Abstract
The pathogenesis of sporadic hemiplegic migraine remains unclear, and perfusion-weighted magnetic resonance imaging (PWI) has been used for characterization of hemodynamic changes in migraine aura. We report a case of sporadic hemiplegic migraine in which magnetic resonance perfusion imaging showed left cerebral hypoperfusion. Dynamic susceptibility contrast (DSC) perfusion maps showed hypoperfusion with posterior predominance in the left cerebral hemisphere. Findings with arterial spin labeling (ASL) perfusion correlated well with DSC perfusion findings.
Conclusion: With unique advantages compared with DSC PWI, ASL has significant potential in the evaluation of the patients with sporadic hemiplegic migraine.
What is Known: • Sporadic hemiplegic migraine is a rare variety of migraine defined by migraine attacks, which include the presence of motor weakness/hemiparesis during the aura phase and where no first- or second-degree relative (parent, sibling, or child) has identical attacks. |
What is New: • Reports on imaging abnormalities described in sporadic hemiplegic migraine are sparse. • To our knowledge, this is the first report to describe arterial spin labeling (ASL) perfusion abnormalities in patients with sporadic hemiplegic migraine, as compared with dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging (PWI). |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporadic hemiplegic migraine is a rare variety of migraine defined by migraine attacks, which include the presence of motor weakness/hemiparesis during the aura phase; affected patients have no first- or second-degree relative (parent, sibling, or child) suffering from identical attacks [4]. The prevalence of hemiplegic migraine in the population is ~1/10,000, and attacks usually start during childhood or adolescence [10]. The pathogenesis of migraine remains unclear, and perfusion-weighted magnetic resonance imaging (PWI) has been used for characterization of hemodynamic changes in migraine aura. Compared with other imaging techniques, MRI with arterial spin labeling (ASL), which measures blood flow using arterial water as an endogenous tracer, offers advantages. We report a case of sporadic hemiplegic migraine with MRI findings suggestive of cerebral hypoperfusion on PWI. To our knowledge, this is the first report to describe perfusion abnormalities detected using ASL in patients with sporadic hemiplegic migraine as compared with dynamic susceptibility contrast (DSC) PWI.
Case report
A 16-year-old boy was admitted with a history of headache, right-sided numbness and hemiparesis, and slurring of speech. He complained of headache with throbbing pain in the left cerebral hemisphere several minutes after the speech disturbance and right-sided weakness. He was admitted to our hospital 3 h 30 min after the onset of symptoms. Right-sided weakness improved gradually on the way to our hospital, and physical examination on admission revealed dysarthria, right-sided facial palsy, and normal fundal appearance. His pediatric National Institutes of Stroke Scale Score was 2 (facial palsy-1, dysarthria-1). Vital signs were as follows: temperature 36.8 °C; pulse 92/min; blood pressure 130/80 mmHg. His echocardiogram and electroencephalogram results were normal.
There was no history of fever, trauma, or seizure preceding the episode. He had a history of headache after transient speech disturbance and mild right arm weakness from the age of 14. There was no history of similar complaints in any of his family members as ascertained by interviewing his mother.
After obtaining written informed consent from the patient’s mother, we performed cerebral MR imaging 4 h after the onset of symptoms using a 3.0 T MR system (Milwaukee, WI, USA) with 16-channel head-neck-spine array receiving coil. ASL scan was performed with pseudo-continuous labeling, background suppression, and a stack of spirals of 3D fast spin-echo imaging sequence (slice thickness: 7 mm, field of view: 21 cm, TR: 4439 ms, TE: 9.7 ms, ET: 1 ms, labeling duration: 1500 ms, post-labeling delay: 1525 ms, number of excitations: 2, spiral matrix: 512 × 8, acquisition time: 3 min 7 s).
DSC PWI was performed using a contrast bolus injection with very rapid EPI-based image acquisition. DSC PWI parameters were TR 2000 ms, TE minimum, FOV 22 × 22 cm, slice thickness 5 mm, spacing 2 mm, matrix 128 × 128, flip angle 60°, NEX 1, and acquisition time 1 min 20 s.
A large-bore 18-gauge intravenous cannula was inserted to administer a 0.2 mmol/kg bolus of gadolinium diethylenetriamine pentaacetic acid at the rate of 4 mL/s using an MR compatible power injector. Post processing was done using the same vendor-specific dedicated software (ADW 4.6 workstation; GE Medical Systems) to generate color-coded and parametric perfusion maps. Regions of interest (ROI) were placed in the left parieto-occipital and fronto-parietal areas. The same ROI used in the ASL map was applied to the relative cerebral blood flow (rCBF) and time-to max (Tmax) map of DSC PWI.
