Abstract
Aim
The necessity of clinical pharmacy services is increasing globally, and their provision is the upcoming direction of hospital pharmacists. The aim of this review was focused on the impact of clinical pharmacy services in the Nepalese healthcare setup.
Subjects and methods
The English-language databases of Web of Science, PubMed, ScienceDirect, and Cochrane Library were searched using the following keywords: ‘pharmacy’, ‘clinical pharmacy’, pharmaceutical services’, ‘pharmacists’, pharmaceutical care’, ‘pharmacy practice’, ‘hospital pharmacy’, ‘pharmaceutical interventions’, and ‘Nepal’. Eligibility for inclusion, risk of bias assessment, and data extraction from the included studies were determined by two authors, and a narrative synthesis was conducted.
Results
This review contained a total of 14 published articles. The included studies involved the counseling and educating of patients, working as a member of the healthcare team, detecting prescribing errors, suggesting clinicians or nurses, reviewing prescriptions on the ward, giving training and monitoring of the implementation of policies, etc. The outcome of these interventions showed a reduction in the direct healthcare cost, excessive use of medicines, and prescription errors, as well as improvement in the drug utilization pattern, knowledge, as well as satisfaction of patients.
Conclusion
In order to implement and enhance clinical pharmacy services, governmental, educational, and administrative support may be needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The necessity of clinical pharmacy services is increasing globally, and their provision is the upcoming direction of hospital pharmacists. In accordance with Barber, clinical pharmacy services are more patient-oriented, which have been developed to promote the rational use of drugs and more precisely maximize therapeutic effect, minimize risk, minimize cost, and respect patient desire (Strand et al. 2004). According to the World Health Organization (WHO), the rational use of drugs is defined as patients who receive drugs appropriate for their clinical requirements, in doses that meet their own individual needs, for an adequate period of time, and at the minimum cost to them and their community (Penm et al. 2014).
In mid-1960s in the USA, an approach was adopted to practice unit dose dispensing. Initially, this was termed “ward pharmacy”. Later, the participation of pharmacists in medical rounds signaled the need for clinical pharmacy to boost the safer use of medication. In 1989, the term “pharmaceutical care” was introduced by Hepler and Strand (1990), which invoked major transformation in clinical pharmacy practice. Pharmaceutical care is a cooperative, patient-centered system. It can be described as the responsible provision of medication treatments with the aim of obtaining specific and positive results for enhancing the patient’s quality of life. In pharmaceutical care, opportunities and responsibilities have been additionally explained by collaborative relations termed as medication therapy management (MTM). MTM defines contemporary models where medication treatment decisions are synchronized collaboratively by clinicians, pharmacists, and other health professionals collectively with the patient (Isetts et al. 2006).
Currently, in the USA, pharmacists can provide immunization and prescribe medication under collaborative practice agreements, where they act as consultants for both patients and healthcare providers and help to reduce medication errors and preventable adverse drug events (Li and Li 2018). Collaborative drug therapy management (CDTM) allows pharmacists to manage medication-related problems according to the general protocol established with related physicians. CDTM can enhance clinical outcomes, medicine safety, patient treatment adherence, and positively influence healthcare expenses (Isetts et al. 2006).
Pharmacists can be asked by clinical primary care or speciality care teams to help medical providers treat large numbers of complex therapy patients. Pharmacists also play a significant role in therapeutic drug monitoring (TDM) by recommending physicians for the most effective treatment regimens (Dingding and Abbas 2017; Li and Li 2018). Clinical pharmacists are introduced as a cost-effective means of addressing drug-related morbidity and mortality (Strand et al. 2004).
Nepal’s National Drug Policy 1995 mentioned that the rational use of drugs will be promoted by involving qualified pharmacists in pharmacy services at all levels of hospital services and other relevant institutions. This was then revised and drafted in the National Medicines Policy 2007, which mentioned that the rational use of medicine will be promoted by constituting a Drugs and Therapeutics Committee and involving pharmacists in the pharmacy services at all levels of hospitals. A further step was taken by the Government of Nepal for the promotion of clinical pharmacy services by legislating the Hospital Pharmacy Guideline 2015, which recommends the presence of at least one pharmacist in hospitals with 26–50 beds and one clinical pharmacist and two pharmacists in hospitals with 51–100 beds (Ranjit 2016).
