Abstract
Medulloblastoma (MB) is a malignant embryonal tumor that develops especially in childhood, with overall survival (OS) at 5 years of up to 70%. The objective of this study is to analyze treatment delivery variables in a retrospective cohort and evaluate the impact of these treatment quality parameters on survival. From 2000 to 2018, 40 pediatric patients with medulloblastoma, treated according to current international protocols, were retrospectively analyzed. Treatment delivery quality indicators were analyzed including the extent of surgery, radiotherapy (RT) parameters, and chemotherapy variables, related with time and dose-intensity deviations. With a median follow-up of 74 months (range, 6–195), OS at 5 years was 74 ± 7%, 81 ± 8% for standard-risk, and 55 ± 16% for high-risk patients (p = 0.090). Disease-free survival at 5 years was not significantly affected by extent of surgery (p = 0.428) and RT-related variables such as surgery-RT interval (p = 0.776) neither RT duration (p = 0.172) or maintenance chemotherapy compliance (p = 0.634). Multivariate analysis identified risk groups predictive of worse DFS (p = 0.032) and leptomeningeal dissemination associated with inferior OS (p = 0.029).
Conclusion: Treatment delivery optimization has improved survival rates of patients with MB. Despite this, in our study, we have not established a clear influence of the considered radiotherapy and chemotherapy treatment quality parameters on outcomes.
What is Known: • Improvement in treatment modalities during the last decades has reached a 5-year OS of up to 70% in these patients. • Extent of resection and radiotherapy parameters such as interval between surgery-radiotherapy and radiotherapy duration has been described as probable survival prognostic factors. What is New: • Differences in medulloblastoma survival rates between prospective studies and retrospective series. • The impact on survival of the three main treatment variables, surgery, radiotherapy and chemotherapy, susceptible to improvement. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is the most frequent malignant brain tumor in children and adolescents accounting for 20–25% of all brain tumors. It can occur at any age, with a peak incidence between 4 and 7 years, although recently its incidence has increased in the group of patients aged 10 to 14 years [1, 2]. Around 30% of patients will present with leptomeningeal disease at diagnosis, but it is rare for MB to spread outside the CNS [3]. Medulloblastoma is a very heterogeneous tumor in terms of biology. It is now stratified into different molecular subgroups depending on methylation pattern: Wingless (WNT), Sonic hedgehog (SHH), group 3, and group 4 [4]. Patients are classified into standard or high-risk, according to demographic and tumor factors such as age, leptomeningeal spread, or extent of surgical resection [5, 6]. This classification is essential for the development of a risk-adapted treatment strategy. Improvement in treatment modalities during the last decades has reached a 5-year OS of up to 70% in these patients [2]. However, there is still room for improvement by optimizing treatment quality, especially in low- and medium-income countries. The objective of this study is to analyze treatment delivery variables in a retrospective cohort and evaluate the impact of these treatment quality parameters on survival.
Material and methods
Patient population
This study was approved by the institutional ethics committee. Between 2000 and 2018, 75 children with medulloblastoma received multimodal treatment with curative intent, at two reference hospitals in Spain. Of these, 59 patients were eligible for initial analysis: twelve patients were omitted from this analysis due to lack of complete treatment information and four patients did not receive radiotherapy. Finally, in order to obtain a more homogeneous population, we excluded infants and young children treated with a radiotherapy deferral strategy; therefore, 40 patients were enrolled for the final analysis.
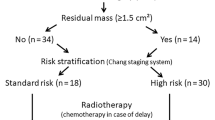
Patient characteristics are shown in Table 1. Mean age was 7.8 years (range, 3–14), with 67.5% being male. All patients had histologic confirmation of medulloblastoma (MB). A craniospinal magnetic resonance imaging (MRI) and cerebrospinal fluid examination to evaluate for disseminated disease were performed at diagnosis. In relation to risk stratification, patients were divided into standard-risk (65%) and high-risk (35%), following the historical risk stratification criteria for medulloblastoma based on clinicopathological variables including age, metastatic stage, and extent of resection. Although since 2012, there is a new classification based in molecular profiling (WNT, SHH, group 3, and group 4); molecular subgrouping was not available for our study [7].
Treatment
All patients underwent surgery, including biopsy alone in 2 patients (5%), subtotal resection (residual tumor by MRI) in 14 patients (35%), and gross total resection in 24 patients (60%). Post-operative MRI was used to determine extent of resection: complete resection (R0), residual disease ≤ 1.5 cm2 (R1), and residual disease > 1.5 cm2 (R2). Patients were treated according to different international protocols, with 67.5% of all patients treated as per SIOP PNET 4 (Table 2).
