Abstract
Population-based data examining recent epidemiological trends in medulloblastoma, the most common pediatric brain malignancy, are limited. Therefore, we sought to examine recent population-level trends in medulloblastoma incidence and survival. Central Brain Tumor Registry of the United States (CBTRUS) data were analyzed from 2001 to 2013. Age-adjusted incidence rates (IR) and annual percent changes (APCs) with 95% confidence intervals (CI) were calculated by age, sex, and race. Relative survival rates were calculated by age, sex, and race using Surveillance, Epidemiology and End-Results (SEER) registries; subsets of CBTRUS data. Kaplan–Meier and Cox proportional hazards models were used to examine survival differences. Medulloblastoma incidence remained relatively stable from 2001 to 2013, with minor fluctuations from 2001 to 2009 (APC = 2.2, 95% CI 0.8, 3.5) and 2009–2013 (APC = −4.1, 95% CI −7.5, −0.6). Incidence was highest in patients aged 1–4 years at diagnosis, but patients aged 10–14 years showed increased incidence from 2000 to 2013 (APC = 3.2, 95% CI 0.6, 5.8). Males displayed higher IR relative to females (males: 0.16 vs. females: 0.12), except in patients <1 year-old. Compared to Whites, Blacks displayed a non-significant increase in incidence (APC = 1.7, 95% CI −0.4, 4.0) and in mortality risk (hazard ratio for survival = 0.74; p = 0.09). The current study reports no overall change in medulloblastoma incidence from 2001 to 2013. Male and female patients <1 year-old had equal medulloblastoma incidence rates and poor 5-year relative survival compared to other ages. Non-significant trends in the data suggest disparities in medulloblastoma incidence and survival by race. Thus, analysis of tumor-specific trends by demographic variables can uncover clinically informative trends in cancer burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system (CNS) tumors are the most common pediatric solid tumors in the United States, accounting for nearly 20% of all cancers in children aged 0–19 [1]. CNS tumors have one of the highest incidence rates among pediatric cancers and represent the second-most common cause of death from pediatric cancer [2]. Medulloblastoma is the most common malignant pediatric tumor in the CNS, accounting for nearly 20% of all childhood brain cancers and ~40% of all childhood tumors in the posterior fossa [3,4,5]. Despite recent advances in understanding the molecular genetics of medulloblastomas [6], we lack a comprehensive picture of recent population-based trends in the incidence and survival of individuals with medulloblastoma.
Medulloblastoma is a type of embryonal tumor, a histologic group that also included medulloepithelioma, CNS neuroblastoma, CNS ganglioneuroblastoma and atypical teratoid/rhabdoid tumor (ATRT) as well as primitive neuroectodermal tumors (PNET) until the 2016 World Health Organization (WHO) reclassification [7]. Embryonal tumors have similarities in histological features and radiographic appearance, but are dissimilar in their demographic characteristics, treatment, and prognosis. Re-categorization of tumors within this group has occurred over time [2], confounding attempts to establish incidence trends and generating potentially misleading data. Importantly, previous work combining medulloblastoma and PNETs for analysis from 1985 to 2002 demonstrated increased incidence rates with an estimated annual percentage change (APC) of 1.6% (95% CI 0.2, 3.1) [8]. However, upon strictly classifying medulloblastomas by International Classification of Diseases for Oncology (ICD-O) codes, medulloblastoma incidence rates were found to be stable [8]. Given the previous uncertainty regarding medulloblastoma incidence, as well as the paucity of in-depth epidemiological data, the aim of this paper is to provide a comprehensive and updated analysis of medulloblastoma population-specific trends by age, sex, and race/ethnicity.
Methods
This study was approved by the University Hospitals Case Medical Center Institutional Review Board. Data were obtained from the Central Brain Tumor Registry of the United States (CBTRUS), which includes incidence data from approximately 99% of the US population. CBTRUS data are derived from 50 state cancer registries and the cancer registry for the District of Columbia. Together, these include 46 National Program of Cancer Registration (NPCR) and five Surveillance Epidemiology and End Results (SEER) central cancer registries [9]. Age-adjusted incidence rates were generated for medulloblastomas, PNET, ATRT, and other embryonal tumors from 2001 to 2013. Medulloblastomas were specified by four specific ICD-O-3 codes: 9470/3—medulloblastoma, NOS, 9471/3—desmoplastic nodular medulloblastoma, 9472/3—medullomyoblastoma and 9474/3—large cell medulloblastomas, as previously reported [10]. Age-adjusted incidence rates were standardized to the 2000 US population [11], and reported per 100,000 population.
