Abstract
The main objective was to analyse the use of rigid laryngotracheoscopy under general anaesthesia (GA) and endoscopic surgery in the management of inspiratory stridor in patients referred to a paediatric ENT outpatient clinic. The secondary objective was to analyse the aetiological diagnoses made and their therapeutic management. This is a prospective study including all newborns and infants, corresponding to 190 patients, presenting for the first time in consultation for inspiratory stridor from January 2015 to December 2017. A consultation form was filled out after each consultation and added to a database; a management algorithm was used to determine which patients required a rigid laryngotracheoscopy. A 17.9% (n = 34) of the patients required rigid laryngotracheoscopy, of whom 12.6% (n = 24) underwent concomitant endoscopic surgery. A 65.8% (n = 125) of the patients were diagnosed with laryngomalacia, 21.1% (n = 40) with isolated posterior excess of mucosa, 9.5% (n = 18) with another diagnosis and 3.7% (n = 7) with a normal examination. The presence of comorbidity was associated (p < 0.001) with the use of rigid laryngotracheoscopy and endoscopic surgery.
Conclusion: Rigid laryngotracheoscopy under GA was required in one in five to six patients. Conservative management with strict follow-up may be appropriate in a large number of patients, especially those with laryngomalacia.
What is Known: • Previous research has established that laryngomalacia is the main aetiology of stridor. • Comorbidities are linked with a poor tolerance of stridor. | |
What is new: • About one in five to six patients seen in consultation for stridor will require a trip to the operative room (and one in eight will require endoscopic surgery). • Laryngomalacia and isolated posterior excess of mucosa account for 85–90% of the patients seen in consultation for stridor. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In English terminology, stridor is a harsh respiratory sound caused by turbulent airflow through a restricted area, with resultant mucosal vibrations. By definition, stridor should correspond to an audible inspiratory sound. However, “stridor” has also been used for expiratory and biphasic sounds in the English medical literature [1]. In this study, only inspiratory stridor has been considered.
Inspiratory stridor is a sign that may reflect different laryngotracheal pathologies. It is typically linked with glottic or supraglottic obstruction, while a deep tone noise rather points to a subglottic obstruction, and the presence of both inspiratory and expiratory noise suggests a tracheal obstacle [2]. Stridor has an estimated incidence of less than 1% of newborns and infants under 6 months of age [3]; laryngomalacia being the most common cause [4].
The aetiological diagnosis of inspiratory stridor is based on the combination of history taking, physical examination including a fibreoptic laryngoscopy, and sometimes a rigid laryngotracheoscopy [5]. The indication for rigid endoscopy must take into account the risks inherent in this exploration, carried out under general anaesthesia (GA), most often during the first 2 years of life.
The literature is rich in studies on particular pathologies but few are interested in the stridor sign in itself, and in the management to be adopted when faced with it. In particular, there is no data available on the number of newborns and infants seen in consultation who will require a rigid laryngotracheoscopy, and very few publications assess the proportion of the aetiological diagnoses of stridor [4, 6].
The main objective of this study was to analyse the use of rigid laryngotracheoscopy under GA in the management of stridor, its indications and its contributions. The secondary objective was to analyse the etiological diagnoses made and their therapeutic management.
Materials and methods
This is a monocentric study prospectively including all patients under 2 years of age referring for the first time for inspiratory stridor at the consultations in the Paediatric ENT outpatient clinic of a tertiary care centre between January 1, 2015 and December 31, 2017. Patients were referred by ENT colleagues, paediatricians and general practitioners.
The exclusion criteria were a breathing noise different from an inspiratory stridor by the breathing time or the quality of the noise; patients seen elsewhere than in consultation, in particular patients seen in the context of an emergency (emergency department or intensive care unit).
Population
One hundred and ninety patients were included over the 3-year period: 111 (58.4%) males and 79 (41.6%) females. The median age at first consultation was 61 (27, 106) days, i.e. 2 months and 1 day. One hundred and seventy-eight (93.7%) patients were less than 6 months old. The median interval between the onset of stridor and the consultation was 47 (17, 87) days, i.e. 1 month and 17 days. The median age at onset of stridor was 3 (0, 18.5) days.
Diagnostic evaluation
All patients underwent medical evaluation by a senior physician. The clinical examination included history taking and a physical examination with a fibreoptic laryngoscopy, looking for the aetiological diagnosis and possible criteria for severity.