Treatment was largely directed by protocol enrollment and risk group stratification. Patients were treated with surgery followed by radiotherapy (standard or hyperfractionation) and maintenance chemotherapy.
All patients were treated with megavoltage X-rays on a linear accelerator. Patients treated before 2016 were treated with 3D conformal radiation therapy (29 patients), and patients treated after, with image-guided radiotherapy (IGRT) volumetric modulated arc therapy (VMAT) techniques (11 patients). Computed tomography (CT) scans with contrast were performed for treatment planning, with 2-mm slices for the posterior fossa, and 5-mm slices for the rest of the brain and spinal axis. When feasible, treatment volume delineation was accomplished with image registration of simulation CT scan and the initial diagnostic MRI. Volume delineation was as per standard of care or per enrolled protocol. The organs at risk were the healthy brain, lens, globes, optic nerves, chiasm, pituitary, cochlea, hippocampus, parotid glands, spinal cord, lungs, thyroid, heart, liver, kidneys, bladder, rectum, testicles, and ovaries. Fourteen (35%) children required sedation during radiotherapy, with a mean age of 6.2 years (range, 3–14 years).
Craniospinal doses ranged from 23.4 to 39.8 Gy (mean, 27.5 Gy). More than half of the patients (n = 27) received reduced dose craniospinal radiation with 23.4 Gy [8, 9]. Posterior fossa doses ranged from 54 to 61.2 Gy (mean, 55.6 Gy), with a boost to the tumor bed or residual tumor of up to 68 Gy in 7 patients (17.5%). In the majority of protocols, radiotherapy doses were delivered once a day using 1.8 Gy per fraction, with the exception of patients included in HIT-SIOP PNET 4, who were treated twice a day with doses of 1.0 Gy per fraction.
Radiotherapy initiation within 40 days after surgery was considered optimal. For ideal treatment compliance, patients had to receive radiotherapy continuously (daily, except weekends and holidays), completing treatment in 45 days or less. According to protocol, some patients received weekly and/or daily chemotherapy during radiotherapy treatment. Maintenance chemotherapy had to start within 6 to 7 weeks after end of radiotherapy for correct timely delivery. For ideal chemotherapy compliance, patients had to receive all cycles, 6 to 8 courses depending on the protocol, within the established timeframe and without dose reduction or drug modification.
In a first analysis, these maintenance chemotherapy parameters were studied separately: agents or dose modifications within each cycle, treatment delays, and total number of courses received. In a second time, we studied the overall maintenance chemotherapy compliance by creating a variable covering all these aspects.
Statistical analysis and outcomes
A statistical analysis was performed using the SPSS 21.0 [10]. Disease-free survival (DFS) and overall survival (OS) was evaluated by Kaplan-Meier non-parametric statistical analysis. Disease-free survival was defined as the time from first diagnosis to first relapse, progression, or last follow-up date. Overall survival was determined as the time from diagnosis to death from any cause or last follow-up. p values less than 0.05 were considered statistically significant. A multivariate analysis was performed although the results were limited due to the small sample size.
Results
With a median follow-up of 74 months (range, 6–195), the 5-year OS of all 40 patients was of 74 ± 7%. When analyzing survival by risk group, 5-year OS was 81 ± 8% for standard-risk and 55 ± 16% for high-risk (p = 0.090) (Fig. 1a/b). Disease-free survival at 5 years was 66 ± 8%, 77 ± 8% for standard-risk, and 40 ± 15% for high-risk (p = 0.024) (Fig. 1c/d). Extent of resection had an effect on 5-year DFS, with patients with R0/R1 having superior outcomes compared with R2, although this was not statistically significant (69 ± 8 versus 48 ± 23%, respectively; p = 0.428).
Regarding radiotherapy, 29 patients (72.5%) started treatment within 40 days of surgery as per protocol with a median interval time of 34 days (range 2–40 days). Radiotherapy treatment delay was mainly due to the following: technical aspects of the linear accelerator (n = 6), post-operative toxicity (n = 3), mostly neurological complications, or unknown for 2 patients. With respect to treatment duration, 57.5% patients (n = 23) received optimal radiotherapy (≤ 45 days). Interruptions were mainly caused by RT and/or concurrent chemotherapy toxicity (n = 3), extended holidays (n = 2), technical linear accelerator problems (n = 3), disease sequelae (n = 1), or unknown cause (n = 8).