Information on patient outcomes was derived from SEER data, since NPCR registries do not provide follow-up data to CBTRUS. SEER data were analyzed to generate survival data for medulloblastomas from 2001 to 2013. The current SEER registry system consists of 18 registries, representing a subset of the population included in the CBTRUS dataset. Of note, some of the 18 registries are dually funded by NPCR and SEER. According to the US 2010 Census, data pulled from the 18 SEER registries provide population-based information for approximately 28% of the US population [2].
Incidence rates, ratios, and other relevant statistics were calculated using SEER*Stat 8.3.2. Figures were generated using GraphPad Prism 6, Adobe Illustrator and Photoshop, and R statistical software. Statistics were excluded for cells containing fewer than 16 counts. Age-adjusted incidence rates and 95% confidence intervals were estimated for medulloblastomas by sex, race and age groupings including: <1, 1–4, 5–9, 10–14, 15–19 and 20+ years in keeping with previous studies reporting CBTRUS data [2]. Race categories for this study include White, Black, and Asian/Pacific Islander (API). American Indian/Alaskan Native (AIAN) rates were excluded from race analyses due to low count numbers for most years. Unknown, unspecified and other race categories were excluded from race-specific incidence rate comparisons. However, these categories are included in statistics that are not race-specific. Joinpoint Regression Program 4.3.1.0 software was used to compute annual percent change (APC) in incidence rates from 2001 to 2013 to examine trends over time. Joinpoint software selects a minimum number of joinpoints to prohibit statistically significant improvement if one additional joinpoint is added (http://surveillance.cancer.gov/joinpoint). APC analyses were included for medulloblastomas by age, race and sex. APCs in incidence for API populations were not calculated due to missing data points. SEER*Stat was used to generate relative survival percentages by age, race, and sex. For survival data, race categories included White, Black and API. Survival by age at diagnosis was examined using the age groupings described above. Given that the 18 SEER registries comprise only 28% of the CBTRUS dataset, an N > 50 was required for group-specific data inclusion to ensure sufficiently stable statistical analyses. Differences in survival were further analyzed in R using Kaplan–Meier and Cox proportional hazards models. Survival curves were generated by race, sex, radiation status, and surgery status and compared using the log-rank test. Adjusted estimates of survival by race, age, radiation status, and surgery status were also performed in R using a multivariable Cox proportional hazards model. Adjusted estimates included all covariates a priori, regardless of individual significance level. Surgery and radiation status were dichotomized by any therapeutic intervention.
Results
Patient demographics and tumor location
Table 1 shows the general demographic information for patients with medulloblastoma included in the CBTRUS and SEER databases identified by the four specific ICD-O-3 codes examined in this report. There were 5764 total patients included in CBTRUS analyses and 1624 included in SEER analyses. The majority of cases occurred in patients from 1 to 14 years old (61.8% in CBTRUS, 59.7% in SEER). There was a male predominance in both datasets (61.9% males in CBTRUS, 62.4% males in SEER). White race was reported for the majority of cases (83.3% in CBTRUS, 83.0% in SEER). Tumors with recorded site locations in the posterior fossa (cerebellum, brain stem and ventricles) comprised 92.3% of the recorded tumor sites in CBTRUS and 95.1% of recorded tumor sites in SEER.
Incidence rates of medulloblastomas by age group
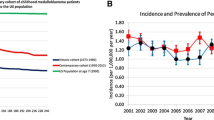
Figure 1a shows the overall age-adjusted incidence rates (IR) for all CNS embryonal tumors and specific histologic diagnoses from 2001 to 2013. Across the years studied, the overall incidence of embryonal tumors peaked in 2008 [IR 0.276 cases per 100,000 (95% CI 0.257, 0.295)] but reached a nadir in 2013 [IR 0.227 (95% CI 0.210, 0.245)]. Of the embryonal tumors, medulloblastoma was more frequently diagnosed than PNET and ATRT [medulloblastoma IR 0.156 (95% CI 0.152, 0.160) vs. PNET IR 0.0496 (95% CI 0.0473, 0.0518) and ATRT IR 0.0228 (95% CI 0.0213, 0.0244)]. Incidence rates for medulloblastomas increased significantly from 2001 to 2009 [annual percentage change (APC) 2.2 (95% CI 0.8, 3.5); Fig. 1b] and then decreased from 2009 to 2013 [APC −4.1 (95% CI −7.5, −0.6); Fig. 1b]. Overall, these changes resulted in minimal absolute variation in medulloblastoma incidence rates between 2001 and 2013.
