel JC, Saggese G, Chaussain JL, Oostdijk W. Effect of gonadotropin-releasing hormone agonist treatment in boys with central precocious puberty: final height results. Horm Res. 2002;58(1):1–7. doi:10.1159/000063209.
Walvoord EC, Mazur T. Behavioral problems and idiopathic central precocious puberty: fact or fiction? Pediatr Endocrinol Rev. 2007;4(S3):306–12.
Schoelwer MJ, Donahue KL, Bryk K, Didrick P, Berenbaum SA, Eugster EA. Psychological assessment of mothers and their daughters at the time of diagnosis of precocious puberty. Int J Pediatr Endocrinol. 2015;2015(1):5. doi:10.1186/s13633-015-0001-7.
Badaru A, Wilson DM, Bachrach LK, Fechner P, Gandrud LM, Durham E, et al. Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab. 2006;91(5):1862–7. doi:10.1210/jc.2005-1500.
Fuld K, Chi C, Neely EK. A randomized trial of 1- and 3-month depot leuprolide doses in the treatment of central precocious puberty. J Pediatr. 2011;159(6):982–7 e1. doi:10.1016/j.jpeds.2011.05.036.
Lee PA, Klein K, Mauras N, Lev-Vaisler T, Bacher P. 36-month treatment experience of two doses of leuprolide acetate 3-month depot for children with central precocious puberty. J Clin Endocrinol Metab. 2014;99(9):3153–9. doi:10.1210/jc.2013-4471.
Miller BS, Shukla AR. Sterile abscess formation in response to two separate branded long-acting gonadotropin-releasing hormone agonists. Clin Ther. 2010;32(10):1749–51. doi:10.1016/j.clinthera.2010.09.009.
Hirsch HJ, Gillis D, Strich D, Chertin B, Farkas A, Lindenberg T, et al. The histrelin implant: a novel treatment for central precocious puberty. Pediatrics. 2005;116(6):e798–802. doi:10.1542/peds.2005-0538.
Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. 2007;92(5):1697–704. doi:10.1210/jc.2006-2479.
Silverman LA, Neely EK, Kletter GB, Lewis K, Chitra S, Terleckyj O, Eugster EA. Long-term continuous suppression with once-yearly histrelin subcutaneous implants for the treatment of central precocious puberty: a final report of a phase 3 multicenter trial. J Clin Endocrinol Metab (Epub 2015 Mar 24).
Lewis KA, Goldyn AK, West KW, Eugster EA. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr. 2013;163(4):1214–6. doi:10.1016/j.jpeds.2013.05.033.
Rahhal S, Clarke WL, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Results of a second year of therapy with the 12-month histrelin implant for the treatment of central precocious puberty. Int J Pediatr Endocrinol. 2009;2009:812517. doi:10.1155/2009/812517.
Davis JS, Alkhoury F, Burnweit C. Surgical and anesthetic considerations in histrelin capsule implantation for the treatment of precocious puberty. J Pediatr Surg. 2014;49(5):807–10. doi:10.1016/j.jpedsurg.2014.02.067.
Vottero A, Pedori S, Verna M, Pagano B, Cappa M, Loche S, et al. Final height in girls with central idiopathic precocious puberty treated with gonadotropin-releasing hormone analog and oxandrolone. J Clin Endocrinol Metab. 2006;91(4):1284–7. doi:10.1210/jc.2005-1693.
Pucarelli I, Segni M, Ortore M, Arcadi E, Pasquino AM. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab. 2003;16(7):1005–10.
Zhao X, Zhang Q. Clinical efficacy of letrozole in boys with idiopathic central precocious puberty. Chin J Contemp Pediatr. 2014;16(4):397–400. doi:10.7499/j.issn.1008-8830.2014.04.018.
Lewis KA, Eugster EA. Random luteinizing hormone often remains pubertal in children treated with the histrelin implant for central precocious puberty. J Pediatr. 2013;162(3):562–5. doi:10.1016/j.jpeds.2012.08.038.
Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, Mattia-Goldberg C, et al. Leuprolide acetate 1-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010;2010:398639. doi:10.1155/2010/398639.
Fisher MM, Lemay D, Eugster EA. Resumption of puberty in girls and boys following removal of the histrelin implant. J Pediatr. 2014;164(4):912–6 e1. doi:10.1016/j.jpeds.2013.12.009.
Magiakou MA, Manousaki D, Papadaki M, Hadjidakis D, Levidou G, Vakaki M, et al. The efficacy and safety of gonadotropin-releasing hormone analog treatment in childhood and adolescence: a single center, long-term follow-up study. J Clin Endocrinol Metab. 2010;95(1):109–17. doi:10.1210/jc.2009-0793.
Poomthavorn P, Suphasit R, Mahachoklertwattana P. Adult height, body mass index and time of menarche of girls with idiopathic central precocious puberty after gonadotropin-releasing hormone analogue treatment. Gynecol Endocrinol. 2011;27(8):524–8. doi:10.3109/09513590.2010.507289.
Franceschi R, Gaudino R, Marcolongo A, Gallo MC, Rossi L, Antoniazzi F, et al. Prevalence of polycystic ovary syndrome in young women who had idiopathic central precocious puberty. Fertil Steril. 2010;93(4):1185–91. doi:10.1016/j.fertnstert.2008.11.016.
Dajani T, Reiner B, Salem G, Shea H, Rappaport M, Alzohaili O, Van Meter Q, Domek D, Bethin K, Kaplowitz P, Klein K, Merritt D, Rose S, Kletter G, Aisenberg J, Brenner D, Rogers D, Silverman L, Lee P, Gomez R, Cassorla F, Yang J, Eugster E, Flores O, Wright N. Efficacy, safety, and pharmacokinetics (PK) of triptorelin 6-month formulation in patients with central precocious puberty. In: ClinicalTrials.gov [Internet]. National Library of Medicine (US), Bethesda (MD). 2014. https://clinicaltrials.gov/ct2/show/NCT01467882.
Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–316. doi:10.1152/physrev.00037.2010.
Hirsch HJ, Lahlou N, Gillis D, Strich D, Rosenberg-Hagen B, Chertin B, et al. Free alpha-subunit is the most sensitive marker of gonadotropin recovery after treatment of central precocious puberty with the histrelin implant. J Clin Endocrinol Metab. 2010;95(6):2841–4. doi:10.1210/jc.2009-2078.
Acknowledgments
None.
Conflict of interest
Dr. Chen has no conflicts of interest to disclose. Dr. Eugster participates in clinical trials investigating treatment of CPP, funded by Endo Pharmaceuticals. No sources of funding were used to support the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, M., Eugster, E.A. Central Precocious Puberty: Update on Diagnosis and Treatment. Pediatr Drugs 17, 273–281 (2015). https://doi.org/10.1007/s40272-015-0130-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-015-0130-8