As for chemotherapy, 35 patients (87.5%) were treated with concomitant chemotherapy during radiotherapy, with vincristine (n = 24), carboplatin (n = 5), or both (n = 6), having five of these patients dose modification due to toxicity. The majority of patients (n = 33) initiated maintenance chemotherapy within 7 weeks after the end of radiotherapy. Regarding maintenance chemotherapy compliance, up to 55% presented agents or dose-intensity deviations, predominantly involving platinum-based drugs (n = 8) or several drugs (n = 8). Despite this, the majority of patients, 92.5%, received all planned number of cycles. When analyzing total compliance, less than half of the patients (n = 17) received maintenance chemotherapy regimen conforming to protocol (all cycles with no interruptions or drug/dose modifications).
When evaluating the impact of radiotherapy parameters on outcomes, DFS at 5 years was 64 ± 9% for patients starting radiotherapy within 40 days of surgery, and 70 ± 15% for patients with treatment delay (p = 0.776). Regarding treatment duration, DFS at 5 years was 59 ± 11% for treatment within 45 days and 75 ± 11% for prolonged treatment (p = 0.172).
Receiving concomitant chemotherapy during radiotherapy did not alter DFS at 5 years (67 ± 8% for patients with concomitant treatment versus 60 ± 22% for patients without it; p = 0.780), although there was a trend towards better 5-year OS for patients with chemotherapy during irradiation (76 ± 8% with concomitant chemotherapy versus 60 ± 22% without it; p = 0.560). The interval between the end of irradiation and the beginning of maintenance chemotherapy, with the established 49 days cut-off point, did not have an impact on DFS (< 49 days 61 ± 9% versus > 49 days interval 86 ± 13%; p = 0.444) neither on OS (< 49 days 71 ± 8% versus >49 days interval 86 ± 13%; p = 0.705). However, when setting the cut-off interval in 41 days, patients with early maintenance chemotherapy initiation (n = 16) showed a statistical significant detriment in DFS (≤ 41 days 36 ± 15% versus > 41 days interval 78 ± 8%; p = 0.008) and in OS (≤ 41 days 42 ± 16% versus > 41 days interval 86 ± 7%; p = 0.010).
When analyzing maintenance chemotherapy compliance, patients who had a drug or dose-intensity modification presented greater 5-year DFS compared with those who received full dose with no drug modification although this was not statistically significant (p = 0.816). However, the number of cycles delivered had an impact on survival rates, with a 5-year OS of 77 ± 7% for patients receiving all cycles versus 33 ± 27% for patients not completing all cycles (p = 0.013). When studying overall maintenance chemotherapy compliance, patients with optimal compliance did not have better 5-year OS compared with those with any treatment delivery deviation (75 ± 11% for optimal compliance versus 73 ± 10% for patients with modifications during treatment delivery; p = 0.948).
On both univariate (Table 3) and multivariate analysis (Table 4), neither extent of surgery, radiotherapy parameters, nor chemotherapy compliance was found to alter significantly OS and DFS. Multivariate analysis identified leptomeningeal dissemination (0.029) and high-risk group (0.032) as negative prognostic factors (Table 4).
When analyzing survival rates for all 59 patients, the 5-year OS was 58 ± 7%, 80 ± 8% in standard-risk group, and 39 ± 10% in high-risk group (p = 0.009). Disease-free survival at 5 years was 51 ± 7%, 73 ± 9% for standard-risk, and 34 ± 9% for high-risk (p = 0.008). On both univariate and multivariate analysis, neither radiotherapy timing nor radiotherapy duration were found to alter significantly OS and DFS. The multivariate analysis identified leptomeningeal dissemination (p = 0.049) and residual disease R2 (p = 0.016) as predictors of worse disease-free survival.
Discussion
Medulloblastoma is treated with multimodal treatment combining surgery, radiotherapy, and chemotherapy. During the past decades, improvements in these treatment modalities have increased long-term survival to a 5-year OS of 60–70% [11, 12]. A 39% reduction in mortality rate was obtained for patients diagnosed from 2000 onwards versus those diagnosed between 1990 and 1999 in a retrospective study from England that included patients diagnosed with medulloblastoma [13].