a Annual age-adjusted incidence rates for medulloblastomas, primitive neuroectodermal tumors (PNET), atypical teratoid/rhabdoid tumors (ATRT) and all embryonal tumors combined. (CBTRUS 2001–2013). b Overall annual percent change (APC) in age-adjusted incidence rates for medulloblastoma. The solid lines depict the trend lines for each group as determined by Joinpoint regression software. The shaded gray regions represent the 95% confidence intervals. (CBTRUS 2001–2013). c Age-adjusted incidence rates for medulloblastomas by age groups studied (CBTRUS 2001–2013)
When stratifying medulloblastoma incidence rates by age groupings, the overall age-adjusted IRs of medulloblastomas from 2001 to 2013 were highest in patients aged 1–4 years (IR 0.629; Fig. 1c) and lowest in patients aged 20+ years (IR 0.058; Fig. 1c). When examining only the 1–4 year age group, no significant change in IRs occurred between 2001 and 2013 [APC 1.0 (95% CI −1.4, 3.5); Fig. 2a, b]. The 20+ age group experienced an increase in incidence from 2001 to 2009 [APC 3.2 (95% CI 0.3, 6.3)] and a significant decrease from 2009 to 2013 [APC −8.5 (95% CI −16.0, −0.2); Fig. 2a, b]. However, the absolute change in incidence rate was moderate in this age group due to low overall incidence. Neither the 5–9 nor the 15–19 age group IRs changed significantly from 2001 to 2013 [5–9 APC −.5 (95% CI −1.9, 0.9); 15–19 APC −1.1 (95% CI −4.2, 2.2); Fig. 2a, b]. Notably, the 10–14 year age group experienced a significant increase in IR across the same time period (APC 3.2, 95% CI 0.6, 5.8; Fig. 2a, b).
Annual age-adjusted incidence rates for medulloblastomas by a 5-year age groups and c by sex. Annual percent change (APC) for medulloblastoma in incidence rates by b 5-year age groups and d by sex. The solid lines depict the trend lines for each group as determined by Joinpoint regression software. The shaded gray regions represent the 95% confidence intervals. Incidence rate ratios of medulloblastomas by 5-year age groups for sex and race are shown in e and f, respectively. (CBTRUS 2001–2013)
Incidence rates and rate ratios according to sex and race
Average annual age-adjusted incidence rates for medulloblastoma by sex are displayed in Fig. 2c. Across all years studied, the overall incidence rates were significantly greater in males than in females. Incidence rates in males increased between 2001 and 2010 [APC 1.4 (95% CI 0.4, 2.5)], but decreased from 2010 to 2013, albeit not significantly [APC −5.4 (95% CI −10.8, 0.2); Fig. 2d]. In females, the incidence rates remained stable across these years [APC 0.7 (95% CI −1.2, 2.6); Fig. 2d]. Incidence rate ratio (IRR) of female to male patients was near 1 in patients aged <1 year but lower in other age groups, which was significant only in patients aged 5–9 years (Fig. 2e).
Whites had significantly higher age-adjusted incidence rates than Blacks across all years studied, except for 2004, 2006, 2012, and 2013 (Fig. 3a). In White populations, incidence rates significantly increased from 2001 to 2008 [APC 2.8 (95% CI 1.0, 4.5); Fig. 3b], then declined significantly until 2013 [APC −3.9 (95% CI −6.6, −10); Fig. 3b]. In contrast, incidence rates for Black populations did not change significantly from 2001 to 2013, although there was a trend towards increasing incidence [APC 1.7 (95% CI −0.4, 4.0); Fig. 3b]. There were no significant differences in incidence rates between Whites and API across the years studied, except in 2010 (Fig. 3c). The IRRs of Black and API populations relative to White populations are shown in Fig. 2f. The Black:White IRR was significantly lower among 5–9 and 10–14 age groups compared to 15–19 year olds. Similarly, the API:White IRR was significantly lower for the 20+ age group compared to 5–9 year olds.