The elements guiding the diagnosis were the type and term of delivery, the date of onset of stridor, the search for comorbidities (in particular cardiac or neurological) and the presence of dysphonia. The physical examination looked for abnormalities of the neck, the oral cavity or the oropharynx and the presence of skin haemangiomas.
The fibreoptic laryngoscopy analysed the pharynx and the larynx and looked for indirect signs of pharyngo-laryngeal reflux (oedema and erythema of the pharyngo-laryngeal wall, of the posterior pharyngeal wall or of the oesophageal inlet).
Stridor was classified as severe when one or more of the following signs were present: respiratory difficulties (dyspnoea, signs of respiratory distress, cyanosis, brief resolved unexplained event, apnoea during sleep) and feeding difficulties (lengthening of bottle-taking time, reduction in amount of nursing bottles, failure to thrive, swallowing difficulty) [7].
Management
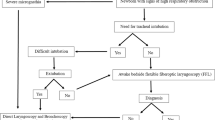
Management could be simple follow-up, medical management (anti-reflux medication, aerosols), or performing a rigid laryngotracheoscopy under GA. The time interval for first follow-up was from 1 to 2 weeks in function of the initial evaluation. The indications for rigid laryngotracheoscopy were a stridor accompanied by the aforementioned signs of severity, a stridor not improving during management, or the presence of an anatomical abnormality visible at fibreoptic laryngoscopy, either to treat or to clarify the diagnosis (Fig. 1). This management algorithm has been used since the start of the study and is based on the previously published algorithms for the management of laryngomalacia [7, 8]. The pharynx, larynx, trachea and main bronchi were analysed under spontaneous ventilation. When endoscopic surgery was necessary, it was performed under spontaneous ventilation when respiratory tolerance allowed it, or after tracheal intubation otherwise. The instruments used were cold micro-instruments or a CO2 laser.
Management algorithm of the patients with stridor. The signs of severity of the stridor were respiratory difficulties (dyspnoea, signs of respiratory distress, cyanosis, brief resolved unexplained event, apnoea during sleep) and feeding difficulties (lengthening of bottle-taking time, reduction in amount of nursing bottles, failure to thrive, swallowing difficulty)
Data analysis
At the end of each consultation, a consultation form containing the elements mentioned above was completed with the parents and added to the medical chart. Informed consent to participate in the study was obtained from the parent or legal guardian. At the end of the collection period, the medical consultation data and rigid laryngotracheoscopy results were included into an anonymized FileMaker Pro® database, allowing descriptive statistical analysis using Microsoft Excel ® 2013 software. The statistical tests consisted of Chi-square tests; the significance threshold being established at p = 0.005 [9].
Results
Use of rigid laryngotracheoscopy
A total of 34 patients (17.9%) required rigid laryngotracheoscopy under GA (Table 1), at the median age of 62 (37, 113) days, i.e. 2 months and 2 days. Twenty-three patients were less than 3 months old; 6 were between 3 and 6 months old and 5 were more than 6 months old. Fifteen (44.1%) rigid laryngotracheoscopies were performed for purely diagnostic purposes and 19 (55.9%) with intention to treat for laryngomalacia (n = 16) and laryngeal cyst (n = 3), the diagnosis of which was made at fibreoptic laryngoscopy. Two rigid endoscopies with intention to treat, performed for laryngomalacia, were not followed by surgical treatment, in front of less severe anatomical anomalies than at fibreoptic laryngoscopy. All diagnostic endoscopies (15/15) were conclusive: laryngomalacia (n = 6), isolated posterior excess of mucosa (n = 2), laryngeal cyst (n = 1), dyskinesia (n = 1), subglottic stenosis (n = 4) and laryngeal cleft (n = 1). The stenoses were congenital (n = 1) or acquired, following prolonged intubation (n = 3). Of the 34 patients who required a laryngotracheoscopy, 25 (73.5%) had a comorbidity, compared to 55 of the 156 (35.3%) patients followed-up (p = 4.2.10−5).