This literature review is the first of its kind on the impact of clinical pharmacy in a developing country like Nepal. The aim of this literature review was concentrated on the impact of clinical pharmacy services or pharmaceutical care in the Nepalese healthcare setup and to propose avenues for the future enhancement of clinical pharmacy practices.
Methods
Design
A systematic review of interventional studies evaluated the impact of pharmacist interventions in the inpatient and/or outpatient wards of healthcare settings. To ensure high-quality reporting, this review is reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al. 2009) and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green 2011) (see the online appendix 1).
Search strategy
The English-language databases of Web of Science, PubMed, ScienceDirect, and Cochrane library were searched with the following keywords: ‘pharmacy’, ‘clinical pharmacy’, ‘pharmaceutical services’, ‘pharmacists’, ‘pharmaceutical care’, ‘pharmacy practice’, ‘hospital pharmacy’, ‘pharmaceutical interventions’, and ‘Nepal’ (see the online appendix 2). The Nepal Health Research Council website, relevant articles’ bibliographies, as well as the authors’ personal files were hand searched. Articles published up to August 9, 2018, were included in this review.
Study selection criteria
All results from database searches were merged by using Endnote software, duplicate studies were removed, and the titles were screened. Then, the search results were reviewed for the title, abstract, and full-text screening levels by the first two investigators independently and ,in case of discrepancy, the other authors were contacted and a consensus was reached by discussion. The studies which were retained after abstract screening were also examined to find other possible significant studies and to reduce publication bias.
The retrieved studies that met all of the following inclusion criteria were included in this systemic review:
- Design:
Randomized controlled trials (RCTs), controlled clinical trials (CCTs), controlled before–after (CBA) studies, interrupted time series (ITS), and pre-post studies without concurrent controls.
- Interventions:
The pharmacist or organization in which the pharmacist played a role in the following intervention(s): patient consultation, taking part in rounds with the healthcare team, medication profile and medical record review, drug monitoring and recommendation of follow-up, presentation of recommendations to the healthcare team or physician, drug therapy management, documentation of clinical interventions or recommendations, patient counseling before discharge and telephonic follow-up after discharge, etc.
- Participants:
Patients, organizations, and healthcare providers.
- Outcome:
Essential outcomes such as mortality, adverse drug reactions (ADRs) (identification of the occurrence and severity of ADRs; prevention of ADRs; ADRs that needed further treatment), drug–drug interactions (DDIs), health facilities use (admission and readmission frequencies due to illness; length of hospitalization; refer to more intensive care; urgent care use after discharge), drug monitoring and pharmacokinetic dosing (appropriateness of drug level; time to therapeutic level; time consumed at the therapeutic level), prescribing error (dose and dosing frequency error; therapy error; number of drugs per prescription; appropriateness of medication), and other measures (quality of life; patient satisfaction; treatment adherence; knowledge of medication regimen; knowledge, attitude, and practice [KAP] of disease).
- Comparator:
Historical or concurrent control groups.
Studies without a description of the comparative outcome before and after the intervention were excluded from this review. Studies for which only abstracts were obtainable, letters to the editor, editorials, reviews, newsletters, and commentaries without comparative outcomes were also excluded from this review.
Risk of bias assessment
The Cochrane’s risk of bias tool was used to assess the risk of bias for all included studies (Higgins and Green 2011), which comprised six bias domains, such as selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Furthermore, the six bias domains contain the following quality items: adequacy of the generation of the allocation sequence, concealment of allocation, blinding procedures, incomplete outcome data, selective outcome reporting, and other sources of bias were checked. Each study was validated by using low risk, high risk, and unclear risk provided by this tool. The first two authors assessed each study independently and disagreements were resolved by discussion with the other authors and reaching a consensus.