a Annual age-adjusted incidence rates for medulloblastomas by race comparing White to Black populations. b Annual percent change (APC) in incidence rates for medulloblastomas by race comparing White to Black populations. The solid lines depict the trend lines for each group as determined by Joinpoint regression software. The shaded gray regions represent the 95% confidence intervals. c Annual age-adjusted incidence rates for medulloblastoma by race comparing White to Asian/Pacific Islander (API) populations. (CBTRUS 2001–2013)
Survival rates by age-group, sex, and race
Relative survival rates up to ten years post-diagnosis, stratified by age group, sex, and race, are displayed in Supplementary Table 1. Patients <1 year old had lower survival rates for each year post-diagnosis compared to patients aged 5–9, 10–14, 15–19, and 20+ years [<1 year old 10 year survival: 48.4% (95% CI 33.2, 64.5)]. However, survival was similar between patients <1 year old and those 1–4 years old, except at 1 year post-diagnosis, when patients <1 year old at diagnosis had a lower survival rate [60.7% (95% CI 45.4, 72.9) vs. 81.1% (95% CI 76.4, 85)]. Patients aged 1–4 years had lower survival rates for each year post-diagnosis relative to patients aged 5–9, 10–14, and 20+ years up to 5 years post-diagnosis. Survival rates for males and females were similar up to 10 years post-diagnosis.
Black patients displayed slightly lower survival rates for each year post-diagnosis compared to White patients (Supplementary Table 1). In a Kaplan–Meier model of survival by race, we observed significantly poorer survival in Black patients compared to White patients (Fig. 4a; Supplementary Table 2; p = 0.039). This difference in survival remained but was no longer statistically significant (hazard ratio = 0.74, p = 0.086) after adjustment for age at diagnosis (p = 0.26), year of diagnosis (p < 0.001), radiation treatment status (p = 0.35), and surgery status (p = 0.20), with Whites demonstrating a ~26% decrease in mortality risk relative to Blacks (Fig. 4b, c). Adjusting only for treatment factors revealed similar non-significant trends in overall survival. Relative survival rates were not able to be generated by age within race groups, as no group had >50 cases to report.
a Kaplan–Meier curve displaying survival functions for Black and White patients with medulloblastoma. Survival functions were compared statistically via a log-rank test. b Survival curves after adjusting for age at diagnosis, year of diagnosis, and radiation status. Adjustment was conducted using a Cox regression model. c Cox proportional hazards analysis of variables associated with mortality
Discussion
Previous studies examining the incidence of medulloblastoma have produced conflicting results, likely due to inconsistencies in tumor classification and differences in sample sizes [12,13,14]. Additionally, to the best of our knowledge, no previous study has utilized the most recent CBTRUS and SEER registry data to conduct a comprehensive evaluation of age-, sex-, and race-based trends in medulloblastoma incidence and survival. In this study, we show that overall medulloblastoma incidence has remained relatively stable between 2001 and 2013, confirming previous CBTRUS-based data [8]. However, age-specific incidence trends reveal an increasing medulloblastoma incidence in children aged 10–14 years (Fig. 2b), which has not been reported previously. Future studies need to determine whether this age-specific increase in medulloblastoma incidence persists, as well as potential etiologies that may include environmental factors [15].
Consistent with previous work [10], our data showed a clear predilection for males to develop medulloblastoma compared to females (Fig. 3a). Interestingly, the observed male predominance was attenuated in patients <1 year-old, with the data instead demonstrating an equal female:male IRR. This finding may be reflective of differences in biologic subgroups between these patients and those in other age groups. For example, medulloblastomas belonging to the SHH subgroup tend to affect males and females equally compared to tumors in patients with group 3 or 4 medulloblastoma [6]. It is possible that a larger proportion of tumors in patients <1 year of age belong to the SHH subgroup. Indeed, previous work has shown that more than half of medulloblastomas in infants (patients up to 3 years old at diagnosis) are SHH pathway-driven [16]. While SHH-subgroup tumors are associated with an approximate 5-year survival of 75% in patients <3 years of age [5, 16], we observed 5-year survival rates of only 48% for children <1 year old and 62% for children between 1 and 4 years of age (Supplementary Table 1). This discrepancy may be explained by the observation that group 3 tumors, which are associated with poor overall survival [5, 17], are the second-most prevalent tumor subtype (present in ~40% of patients) in patients <4 years old [16]. Therefore, the favorable survival rates associated with SHH tumors may be offset by the poor survival rates for group 3 tumors. However, this report was unable to assess differences in survival by molecular subgroup due to the limitations in CBTRUS-derived data.