In these 34 patients, the indication for rigid laryngotracheoscopy was established after the first consultation for 23 (12.1%) of them, of which 15 had concomitant endoscopic surgery and 2 were operated on secondarily after further assessment: one for a laryngeal cleft, operated after Nissen fundoplication and gastrostomy, and one for laryngeal cyst, operated after MRI (Table 1). For the 11 (5.8%) other patients, the endoscopy was indicated during the follow-up, due to worsening of symptoms or lack of improvement (Fig. 2).
Use of endoscopic surgery
Twenty-four patients (12.6%), 12 female and 12 male, were operated on endoscopically; 22/24 (91.7%) were less than 6 months old at surgery. All operated patients had at least one criterion of severity: dyspnoea (n = 13), signs of respiratory distress (n = 11), difficulty swallowing (n = 9), broken weight curve (n = 9), apnoea (n = 3), brief resolved unexplained event (n = 1). Of the 24 operated patients, 19 (79.2%) had a comorbidity, compared to 61 of 166 (36.7%) not operated (p = 8.10−5). The history of operated patients was preterm birth (n = 5), tracheal intubation (n = 5), syndromic association (n = 5), cardiac disease (n = 4).
The pathologies operated on were laryngomalacia (n = 16), laryngeal cyst (n = 4), laryngeal subglottic stenosis (n = 3), laryngeal cleft (n = 1). Patients with laryngomalacia were operated on for a supraglottoplasty, using micro-instruments (n = 7), CO2 laser (n = 6), or using laser and micro-instruments (n = 3). The laryngeal cysts were opened with a CO2 laser (n = 3) or with micro-scissors (n = 1). The subglottic stenoses were incised with micro-instruments (n = 2) or with a thulium fibre laser (n = 1) and then balloon-dilated (n = 3). The cleft was closed using CO2 laser (n = 1). No open surgery was performed. The laser was used in 14 cases (58.3%) and the micro-instruments alone in 10 cases (41.7%). Four patients required secondary laryngotracheoscopy: one for laryngomalacia in the face of persistent symptoms; and all (n = 3) operated on for stenosis, for endoscopic control and removal of fibrinous deposits. No complications were noted in the operated patients.
Analysis of the entire population
Laryngomalacia was the most frequent diagnosis, in 65.8% (n = 125) of patients (Table 1). Isolated posterior excess of mucosa was the second most common aetiology, in 21.1% (n = 40) of patients. Across all diagnoses, signs of reflux were present in 72.6% (n = 138) of patients (including all isolated posterior excess of mucosa). Other diagnoses were rare (Table 1).
One hundred and sixty-seven (87.9%) patients were initially followed up, of whom 11 required secondarily a rigid laryngotracheoscopy due to worsening or lack of improvement in symptoms. Seven of these 11 patients were operated on endoscopically. In the end, 155 patients (81.6%) were followed up with or without medical treatment and improved; one patient was lost to follow-up.
All patients (38/38) with a diagnosis of isolated posterior excess of mucosa at fibreoptic laryngoscopy, treated with PPI, improved during follow-up. 103/125 (81.6%) patients with a diagnosis of laryngomalacia were monitored and treated with PPI without requiring laryngotracheoscopy. All diagnoses combined, proton pump inhibitors (PPIs) were prescribed in 84.7% of patients.
Discussion
Stridor of newborns and infants is a sign with different possible aetiologies. It may be linked to the presence of anatomical abnormalities, of pharyngo-laryngeal reflux altering the laryngeal mucosa or of abnormal pharyngeal and laryngeal tone in the case of neuromuscular diseases or immaturities [10]. Once the stridor is installed, the vibrations of the laryngeal mucosa themselves cause oedema. The principle of management is to break this cycle by acting on the causes listed above; hence, the importance of making the right diagnosis.
Of the 190 patients who consulted for stridor, about one in five to six required a rigid laryngotracheoscopy. This is the first study to analyse the use of endoscopy in front of the stridor sign seen in consultation, all aetiologies taken together. About a third of the rigid laryngotracheoscopies were indicated secondarily, in the absence of improvement or worsening of symptoms, which highlights the need for rigorous follow-up with adaptation of the management strategy when required. When it is for diagnostic purposes, rigid laryngotracheoscopy allows to examine under the vocal cords, to visualize the subglottic larynx and to identify abnormalities that are difficult to see at fibreoptic laryngoscopy, such as stenosis or cleft. It is often necessary to differentiate laryngeal palsy (where the vocal cords remain closed during anaesthesia) from dyskinesia (where the vocal cords open at anaesthetic induction). It also unmasks laryngomalacia with appears during sleep, which does not usually present in neonates and infants, but in older children.