Data extraction
Data collection was performed using a predesigned form that was piloted and refined before the data extraction phase. Essential constituents of these studies were extracted by the first two investigators in discussion with the other investigators by a standardized procedure. The resulting information was extracted from the included studies: first author, year of publication, participants’ characteristics, study area, study design, follow-up period, intervention used with specifications, sample size, main analytical method, major selected outcomes, and conclusive remarks. Additionally, these studies were organized by patient care setting, therapeutic area, and publication trends.
Results
Number of studies
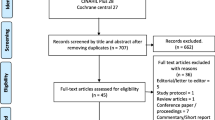
The initial literature search of all databases identified 1000 articles and a further additional search lead to three potentially relevant articles being identified. After the removal of duplicates and title screening, 61 full-text articles were reviewed and screened. Further refining on the basis of inclusion criteria lead to a total of 14 articles, which were fully reviewed (Fig. 1). The excluded studies, along with their reason for exclusion, are presented in the online appendix 4.
Study setting and intervention used
Most of the studies were conducted in both inpatient and outpatient settings (42%), while an equal proportion of studies were conducted in inpatient (29%) or outpatient settings (29%) (Table 1). In the outpatient settings, the majority of studies involved counseling of the patients (Sharma et al. 2014; Poudel et al. 2016), educating patients (Sharma et al. 2014; Poudel et al. 2016), detecting prescribing errors (Poudel et al. 2015), contacting the prescriber if there is any severe prescription error and providing a suggestion for correction or discontinuation (Poudel et al. 2015), and enlightening the prescriber about the rational use of drugs (Rauniar et al. 2014). Most of the studies conducted in inpatient settings involved interventions such as the pharmacist providing consultation and pharmaceutical care to the patients (Upadhyay et al. 2015, 2016), educating patients regarding types, signs, and symptoms, causes, risk factors, pharmacological and non-pharmacological management of disease (Upadhyay et al. 2015, 2016), giving training about how to use medication kits (Upadhyay et al. 2015, 2016), participating in daily rounds with the prescriber (Bista et al. 2009), giving a presentation or suggestions to the clinician or nurses and reviewing prescriptions on the ward (Bista et al. 2009), enhancing the KAP of patients (Ghimirey et al. 2013), and most of the studies conducted in both inpatient and outpatient settings involved providing consultations regarding the use of medical kits (Ansari et al. 2005; Khan et al. 2016), giving training to the prescribers for promoting rationality of drug use (Shrestha et al. 2006; Kafle et al. 2009), monitoring the implementation of training to the prescribers and implementation in hospital policies (Holloway et al. 2001, 2008; Kafle et al. 2009) (Table 2).
Studies were categorized according to their study area of pharmacist intervention. Twenty-nine percent of studies were focused on respiratory care, 14% studies on diabetes care, and 7% of studies each on hypertension, nephrology, dental, and internal medicine wards. Likewise, 29% of studies was conducted on multiple therapeutic areas or was an institution which was not specific in one ward or field (Table 1).
Risk of bias in the included studies
In general, all of the included studies showed a high risk of bias. Out of the 14 studies, eight studies were pre-post studies without concurrent control groups and bounded by severe methodological limitations. The remaining six studies, however, used comparatively vigorous research methods (CBA and RCT) but also suffered from a high risk of bias. Both CBA studies by Holloway et al. (2001, 2008) and one by Ghimirey et al. (2013) suffered from the lack of random allocation of the intervention and control groups. These studies failed to provide baseline outcome measurements and characteristics of the regions and their similarity. Moreover, these studies also failed to provide information regarding knowledge of the allocated interventions adequately prevented during the study. The included RCT studies were also not free from important biases (Shrestha et al. 2006; Upadhyay et al. 2015, 2016). These studies have an unclear description of the random component in the sequence generation process, as well as no information regarding sequence allocation. Both of the studies by Upadhyay et al. (2015, 2016) suffer from a lack of information regarding the similarity of baseline characteristics and baseline outcome measures across study groups, while in the study by Shrestha et al. (2006), knowledge of the allocated interventions adequately prevented during the study is not specified in the paper. The included controlled trial studies have lower quality because of the lack of the impact of confounding factors and different baseline characteristics of states and of the matching process. All of the other included studies suffered from the lack of a control group; therefore, their outcomes are subject to important biases (see the online appendix 3).