When examining incidence rates by race/ethnicity, we found that White individuals were more frequently affected by medulloblastomas than Black individuals from 2001 to 2013 across all age groups. Interestingly, we observed a trend towards increasing incidence in Black individuals from 2001 to 2013 that was distinct from the trends observed in White populations (Fig. 3b). In contrast, incidence in White individuals underwent a significant increase and then decrease during the same period, resulting in a similar incidence rate in 2013 as was observed in 2001 (Fig. 3b). Despite lower incidence rates, we observed poorer survival for Black compared to White patients (Fig. 4 and Supplementary Table 2). These findings are consistent with previous work reporting that Black patients with primary brain tumors had an increased risk of death due to any cause relative to White patients [18]. In our study, differences in survival between Black and White patients persisted but became non-significant after adjusting for age, year of diagnosis and treatment. Previous reports have shown that Black patients have significantly worse access to high-quality neuro-oncologic care relative to White patients [19]. Adjusting for general treatment factors alone in our data eliminated any significant difference in survival between Black and White patients, suggesting that access to care may be an important factor in outcome disparities. However, these disparities may also be influenced by differences in tumor biology, stage at diagnosis, variations in treatment regimens, socioeconomic status, and health beliefs [20]. Indeed, disparities in mortality and survival rates between Black and White patients remain present for all cancer types, despite advances in preventative care and adoption of early detection methods [21]. Further study of the factors leading to such disparities is warranted. Due to the limited number of cases reported to the 18 SEER registries, we were unable to compare survival rates between different ethnicities when stratifying by age groups. In the future, it will be important to examine how pediatric patients from various ethnic backgrounds are differentially impacted by medulloblastoma.
The current study has several limitations that must be considered. First, the data used to generate our survival analyses were collected from the most recent update of all 18 SEER registries. Despite containing high-quality data, these registries represent only about 28% of the US population. The survival trends described in this paper may therefore not fully reflect trends throughout the entire US. Second, our study dataset included tumors with reported locations outside the posterior fossa (1.2% of CBTRUS data and 0.6% of SEER data). The majority of tumors not specifically assigned to the posterior fossa were reported as “brain—not otherwise specified (NOS)” (6.5% of the 7.7% CBTRUS non-posterior fossa tumors and 4.3% of the 4.9% SEER non-posterior fossa tumors; ICD-O-3 site code: C71.8). This category includes the posterior fossa among other brain regions, and may represent tumors originating in the posterior fossa with extension to other regions. Tumors reported as ‘brain-NOS’ were incorporated to ensure inclusion of all posterior fossa tumors. Slight differences between the SEER- and CBTRUS-reported tumor location suggests that the degree of misclassification of tumor type or location is small, so we chose to include all tumors classified as medulloblastoma without regard to location. Lastly, the population-based data available to us lacked information on the molecular profile specific to each patient’s tumor. The recent 2016 update of the WHO classification for tumors of the central nervous system reflects major restructuring of medulloblastomas [7], with the incorporation of molecular subgroups alongside existing histological schema. Because this information was not available in the dataset, we were unable to examine incidence and survival trends in the context of tumors belonging to specific molecular subgroups. As genetic profiling of medulloblastomas becomes well-established into routine clinical algorithms, inclusion of this information into population-level databases such as CBTRUS and SEER will be important to further our understanding of the disease.
Conclusion
The present study provides a comprehensive and up-to-date population-based analysis of recent incidence and survival trends in medulloblastoma by age, sex, and race. In addition to showing stability of medulloblastoma incidence from 2001 to 2013, our results demonstrate poorer survival outcomes for infants and highlight race as a potential variable in medulloblastoma survival. Future studies that are able to incorporate molecular information may provide insight into these survival differences.