In our study, survival rates are in line with those present in other European countries and slightly inferior to prospective trial survival outcomes [8, 14,15,16]. When analyzing survival in patients with high-risk MB, our results are in line with the ones described in previous studies, including those with deferred radiotherapy treatment strategy, with 5-year OS rates ranging between 40 and 75% [17,18,19,20,21,22]. In our study, 6 patients (15%) were classified as high-risk MB because of M2–M3 involvement, with a 5-year DFS of 21%. In this subgroup with disease dissemination, the 10-year DFS rate reported by Van Hoff et al. [17] was 32%. Prior phase III studies analyzing patients with high-risk MB published survival rates above 70%, rates that have not been reproduced in retrospective series [17,18,19]. The rigorous selection of patients from prospective clinical trials, the required start time of radiation within 28 days after surgery (COG trials), the proportion of enrolled M1–M3 patients, and the size of the residual tumor are factors that can explain these differences in survival between prospective and retrospective studies [6, 23,24,25].
More agreement is found when analyzing the impact of post-surgical residual tumor in survival. Lannering et al. published in a prospective randomized trial that a post-operative residual tumor > 1.5 cm2 based on post-operative CT scan had a profound, detrimental impact on survival (p < 0.01) [12]. A population-based study from the Oslo University Hospital, which included 175 patients with MB or CNS PNET, also found an improvement in 5-year OS in patients with gross total resection versus subtotal resection (64 versus 22%, respectively) [26]. Nonetheless, Thompson et al., in the molecular era, published a retrospective study, assessing the effect of surgery extent on survival within the different molecular subgroups (WNT, SHH, group 3, and group 4) [27]. Extent of resection was classified into three categories based on the post-operative imaging (MRI for most cases): “gross total resection” (no residual tumor), “near-total resection” (< 1.5 cm2 of residual disease), and “sub-total resection” (1.5 cm2 or above). Significant survival benefit was observed for gross total resection over subtotal resection. Interestingly, in a molecular subgroup sub analysis, maximum resection provided benefit in progression-free survival only for patients with group 4 medulloblastoma (total versus subtotal resection; HR 1.97, 1.22–3.17, p = 0.006). In our study, although there is a numerical detriment in survival for those patients undergoing suboptimal surgery, this was not statistically significant, which could be in relation to the small sample size.
Regarding the influence of radiotherapy parameters, the interval between surgery and radiotherapy, as well as duration of radiotherapy, has been described as probable survival prognostic factors. One of the first studies to observe a relation between radiotherapy duration and outcome was a 30-year review, which included 53 patients treated with radiotherapy with curative intent at the University of Florida [28]. Five-year posterior fossa control was 89% for those treated within 45 days versus 68% for prolonged duration (p = 0.010). Multivariate analysis for local control identified radiotherapy duration as the only statistically significant prognostic factor (p = 0.030). Taylor et al. reported the impact of radiotherapy parameters on outcome, including radiotherapy duration, surgery-radiotherapy interval, dose, and targeting deviations [29]. Three-year OS and DFS were better for those who completed treatment within 50 days as established in PNET-3 protocol recommendations (OS 84 versus 71%, p = 0.036 and DFS 79 versus 54%, p = 0.009). The main cause of delay was treatment toxicity (mainly myelosuppression in the chemotherapy group) followed by technical problems related to the facility or holidays; similar to our results. Multivariate analysis revealed use of chemotherapy (p = 0.025) and radiotherapy duration (p = 0.010) as the only parameters predictive of better DFS. Kann et al. retrospectively analyzed radiotherapy timing in young children (3 to 8 years old) [30]. Patients were divided into two groups, those receiving upfront post-operative radiotherapy (treatment within 90 days of surgery) and those having delayed post-operative radiotherapy. Although radiotherapy deferral has gained acceptance in children under 3 years old, for patients in this study, delayed radiotherapy was associated with poorer OS in multivariable analysis (HR 1.95; 95% CI, 1.04–2.94).
Contrary to the aforementioned, results from a series published by Frost et al. proved no relation between disease-free survival and radiotherapy variables (doses or radiotherapy treatment duration) [31]. However, it is important to recall that patients in this study were over 16 years old and were treated in some cases with different radiotherapy techniques (Cobalt-60) and doses. Back et al. analyzed treatment prognostic factors such as radiotherapy duration for patients treated from 1980 to 1993 [32]. On this retrospective study, median radiotherapy dose to the posterior fossa, radiotherapy timing and radiotherapy duration were 55 Gy, 42 days, and 45 days, respectively. On multivariate analysis, the only treatment prognostic factor strongly associated with local control was radiotherapy dose to posterior fossa (p = 0.004). However, in this analysis, extended radiotherapy duration, especially above 46 days, was nearly associated with poorer disease control (OR of 1.02 [95% CI 0.96–1.08]; p = 0.049). In our series, survival in relation to radiation doses was analyzed but was difficult to interpret as delivered doses depended mainly on protocol inclusion, which in turn depended on risk group; therefore, high-risk patients generally received higher craniospinal and posterior fossa doses than standard-risk patients.