Impact of pharmaceutical care
Most of the included studies showed that an impact of pharmacist intervention (Upadhyay et al. 2016) or monitoring implementation of training to the prescribers and implementation in hospital policies (Holloway et al. 2001, 2008; Shrestha et al. 2006; Kafle et al. 2009) reduced the direct healthcare cost of admitted patients. Pharmacist intervention minimized the incidence of DDIs, use of excessive medicines (Bista et al. 2009), and the occurrence of prescription errors (Poudel et al. 2015), as well as improved the prescribing behaviors and rationalized drug utilization patterns (Rauniar et al. 2014). Furthermore, the intervention carried out by pharmacists considerably improved the knowledge of the patient on inhalation technique (Ansari et al. 2005; Khan et al. 2016; Poudel et al. 2016), improved the KAP of patients (Ghimirey et al. 2013; Sharma et al. 2014), and significantly enhanced the patient satisfaction level (Upadhyay et al. 2015) (Table 2).
Publication trends
All included studies were differentiated on the basis of their year of publication and the results are plotted in a line chart (Fig. 2). The publication trend started from the year 2001 and 2016 reported the highest number of publications on the impact of pharmaceutical care.
Discussion
This review is the first of its kind to highlight the impact of clinical pharmacy services in Nepal. Fourteen studies met the inclusion criteria. Most of the studies were pre-post interventional studies. Only six studies employed a research design with the lower risk of bias, i.e., three CBA and three controlled trials studies (Holloway et al. 2001, 2008; Shrestha et al. 2006; Ghimirey et al. 2013; Upadhyay et al. 2015, 2016).
The conducted study reveals that intervention made by pharmacists positively influenced patient care, enhanced appropriate prescribing and medications use, enhanced patient understanding on drug therapy, increased patient satisfaction and knowledge, and lowered the overall cost of medication. Three studies conducted by the WHO and the International Network for Rational Use of Drugs (INRUD), Nepal, did not clearly mention the presence of pharmacists being directly involved in training/educational intervention (Holloway et al. 2001, 2008; Kafle et al. 2005), nor did they describe the role of pharmacists for the implementation of Practical Approach to Lung Health (PAL) guidelines (Shrestha et al. 2006). However, the intervention for pharmaceutical care, educating prescribers for better prescribing, implementing rationality of medication use, and promoting unit dose dispensing describes the role of the clinical pharmacist. All of the reported studies showed that the intervention made by clinical pharmacists positively influenced patient care.
Concerning the impact of clinical pharmacy services, our studies from Nepal, a low-income country, showed a reduction in a prescription error, DDIs, inappropriate prescriptions, and overall costs of medications, as well as an improvement in patient understanding about the state of disease and medication use, patient satisfaction, and rationality of drug use. This is consistent with previous literature reviews, such as Penm et al. (2014), who studied China, Pickard and Hung (2006), Strand et al. (2004), Schumock et al. (2003), Perez et al. (2009), and Kaboli et al. (2006) studying the USA, De Rijdt et al. (2008) studying Belgium, and Viktil and Blix (2008), who studied Norway. However, our study did not report the outcome-related mortality, morbidity, and ADRs, as reported by Penm et al. (2014), Schumock et al. (2003), Perez et al. (2009), De Rijdt et al. (2008), etc. The reasons for this may be due to the fact that the clinical pharmacy service is at a very early phase of development, poor implementation of Hospital Pharmacy Guideline 2015, lesser acceptance by healthcare providers, lack of policies for strengthening and empowering the role of clinical pharmacists as per international practices, lack of trained clinical pharmacists, unstable government, lack of government determination, etc.