References
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A (2014) Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64:83–103. doi:10.3322/caac.21219
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18:v1–v75. doi:10.1093/neuonc/now207
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 19:1541–1544. doi:10.1016/j.jocn.2012.04.009
Koeller KK, Rushing EJ (2003) From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics 23:1613–1637. doi:10.1148/rg.236035168
Gajjar AJ, Robinson GW (2014) Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol 11:714–722. doi:10.1038/nrclinonc.2014.181
Northcott PA, Korshunov A, Pfister SM, Taylor MD (2012) The clinical implications of medulloblastoma subgroups. Nat Rev Neurol 8:340–351. doi:10.1038/nrneurol.2012.78
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Partap S, Curran EK, Propp JM, Le GM, Sainani KL, Fisher PG (2009) Medulloblastoma incidence has not changed over time: a CBTRUS study. J Pediatr Hematol Oncol 31:970–971. doi:10.1097/MPH.0b013e3181bbc502
Gittleman HR, Ostrom QT, Rouse CD, Dowling JA, de Blank PM, Kruchko CA, Elder JB, Rosenfeld SS, Selman WR, Sloan AE, Barnholtz-Sloan JS (2015) Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 121:102–112. doi:10.1002/cncr.29015
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS (2015) Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 10):x1–x36. doi:10.1093/neuonc/nou327
Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) American Brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 18(Suppl 1):i1–i50. doi:10.1093/neuonc/nov297
Alston RD, Newton R, Kelsey A, Newbould MJ, Birch JM, Lawson B, McNally RJ (2003) Childhood medulloblastoma in northwest England 1954 to 1997: incidence and survival. Dev Med Child Neurol 45:308–314
McNeil DE, Cote TR, Clegg L, Rorke LB (2002) Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol 39:190–194. doi:10.1002/mpo.10121
Patel S, Bhatnagar A, Wear C, Osiro S, Gabriel A, Kimball D, John A, Fields PJ, Tubbs RS, Loukas M (2014) Are pediatric brain tumors on the rise in the USA? Significant incidence and survival findings from the SEER database analysis. Childs Nerv Syst 30:147–154. doi:10.1007/s00381-013-2307-1
von Ehrenstein OS, Heck JE, Park AS, Cockburn M, Escobedo L, Ritz B (2016) In utero and early-life exposure to ambient air toxics and childhood brain tumors: a population-based case-control study in California, USA. Environ Health Perspect 124:1093–1099. doi:10.1289/ehp.1408582
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM (2012) Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484. doi:10.1007/s00401-012-0958-8
DeSouza RM, Jones BR, Lowis SP, Kurian KM (2014) Pediatric medulloblastoma—update on molecular classification driving targeted therapies. Front Oncol 4:176. doi:10.3389/fonc.2014.00176
Barnholtz-Sloan JS, Sloan AE, Schwartz AG (2003) Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer 98:603–609. doi:10.1002/cncr.11534
Mukherjee D, Zaidi HA, Kosztowski T, Chaichana KL, Brem H, Chang DC, Quinones-Hinojosa A (2010) Disparities in access to neuro-oncologic care in the United States. Arch Surg 145:247–253. doi:10.1001/archsurg.2009.288
Kann BH, Park HS, Lester-Coll NH, Yeboa DN, Benitez V, Khan AJ, Bindra RS, Marks AM, Roberts KB (2016) Postoperative radiotherapy patterns of care and survival implications for medulloblastoma in young children. JAMA Oncol 2:1574–1581. doi:10.1001/jamaoncol.2016.2547
O’Keefe EB, Meltzer JP, Bethea TN (2015) Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health 3:51. doi:10.3389/fpubh.2015.00051
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 200-2016-M-90304, The Sontag Foundation, Genentech, Novocure, Celldex, AbbVie, along with the Musella Foundation, Voices Against Brain Cancer, and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report. Contents are solely the responsibility of the authors and do not necessarily represent the official view of the CDC.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board at University Hospitals Case Medical Center. For this type of study, formal consent was not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khanna, V., Achey, R.L., Ostrom, Q.T. et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol 135, 433–441 (2017). https://doi.org/10.1007/s11060-017-2594-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2594-6