On a recent analysis of the National Cancer Database (NCDB), data from 1338 patients with medulloblastoma treated with curative intent, multimodal therapy was collected [33]. The ideal interval between surgery and the start of radiation was set on 3.1–4 weeks. It was shown that patients starting radiotherapy within 3 weeks from surgery had a decreased 5-year OS compared with those treated within the stipulated timing both in the univariate (p = 0.003) and multivariate analysis (p = 0.004). These results could be related to the fact that patients with early radiotherapy initiation tended to be those with worse prognostic factors (younger patients, M1–M3 disease, or/and subtotal resection). However, in the remaining patients, a delay on radiotherapy treatment (> 5 weeks) did not have a significant impact on survival, as long as the interval did not exceed 90 days (p = 0.563). Therefore, radiotherapy timing should allow adequate post-surgery recovery instead of focusing on meeting the 4 to 6 weeks window required by the majority of trials.
To summarize, the impact on survival of radiotherapy treatment variables remains a controversial issue, although there is a general agreement on beginning radiotherapy within 4 to 6 weeks from surgery and minimizing delays during radiotherapy. In fact, the National Cancer Institute for pediatric tumors includes, as priority matter, radiation issues such as treatment delay or omission [34].
In a recent study, Rojas et al. defined, as quality indicators for radiotherapy treatment, the percentage of patients initiating radiotherapy within 40 days after surgery (68%) but they did not analyze if this had an impact on survival [35]. For maintenance chemotherapy treatment delivery, they considered the number of patients with dose-intensity modification (26%) or time-intensity modifications (42%) as negative quality indicators. However, we did not find in the reviewed literature any reference regarding the prognostic influence of chemotherapy compliance. According to SIOP PNET 4 and 5 protocols, patients should start maintenance chemotherapy 6 weeks after the end of radiation treatment, and administered doses of both concomitant and maintenance chemotherapy should be tested for prognostic relevance [36, 37]. In the present study, optimal chemotherapy compliance was not associated with better outcomes. Although delay in chemotherapy starting date after radiotherapy did not resulted in worse outcomes, early initiation (< 6 weeks after end of radiotherapy) was associated significantly with a survival detriment. This could be related to the fact that patients who initiated maintenance chemotherapy before the established 6 weeks period tended to have more advanced disease at diagnosis or worse response to initial treatment.
The main objective of this study was to establish treatment delivery quality in medulloblastoma patients and further analyze their prognostic impact. Nonetheless, the limitations of our study need to be acknowledged, starting with the issues inherent in any retrospective analysis, in a period of 15 years, during which time different treatment protocols have emerged. Furthermore, in relation to patient classification, patients treated in the first decade (2000–2010) could have been misclassified as no molecular biological analysis was performed in them. Due to the small sample size, most of the results obtained regarding treatment quality parameters did not reach statistical significance.
The strengths of the study relate to the inclusion of patients treated in the same radiation oncology center, with international protocols and all with megavoltage techniques and CT treatment simulation. We analyzed the three main treatment variables, susceptible to improvement, and their impact on survival. Hopefully, the improvement of these treatment variables (complete resection, early initiation of radiotherapy after surgery, optimal treatment duration, and total chemotherapy compliance) along with the systematic application of quality control programs such as RTQA (radiation therapy quality assurance) will optimize survival rates and bring them closer to those achieved in prospective clinical trials [38].
In absence of studies that can confirm the influence of radiotherapy timing and chemotherapy compliance on survival for patients with medulloblastoma, our study has not found a prognostic association. Subtotal resection is the only treatment parameter associated with worse survival outcomes, although in the present study this finding did not reach statistical significance. Although higher survival rates have been published by prospective studies that recommend early radiotherapy initiation (preferably within 28 days), and duration within 45–50 days, its prognostic impact has not been proven in the majority of institutional series. The current evolution of radiotherapy techniques, allowing greater precision, together with mandatory daily image-guided quality control and the emphases on radiotherapy timing compliance are elements that can be easily improved in order to potentially optimize survival outcomes.