None of the studies included in this review reported on the complete implementation of a clinical pharmacy service in any hospital throughout the country. Implementing a clinical pharmacy service as mentioned in the Hospital Pharmacy Guideline 2015 is essential in Nepal. As Nepal is one of the poorest countries in the world, increasing healthcare costs will affect the reach of standard healthcare to the general public. Hospitals should be encouraged to implement clinical pharmacy services. Several studies from a neighboring country China highlighted that implementing clinical pharmacy services does not always need additional assets but can happen by the reallocation of existing resources (Penm et al. 2014). The Government of Nepal needs to be more focused on implementing clinical pharmacy services and provide a platform for newly graduated clinical pharmacists and PharmD graduates. More skilled clinical pharmacists would be needed for all hospitals in order to meet the requirements set by the Government of Nepal to ensure the presence of at least one pharmacist in hospitals with 26–50 beds and one clinical pharmacist and two pharmacists in hospitals with 51–100 beds (Ranjit 2016). Currently, the Ministry of Health and Population of Nepal consists of 102 hospitals and 208 PHCs, as well as 305 health centers based on Ayurveda, an alternative healthcare practice (Ranjit 2016). Pokhara University started producing manpower in clinical pharmacy practice/pharmaceutical care and PharmD graduates are available from Kathmandu University or other universities of different countries. It, therefore, seems inescapable that Nepal will be capable of meeting the clinical pharmacy manpower requirements for providing clinical pharmacy services as a routine part of the hospital system.
Furthermore, current hospital pharmacy guidelines seem to be lacking in providing authority and obvious guidelines for performing clinical pharmacy practices. This needs to be amended as per international policies as well as establishing settings for research and development to further enhance the knowledge, innovation, and growth in this field. An effective management and monitoring system needs to be implemented. Finally, the clinical pharmacist should prove themselves to be accepted by healthcare providers and healthcare providers should accept and encourage the outcomes brought with them for better patient care.
Strengths of this review
This review is perhaps the first perception towards the impact of clinical pharmacy services from a low-income country. Nepal’s new hospital pharmacy directive is in favor of implementing clinical pharmacy services in Nepalese hospitals. This review will mobilize government officials to implement clinical pharmacy services and to make the decision for further improvement and issuance of rules and regulations regarding clinical pharmacists. This review also provides a view of the current scenario and outcomes of clinical pharmacy services in Nepalese healthcare setups and will guide the new researcher to report further research to identify such outcomes which were not obtained in this review.
Limitations of this study
The limitations of this review and its included studies are numerous. Many pharmacist intervention studies have a small sample size, short follow-up period, and several are single-institution or -ward studies, limiting generalizability. The study designs of the included studies also have limitations; scant information was given on the precise nature of the intervention used, some studies are limited to one technique, while some use multiple interventions simultaneously and some are unable to provide clear outcomes. A comparable limitation in a similar study design has been identified in the reviews of Penm et al. (2014), Kaboli et al. (2006), and De Rijdt et al. (2008). Due to outcome variation, methodological limitations and discrepancies within the included studies prevented carrying out a meta-analysis. Inadequate randomization in studies, lack of concurrent control groups, and pre-post studies without control groups were major concerns when assessing the risk of bias. The nature of systemic reviews is subject to publication bias. This review is retrospective and observational, and, due to prospective defects in the search strategy, there might be some systematic and random errors, which makes it uncertain that all related articles published were included. However, this review revealed that clinical pharmacy facilities had a positive outcome towards patient care and appropriate use of medications within Nepal’s territory.
Conclusion
From the conducted studies, it can be elucidated that clinical pharmacy services appear to positively influence patient care, appropriate use of medications, as well as decreasing overall medication cost and improving patient satisfaction. They can make a valuable contribution to Nepal’s healthcare system. In order to implement and enhance clinical pharmacy services, governmental, educational, and administrative support may be needed.