In contrast, the management consisted of follow-up with or without medical treatment for 82.1% of the patients, after explanations given to the parents of the severity signs that should lead to anticipated consultation. In these cases, stridor improves with the growth of the child’s larynx, the acquisition of better neuromuscular tone, and the decrease in pharyngo-laryngeal reflux.
The inclusion of 190 patients consulting for stridor over a 3-year period equates to approximately one new patient per week. As in the other studies, a slight male predominance was found (58.4%), without a clear explanation [4,5,6, 11]. A 93.7% of the patients were less than 6 months old at the first consultation, in the line of the preceding observations, where the diagnosis of stridor is exceptional after 2 years and made before 1 year in almost 90% of the cases [4, 6]. The median age of onset of stridor was 3 days. This age is important in the aetiological analysis: a stridor present from birth rather evokes a congenital anomaly such as a disorder of the laryngeal mobility, a congenital subglottic stenosis or a cleft; a stridor appearing in the very first days of life and progressively worsening rather evokes a laryngomalacia or an isolated posterior excess of mucosa; a stridor appearing around the first month of life rather points to a subglottic haemangioma. A stridor after tracheal intubation points to a subglottic pathology decompensated or induced by intubation (acquired stenosis or cyst). This history taking data is subject to recall bias; the age of onset of stridor is probably announced as earlier than reality: “from birth” is an answer frequently given by the parents that does not automatically rule out the diagnosis of laryngomalacia.
The median age at consultation was 2 months, differed from the onset of stridor, but with a delay less than the 3 months usually described in the literature [4]. This delay between the consultation and the first symptoms could be explained by a period of observation generally practiced by paediatricians and general practitioners before requesting a specialized consultation, in presence of a stridor without criteria of severity. Once requested, consultations for stridor are quickly scheduled with senior physicians, due to the risk of worsening.
After this consultation, and sometimes after rigid laryngotracheoscopy, the aetiological diagnosis of stridor could be established. Laryngomalacia was the most common cause of stridor (65.8%), which corresponds to data from the literature [1, 4, 6]. It is characterized by an inspiratory collapse linked to an oedematous posterior mucosa, prominent arytenoid cartilages, aspiration of the epiglottis, short aryepiglottic folds and/or an omega tubular epiglottis, most of the times seen at fibreoptic laryngoscopy [10]. In our series, laryngotracheoscopy was performed in 22/125 (17.6%) of patients with laryngomalacia, and supraglottoplasty performed in 16 (12.8%) of them. These data are in line with those of the literature: severe forms represent from 5 to 28.2% of cases of laryngomalacia [12,13,14]. Non-irradiating imaging, such as ultrasound, may be useful when a child is difficult to examine, but its performance in terms of sensitivity remains lower than that of fibreoptic laryngoscopy [15].
Isolated posterior excess of mucosa was the second cause of stridor found in this series (21.1% of patients), most likely induced by pharyngo-laryngeal reflux. It corresponds to a mucosal oedema of the posterior part of the larynx, on the arytenoids, without supraglottic collapse during inspiration (Fig. 3). The vibration of the posterior mucosa is responsible for the stridor, but in the absence of supraglottic collapse, the respiratory symptomatology is less important than in laryngomalacia. The individualization of “isolated posterior excess of mucosa” is not conventionally found in other studies, where it is likely that these patients are considered to have a normal fibreoptic laryngoscopy or minimal laryngomalacia. It is also likely that some of them develop into laryngomalacia in the absence of treatment. These patients without criteria of severity were treated with PPI and measures to limit the reflux, detailed below. They all improved during follow-up, without surgery, which suggests an interesting diagnostic precision of history taking coupled with fibreoptic laryngoscopy.
Other laryngotracheal pathologies were rarer, which prohibits any extrapolation to the general population. It is important to note that this series does not reflect the entire laryngotracheal pathology associated with a stridor. In particular, stenoses represent a greater part of our activity in paediatric laryngology than what is observed here. This is explained by the selected inclusion criteria: this study excludes severe forms with dyspnoea, which are not seen in clinics, and stenoses with low-pitched noise.