Up to now, complete resection without neurological sequelae, optimal chemotherapy, and radiation treatment, along with the fundamental quality control in the delivery of these treatments, are the mainstays to achieve the survival rates obtained in reference centers. In the near future, individualized treatment strategies based on molecular subgroups, the introduction of new drugs and the real possibility of irradiating all patients with proton therapy within the appropriate time, will help improve the survival of children with medulloblastoma.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CG:
-
Children’s Oncology Group
- ChT:
-
Chemotherapy
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- CSI:
-
Craniospinal irradiation
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DFS:
-
Disease-free survival
- Gy:
-
Gray
- HR:
-
Hazard ratio
- HTRT:
-
Hyperfractionated radiotherapy
- IGRT:
-
Image-guided radiation therapy
- MB:
-
Medulloblastoma
- MRI:
-
Magnetic resonance imaging
- NCDB:
-
National Cancer Database
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PF:
-
Posterior fossa
- PNET:
-
Primitive neuroectodermal tumors
- RT:
-
Radiotherapy
- RTQA:
-
Radiation therapy quality assurance
- SE:
-
Standard error
- SHH:
-
Sonic hedgehog
- SIOP:
-
International Society of Paediatric Oncology
- SPSS:
-
Statistical Package for the Social Sciences
- STRT:
-
Standard radiotherapy
- VMAT:
-
Volumetric modulated arc therapy
- WNT:
-
Wingless
References
RivasVilela S, Rubio-Casadevall J, Fabrega-Ribas A, Joly-Torta C, Vilardell L, Marcos-Gragera R (2019) Incidence and survival of central nervous system tumors in childhood and adolescence in Girona (Spain) 1990–2013: national and international comparisons. Clin Transl Oncol 21(9):1177–1185
Khanna V, Achey RL, Ostrom QT, Block-Beach H, Kruchko C, Barnholtz-Sloan JS, de Blank PM (2017) Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neuro-Oncol 135(3):433–441
Louis DN, Ohgaki H, Wiestler O, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Sengupta S, Pomeranz Krummel D, Pomeroy S (2017) The evolution of medulloblastoma therapy to personalized medicine. F1000Res 6:490
Chang CH, Housepian EM, Herbert C Jr (1969) An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93(6):1351–1359
Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ (1999) Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 17(3):832–845
Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL (2016) Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol 131(6):821–831
Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R (2006) Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24(25):4202–4208
Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, Ashley DM, Sexton M, Kellie SJ, Ahern V, Gajjar A (2008) Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 70(3):782–787
IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp
Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, Pastore G, Peris-Bonet R, Stiller CA; EUROCARE Working Group (2009) Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer 45(6):992–1005
Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, Massimino M, Reddingius R, Benesch M, Carrie C, Taylor R, Gandola L, Björk-Eriksson T, Giralt J, Oldenburger F, Pietsch T, Figarella-Branger D, Robson K, Forni M, Clifford SC, Warmuth-Metz M, von Hoff K, Faldum A, Mosseri V, Kortmann R (2012) Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 Trial. J Clin Oncol 30(26):3187–3193
Fairley L, Picton SV, Mc Nally RJ, Bailey S, Mc Cabe MG, Feltbower RG (2016) Incidence and survival of children and young people with central nervous system embryonal tumours in the North of England, 1990–2013. Eur J Cancer 61:36–43
Stensvold E, Myklebust TÅ, Cappelen J, Due-Tønnessen BJ, Due-Tønnessen P, Kepka A, Johannesen TB, Krossnes B, Lundar T, Maric S, Miletic H, Moholdt V, Myrmel KS, Nordberg T, Rydland J, Stokland T, Solem K, Solheim O, Torsvik I, Wikran GC, Zeller B, Wesenberg F, Bechensteen AG, Brandal P (2019) Children treated for medulloblastoma and supratentorial primitive neuroectodermal tumor in Norway from 1974 through 2013: unexplainable regional differences in survival. Pediatr Blood Cancer 66(10):e27910