Change history
22 May 2019
The Fig. 1 was incompletely filled. The correction and corresponding data are shown below. Addition to Fig. 1 “Records identified through database searching”: <Emphasis Type="Bold">Cochrane library (</Emphasis><Emphasis Type="BoldItalic">n</Emphasis> <Emphasis Type="Bold">= 6)</Emphasis>. The original article has been corrected.
References
Ansari M, Rao BS, Koju R, Shakya R (2005) Impact of pharmaceutical intervention on inhalation technique. Kathmandu Univ J Sci Eng Technol 1:1–8
Bista D, Saha A, Mishra P, Palaian S, Shankar PR (2009) Impact of educational intervention on the pattern and incidence of potential drug–drug interactions in Nepal. Pharm Pract (Granada) 7:242–247
De Rijdt T, Willems L, Simoens S (2008) Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm 65:1161–1172. https://doi.org/10.2146/ajhp070506
Dingding C, Abbas M (2017) A critical review; the quest of clinical pharmacist in Pakistan government hospital, references USA and China hospital setting, to salvage the public health problems like diabetes. J Basic Clin Pharm 8:1–6
Ghimirey A, Sapkota B, Shrestha S, Basnet N, Shankar PR, Sapkota S (2013) Evaluation of pharmacist counseling in improving knowledge, attitude, and practice in chronic kidney disease patients. SAGE Open Med 1:2050312113516111. https://doi.org/10.1177/2050312113516111
Hepler CD, Strand LM (1990) Opportunities and responsibilities in pharmaceutical care. Am J Health Syst Pharm 47:533–543
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration
Holloway KA, Gautam BR, Reeves BC (2001) The effects of different kinds of user fee on prescribing costs in rural Nepal. Health Policy Plan 16:421–427
Holloway KA, Karkee S, Tamang A, Gurung YB, Pradhan R, Reeves BC (2008) The effect of user fees on prescribing quality in rural Nepal: two controlled pre-post studies to compare a fee per drug unit vs. a fee per drug item. Trop Med Int Health 13:541–547. https://doi.org/10.1111/j.1365-3156.2008.02032.x
Isetts BJ, Schondelmeyer SW, Heaton AH, Wadd WB, Hardie NA, Artz MB (2006) Effects of collaborative drug therapy management on patients’ perceptions of care and health-related quality of life. Res Social Adm Pharm 2:129–142
Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL (2006) Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med 166:955–964
Kafle KK, Shrestha N, Karkee SB, Prasad RR, Bhuju GB, Das PL (2005) Intervention studies on rational use of drugs in public and private sector in Nepal. Nepal Med Coll J 7:47–50
Kafle KK, Bhuju GB, Karkee SB, Prasad RR, Shrestha N, Shrestha AD, Das PL, Chataut BD, Daud M (2009) An intervention improving prescribing practices and monitoring drugs availability in a district. Nepal Med Coll J 11:217–221
Khan GM, Badri P, Parbati T, Anita D, Atul A, Deepak P, Dipendra R, Himal B, Kabita G, Nirmala K (2016) Intervention on inhalation technique of rotahaler in patients with chronic obstructive pulmonary disease and asthma. Asian J Pharm Sci 11:81–82. https://doi.org/10.1016/j.ajps.2015.11.112
Li J, Li Z (2018) Differences and similarities in clinical pharmacy practice in China and the United States: a narrative review. Eur J Hosp Pharm 25:2–5. https://doi.org/10.1136/ejhpharm-2016-001195
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Penm J, Li Y, Zhai S, Hu Y, Chaar B, Moles R (2014) The impact of clinical pharmacy services in China on the quality use of medicines: a systematic review in context of China’s current healthcare reform. Health Policy Plan 29:849–872. https://doi.org/10.1093/heapol/czt067
Perez A, Doloresco F, Hoffman JM, Meek PD, Touchette DR, Vermeulen LC, Schumock GT (2009) Economic evaluations of clinical pharmacy services: 2001–2005. Pharmacotherapy 29:128