The presence of comorbidities is known to be a factor in poor tolerance of stridor, and therefore of higher use of rigid laryngotracheoscopy and endoscopic surgery [8]. This was found in our study, which confirms that newborns and infants with comorbidities must be carefully followed up. The presence of comorbidities is also responsible for surgical failure. With regard to supraglottoplasty for laryngomalacia, the study by Denoyelle et al. found a 8.8% failure or only partial improvement rate, mainly in patients with comorbidities [12]. Complications from endoscopic surgery are rare but can be very difficult to treat, like supraglottic stenosis by excessive scarring of the arytenoids [12]; none was encountered in this series.
Pharyngo-laryngeal reflux has to be taken into account when faced with stridor, where it is found in more than half of newborns and infants [16]. It may result in an inflammation and oedema of supraglottic tissues, allowing or exacerbating mucosal vibrations with inspiration. In laryngomalacia, pharyngo-laryngeal reflux seems to affect more than 80% of the patients whether diagnosed using pH probes or the presence of pepsin in saliva [17,18,19]. It is also known to be an aggravating factor for laryngotracheal pathologies and a risk of surgical failure, like in dyskinesia [20], laryngomalacia [18, 21] or subglottic stenosis [22]. An evaluation by pHmetry or impedancemetry was not systematic, given the difficulty of access to these examinations, which constitutes one of the limits of our study for making this diagnosis. It was suspected at fibreoptic laryngoscopy in the presence of erythema or oedema of the posterior laryngeal mucosa, posterior pharyngeal wall, oesophageal inlet or in the presence of endolaryngeal erythema at rigid endoscopy. Medical treatment with PPI (generally esomeprazole 1 mg/kg/day) was introduced in more than 80% of patients to reduce laryngeal inflammation. In our protocol, anti-reflux drugs were prescribed for 3 to 6 weeks, only to patients with suspected pharyngo-laryngeal reflux, i.e. to patients with laryngomalacia, isolated posterior excess of mucosa, indirect signs of pharyngo-laryngeal reflux, to avoid worsening of the stridor, and systematically after surgery to prevent scarring disorders. Short-term adverse effects of PPI are frequent but usually mild and mainly include nausea, vomiting or regurgitation, diarrhoea, abdominal pain, fever and pharyngitis [23]. The appearance of adverse effects should lead to discontinuation of PPI treatment, given the lack of strong evidence of its efficacy in stridor. PPI should also not be used long term in this indication, given the risk of adverse effects such as hypochlorhydria, hypergastrinemia, increased risk of infections, vitamin and mineral deficiencies, adverse bone health, food allergy and drug interactions [24]. The measures to limit the reflux were also explained to the parents: reflux can be improved by postural measures, eating measures, clothing and diapers that do not compress the abdomen and an avoidance of passive smoking.
In conclusion, a trip to the operative was required in 17.9% of patients seen for stridor, with concomitant surgery performed in 12.6%. Having comorbidity was associated with a greater risk of undergoing laryngotracheoscopy. The most common aetiologies of stridor were laryngomalacia and isolated posterior excess of mucosa; these diagnoses being made in 86.9% of patients. Given the complexity of stridor management and the frequent use of rigid laryngotracheoscopy, follow-up by specialists with perfect knowledge of possible diagnoses, of the signs of severity and of possible treatments is essential.
Data availability
Yes.