Desandes E, Guissou S, Chastagner P, Lacour B (2014) Incidence and survival of children with central nervous system primitive tumors in the French National Registry of Childhood Solid Tumors. Neuro-Oncology 16(7):975–983
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7(10):813–820
Hoff KV, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S (2009) Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer 45(7):1209–1217
Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, Burger P, Barnes P, Kun L (2013) High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol 31(23):2936–2941
von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Müller K, Goschzik T, Mühlen AZ, Gerber N, Warmuth-Metz M, Soerensen N, Deinlein F, Benesch M, Zwiener I, Kwiecien R, Faldum A, Bode U, Fleischhack G, Hovestadt V, Kool M, Jones D, Northcott P, Kuehl J, Pfister S, Pietsch T, Rutkowski S (2016) Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol 34(34):4151–4160
Vivekanandan S, Breene R, Ramanujachar R, Traunecker H, Pizer B, Gaze MN, Saran F, Thorp N, English M, Wheeler KA, Michalski A, Walker DA, Saunders D, Cowie F, Cameron A, Picton SV, Parashar D, Horan G, Williams MV (2015) The UK experience of a treatment strategy for pediatric metastatic medulloblastoma comprising intensive induction chemotherapy, hyperfractionated accelerated radiotherapy and response directed high dose myeloablative chemotherapy or maintenance chemotherapy (Milan strategy). Pediatr Blood Cancer 62(12):2132–2139
Sirachainan N, Nuchprayoon I, Thanarattanakorn P, Pakakasama S, Lusawat A, Visudibhan A, Dhanachai M, Larbcharoensub N, Amornfa J, Shotelersuk K, Katanyuwong K, Tangkaratt S, Hongeng S (2011) Outcome of medulloblastoma in children treated with reduced-dose radiation therapy plus adjuvant chemotherapy. J Clin Neurosci 18(4):515–519
Sirachainan N, Pakakasama S, Anurathapan U, Hansasuta A, Dhanachai M, Khongkhatithum C, Jinawath A, Mahachoklertwattana P, Hongeng S (2018) Outcome of newly diagnosed high risk medulloblastoma treated with carboplatin, vincristine, cyclophosphamide and etoposide. J Clin Neurosci 56:139–142
Brandes AA, Paris MK (2004) Review of the prognostic factors in medulloblastoma of children and adults. Crit Rev Oncol Hematol 50(2):121–128
Gerber NU, Mynarek M, von Hoff K, Friedrich C, Resch A, Rutkowski S (2014) Recent developments and current concepts in medulloblastoma. Cancer Treat Rev 40(3):356–365
Esbenshade AJ, Kocak M, Hershon L, Rousseau P, Decarie JC, Shaw S, Burger P, Friedman HS, Gajjar A, Moghrabi A (2017) A phase II feasibility study of oral etoposide given concurrently with radiotherapy followed by dose intensive adjuvant chemotherapy for children with newly diagnosed high-risk medulloblastoma (protocol POG 9631): a report from the Children’s Oncology Group. Pediatr Blood Cancer 64(6)
Stensvold E, Krossnes BK, Lundar T, Due-Tønnessen BJ, Frič R, Due-Tønnessen P, Bechensteen AG, Myklebust TÅ, Johannesen TB, Brandal P (2017) Outcome for children treated for medulloblastoma and supratentorial primitive neuroectodermal tumor (CNS-PNET) – a retrospective analysis spanning 40 years of treatment. Acta Oncol 56(5):698–705
Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, McLendon RE, Bigner DD, Lipp ES, Perreault S, Cho YJ, Grant G, Kim SK, Lee JY, Rao AAN, Giannini C, KKW L, Ng HK, Yao Y, Kumabe T, Tominaga T, Grajkowska WA, Perek-Polnik M, Low DCY, Seow WT, Chang KTE, Mora J, Pollack IF, Hamilton RL, Leary S, Moore AS, Ingram WJ, Hallahan AR, Jouvet A, Fèvre-Montange M, Vasiljevic A, Faure-Conter C, Shofuda T, Kagawa N, Hashimoto N, Jabado N, Weil AG, Gayden T, Wataya T, Shalaby T, Grotzer M, Zitterbart K, Sterba J, Kren L, Hortobágyi T, Klekner A, László B, Pócza T, Hauser P, Schüller U, Jung S, Jang WY, French PJ, Kros JM, van Veelen MC, Massimi L, Leonard JR, Rubin JB, Vibhakar R, Chambless LB, Cooper MK, Thompson RC, Faria CC, Carvalho A, Nunes S, Pimentel J, Fan X, Muraszko KM, López-Aguilar E, Lyden D, Garzia L, Shih DJH, Kijima N, Schneider C, Adamski J, Northcott PA, Kool M, Jones DTW, Chan JA, Nikolic A, Garre ML, Van Meir EG, Osuka S, Olson JJ, Jahangiri A, Castro BA, Gupta N, Weiss WA, Moxon-Emre I, Mabbott DJ, Lassaletta A, Hawkins CE, Tabori U, Drake J, Kulkarni A, Dirks P, Rutka JT, Korshunov A, Pfister SM, Packer RJ, Ramaswamy V, Taylor MD (2016) Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 17(4):484–495