Pickard AS, Hung S-Y (2006) An update on evidence of clinical pharmacy services’ impact on health-related quality of life. Ann Pharmacother 40:1623–1634. https://doi.org/10.1345/aph.1G653
Poudel RS, Shrestha S, Khatiwada D, Thapa S, Prajapati A, Thapa L, Baral R (2015) Prescription errors and pharmacist’s intervention at outpatient pharmacies of two teaching hospitals of central Nepal. World J Pharm Sci 3:448–452
Poudel RS, Piryani RM, Shrestha S, Prajapati A (2016) Benefit of hospital pharmacy intervention on the current status of dry powder inhaler technique in patients with asthma and COPD: a study from the Central Development Region, Nepal. Integr Pharm Res Pract 6:7–13. https://doi.org/10.2147/IPRP.S119202
Ranjit E (2016) Pharmacy practice in Nepal. Can J Hosp Pharm 69:493–500
Rauniar GP, Das BP, Manandhar TR, Bhattacharya SK (2014) Effectiveness of an educational feedback intervention on drug prescribing in dental practice. Kathmandu Univ Med J 10:30–35. https://doi.org/10.3126/kumj.v10i4.10991
Schumock GT, Butler MG, Meek PD, Vermeulen LC, Arondekar BV, Bauman JL (2003) Evidence of the economic benefit of clinical pharmacy services: 1996–2000. Pharmacotherapy 23:113–132
Sharma S, Bhuvan KC, Alrasheedy AA, Kaundinnyayana A, Khanal A (2014) Impact of community pharmacy-based educational intervention on patients with hypertension in Western Nepal. Australas Med J 7:304–313. https://doi.org/10.4066/AMJ.2014.2133
Shrestha N, Samir KC, Baltussen R, Kafle KK, Bishai D, Niessen L (2006) Practical approach to lung health in Nepal: better prescribing and reduction of cost. Trop Med Int Health 11:765–772. https://doi.org/10.1111/j.1365-3156.2006.01599.x
Strand LM, Cipolle RJ, Morley PC, Frakes MJ (2004) The impact of pharmaceutical care practice on the practitioner and the patient in the ambulatory practice setting: twenty-five years of experience. Curr Pharm Des 10:3987–4001
Upadhyay DK, Ibrahim MIM, Mishra P, Alurkar VM (2015) A non-clinical randomised controlled trial to assess the impact of pharmaceutical care intervention on satisfaction level of newly diagnosed diabetes mellitus patients in a tertiary care teaching hospital in Nepal. BMC Health Serv Res 15:57. https://doi.org/10.1186/s12913-015-0715-5
Upadhyay DK, Ibrahim MIM, Mishra P, Alurkar VM, Ansari M (2016) Does pharmacist-supervised intervention through pharmaceutical care program influence direct healthcare cost burden of newly diagnosed diabetics in a tertiary care teaching hospital in Nepal: a non-clinical randomised controlled trial approach. Daru 24:6. https://doi.org/10.1186/s40199-016-0145-x
Viktil KK, Blix HS (2008) The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol 102:275–280. https://doi.org/10.1111/j.1742-7843.2007.00206.x
Acknowledgements
The authors greatly acknowledge Dr. Muhammad Abbas (School of Life Sciences, Nanjing University, People’s Republic of China) for his guidance and insightful comments on this manuscript. The lead author (Reyaj Mikrani) is thankful to the China Scholarship Council (CSC) for its generous financial support for him to study in China.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 30973003 and 30901993) and the Administration of Traditional Chinese Medicine of Jiangsu Province (grant number LZ11093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare that they have no conflicting interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Mikrani, R., Naveed, M., Mikrani, A. et al. The impact of clinical pharmacy services in Nepal in the context of current health policy: a systematic review. J Public Health (Berl.) 28, 245–255 (2020). https://doi.org/10.1007/s10389-019-01042-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-019-01042-y