Abbreviations
- ENT:
-
Ear, nose throat
- GA:
-
General anaesthesia
- PPI:
-
Proton pump inhibitor
References
Ida JB, Thompson DM (2014) Pediatric stridor. Otolaryngol Clin N Am 47:795–819
Mondain M, Blanchet C (2010) Stridor or not stridor. Arch Pediatr 17:602–603
Thornton AJ, Morley CJ, Hewson PH, Cole TJ, Fowler MA, Tunnacliffe JM (1990) Symptoms in 298 infants under 6 months old, seen at home. Arch Dis Child 65:280–285
Zoumalan R, Maddalozzo J, Holinger LD (2007) Etiology of stridor in infants. Ann Otol Rhinol Laryngol 116:329–334
Botma M, Kishore A, Kubba H, Geddes N (2000) The role of fibreoptic laryngoscopy in infants with stridor. Int J Pediatr Otorhinolaryngol 55:17–20
Holinger LD (1980) Etiology of stridor in the neonate, infant and child. Ann Otol Rhinol Laryngol 89:397–400
Carter J, Rahbar R, Brigger M, Chan K, Cheng A, Daniel SJ, De Alarcon A et al (2016) International Pediatric ORL Group (IPOG) laryngomalacia consensus recommendations. Int J Pediatr Otorhinolaryngol 86:256–261
Thompson DM (2010) Laryngomalacia: factors that influence disease severity and outcomes of management. Curr Opin Otolaryngol Head Neck Surg 18:564–570
Ioannidis JPA (2018) The proposal to lower P value thresholds to .005. JAMA 319:1429–1430
Ayari S, Aubertin G, Girschig H, Van Den Abbeele T, Mondain M (2012) Pathophysiology and diagnostic approach to laryngomalacia in infants. Eur Ann Otorhinolaryngol Head Neck Dis 129:257–263
Mancuso RF (1996) Stridor in neonates. Pediatr Clin N Am 43:1339–1356
Denoyelle F, Mondain M, Gresillon N, Roger G, Chaudre F, Garabedian EN (2003) Failures and complications of supraglottoplasty in children. Arch Otolaryngol Head Neck Surg 129:1077–1080
Landry AM, Thompson DM (2012) Laryngomalacia: disease presentation, spectrum, and management. Int J Pediatr 2012:753526
Ribeiro J, Julio S, Dias C, Santos M, Spratley J (2018) Supraglottoplasty in children with laryngomalacia: a review and parents’ appraisal. Am J Otolaryngol 39:613–617
Shirley F, Oshri W, Ari D, Gad F (2019) The role of laryngeal ultrasound in the assessment of pediatric dysphonia and stridor. Int J Pediatr Otorhinolaryngol 122:175–179
Hartl TT, Chadha NK (2012) A systematic review of laryngomalacia and acid reflux. Otolaryngol Head Neck Surg 147:619–626
Klimara MJ, Samuels TL, Johnston N, Chun RH, McCormick ME (2020) Detection of pepsin in oral secretions of infants with and without laryngomalacia. Ann Otol Rhinol Laryngol 129:224–229
Luebke K, Samuels TL, Chelius TH, Sulman CG, McCormick ME, Kerschner JE, Johnston N, Chun RH (2017) Pepsin as a biomarker for laryngopharyngeal reflux in children with laryngomalacia. Laryngoscope 127:2413–2417
Matthews BL, Little JP, McGuirt WF Jr, Koufman JA (1999) Reflux in infants with laryngomalacia: results of 24-hour double-probe pH monitoring. Otolaryngol Head Neck Surg 120:860–864
Denoyelle F, Garabedian EN, Roger G, Tashjian G (1996) Laryngeal dyskinesia as a cause of stridor in infants. Arch Otolaryngol Head Neck Surg 122:612–616
Bibi H, Khvolis E, Shoseyov D, Ohaly M, Ben Dor D, London D, Ater D (2001) The prevalence of gastroesophageal reflux in children with tracheomalacia and laryngomalacia. Chest 119:409–413
Halstead LA (1999) Gastroesophageal reflux: a critical factor in pediatric subglottic stenosis. Otolaryngol Head Neck Surg 120:683–688
Cohen S, Bueno de Mesquita M, Mimouni FB (2015) Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol 80:200–208
De Bruyne P, Ito S (2018) Toxicity of long-term use of proton pump inhibitors in children. Arch Dis Child 103:78–82
Author information
Authors and Affiliations
Contributions
Eric Moreddu: study conception and design, material preparation, data collection, analysis, draft and article writing, review.
Maeva Montero: study conception and design, material preparation, data collection, analysis, draft writing, review.
Laurent Gilain: study conception and design, resources, supervision, review.
Jean-Michel Triglia: study conception and design, resources, supervision, review.
Richard Nicollas: study conception and design, data collection, analysis, resources, supervision, review.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent for publication was obtained from all individual participants included in the study.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreddu, E., Montero, M., Gilain, L. et al. Inspiratory stridor of newborns and infants admitted to a paediatric ENT outpatient clinic: diagnostic approach and therapeutic outcome. Eur J Pediatr 180, 1177–1183 (2021). https://doi.org/10.1007/s00431-020-03858-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03858-3