Del Charco JO, Bolek TW, McCollough WM, Maria BL, Kedar A, Braylan RC, Mickle JP, Buatti JM, Mendenhall NP, Marcus RB Jr (1998) Medulloblastoma: time–dose relationship based on a 30-year review. Int J Radiat Oncol Biol Phys 42(1):147–154
Taylor RE, Bailey CC, Robinson KJ, Weston CL, Ellison D, Ironside J, Lucraft H, Gilbertson R, Tait DM, Saran F, Walker DA, Pizer BL, Lashford LS, United Kingdom Children’s Cancer Study Group Brain Tumour Committee; International Society of Paediatric Oncology (2004) Impact of radiotherapy parameters on outcome in the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int J Radiat Oncol Biol Phys 58(4):1184–1193
Kann BH, Park HS, Lester-Coll NH, Yeboa DN, Benitez V, Khan AJ, Bindra RS, Marks AM, Roberts KB (2016) Postoperative radiotherapy patterns of care and survival implications for medulloblastoma in young children. JAMA Oncol 2(12):1574–1581
Frost PJ, Laperriere NJ, Wong CS, Milosevic MF, Simpson WJS, Pintilie M (1995) Medulloblastoma in adults. Int J Radiat Oncol Biol Phys 32(4):951–957
Back M, Ahern V, Berry M, Borg M, Sexton M, Cameron F, Stevens G, Allison R, Childs J, Barton M (2005) Importance of radiation time and dose factors on outcome for childhood medulloblastoma. Austral Radiol 49(4):298–303
Chin AL, Moding EJ, Donaldson SS, Gibbs IC, Soltys SG, Hiniker SM, Pollom EL (2018) Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro-Oncology 20(8):1133–1141
Kunos CA, Coleman CN (2018) Current and future initiatives for radiation oncology at the National Cancer Institute in the era of precision medicine. Int J Radiat Oncol Biol Phys 102(1):18–25
De Rojas T, Puertas M, Bautista F, de Prada I, López-Pino MÁ, Rivero B, Gonzalez-San Segundo C, Gonzalez-Vicent M, Lassaletta A, Madero L, Moreno L (2019) Improving the quality of care in the molecular era for children and adolescents with medulloblastoma. Clin Transl Oncol 21(12):1687–1698
A prospective randomised controlled trial of hyperfractionated versus conventionally fractionated radiotherapy in standard risk medulloblastoma HIT – SIOP PNET 4. HIT-SIOP PNET 4 (NCT01351870). Protocol Version 3.0, 27th July 2010 (RG_10–034).
Rutkowski et al. An international prospective study on clinically standard-risk medulloblastoma in children older than 3 to 5 years with low-risk biological profile (PNET 5 MB - LR) or average-risk biological profile (PNET 5 MB –SR). SIOP PNET 5 Medulloblastoma (NCT02066220). PROTOCOL VERSION, November 21, 2011
De Rojas T, Clementel E, Giralt J, Cruz O, Boterberg T, Kortmann RD, Gaze MN, Moreno L, Janssens GO, SIOP-Europe QUARTET Project and of the EORTC (2019) Radiotherapy practice for paediatric brain tumours across Europe and quality assurance initiatives: current situation, international survey and future perspectives. Eur J Cancer 114:36–46
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Paula Sedano, Carmen González-San Segundo, Lourdes De Ingunza, Pedro Cuesta-Alvaro, and Alvaro Lassaletta. The first draft of the manuscript was written by Paula Sedano, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was waived by the local Ethics Committee of Hospital Universitario Niño Jesus linked to University Autonoma de Madrid. In view of the retrospective nature of the study, all the procedures being performed were part of the routine clinical care.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sedano, P., Segundo, C.GS., De Ingunza, L. et al. Real-world data for pediatric medulloblastoma: can we improve outcomes?. Eur J Pediatr 180, 127–136 (2021). https://doi.org/10.1007/s00431-020-03722-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03722-4