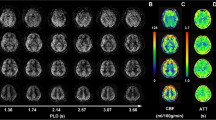
ASL perfusion map showed hypoperfusion with posterior predominance in the left cerebral hemisphere (Fig. 1). CBF values obtained using ASL in the parieto-occipital and fronto-parietal areas of the left cerebral hemisphere were 17.5 and 27.4 mL/100 g tissue/min, respectively. The ROI placed over the left parieto-occipital area on the rCBF map showed a mean CBF value of 44.15 mL/100 g tissue compared with the left fronto-parietal area mean CBF of 47.18 mL/100 g tissue/min. In regions of the parieto-occipital area, the Tmax delay of 4.5 s was higher compared with the fronto-parietal area in which the delay was 3.3 s.
a The arterial spin labeling (ASL) perfusion map showed the hypoperfusion areas with posterior predominance in the left cerebral hemisphere. Region of interest (ROI) placed over the parieto-occipital area showed a mean cerebral blood flow (CBF) value of 17.5 compared with 27.4 mL/100 g tissue/min of the fronto-parietal area. b In regions of parieto-occipital area, the time-to max (Tmax) delay of 4.5 s was higher compared to the fronto-parietal area, 3.3 s. c ROI placed over the left parieto-occipital area on a relative CBF map showed a mean CBF of 44.15 mL/100 g tissue compared with 47.18 mL/100 g tissue/min of the left fronto-parietal area. d With the fusion technique, hypoperfusion in ASL perfusion map corresponded well with the hypoperfusion area seen on the Tmax map of dynamic susceptibility contrast (DSC) perfusion-weighted magnetic resonance imaging (PWI). e Magnetic resonance angiography demonstrated subtle vasoconstriction of peripheral branches of the left middle and posterior cerebral arteries (arrows)
With the fusion technique, hypoperfusion observed in ASL perfusion map corresponded well with the hypoperfusion area seen on the rCBF and Tmax maps of DSC PWI. MR angiography showed subtle vasoconstriction of the left middle and posterior cerebral artery branches. DWI, ADC, and FLAIR showed no abnormality.
The patient’s dysarthria and facial palsy improved significantly soon after the MRI study; however, dull headache persisted. Vital signs were stable. The patient was administered nimodipine and showed gradual and complete recovery over the next 5 days. He did not have any further episodes of headache or neurological deficit over a 1-month follow-up. Unfortunately, follow-up imaging studies were not performed.
Discussion
Our patient presented with a history of sensory symptoms and dysarthria accompanied with headache and associated hemiparesis. None of his family members had experienced similar symptoms. The clinical diagnosis of migraine is not easily established because its symptoms can mimic cerebral ischemia. However, hemodynamic changes on PWI did not represent any single arterial territory, DWI findings were negative, and MR angiography did not show vascular occlusion. Thus, our patient fulfilled the criteria for the diagnosis of sporadic hemiplegic migraine [4].
Sporadic hemiplegic migraine is a rare variant of migraine and a possible common pathophysiology in different types of migraines is a vascular or neurogenic dysfunction. The vascular theory of migraine proposes that migraines are dynamic forms of cerebral pathologic disorders with clinical features and perfusion abnormalities evolving with time, which suggest that migraine headache is caused by dilatation of blood vessels, while the aura of migraine results from vasoconstriction [9]. A popular theory on the causation of migraine is that a depolarizing neuroelectric or metabolic stimulus results in a wave of spreading depolarization analogous to the spreading depression [1]. This could be excessive or unusually prolonged in the presence of abnormal calcium or sodium channels. The altered membrane permeability and ensuing dysfunction might be clinically evident as auras or hemiplegia [7].
Literature on imaging abnormalities associated with sporadic hemiplegic migraine is sparse. Most findings consist of either restricted diffusion, normal or increased diffusion based on DWI, and ADC values involving a hemisphere opposite to the side of deficit associated with normal T2- and T1-weighted images [8]. T2-weighted imaging during hemiplegic migraine typically shows cortical edema without infarction [5, 9]. Magnetic resonance spectroscopy has suggested a reduced myoinositol/creatinine ratio or reduced N-acetylaspartate (NAA) levels on the affected side with a normal choline/creatinine ratio [7]. Migraine aura is usually associated with a deficit in perfusion not limited to a specific vascular territory, and MR angiography may demonstrate subtle vascular abnormalities such as dilated vessels or reduced visualization of peripheral branches in a subset of patients with migraine aura [2].
Our patient showed abnormalities on PWI and MR angiography. These findings demonstrate the usefulness of PWI for understanding the pathogenesis of migraine in patients with acute hemiparesis. The perfusion map we obtained with ASL corresponded well with the DSC perfusion maps. ASL perfusion map showed hypoperfusion with posterior predominance in the left cerebral hemisphere. These results are in concordance with findings by Förster who reported posterior predominance of hypoperfusion during the aura [2]. The theory of migraine pathogenesis that neuronal depolarization spreads forward from the posterior cortex inducing secondary cerebral vasculature changes may explain why and how posterior predominance of hypoperfusion occurs during the aura [3]. MR angiography demonstrated vasoconstriction of peripheral branches of the left middle and posterior cerebral arteries. This is of clinical importance because it supports the notion that triptans, which are usually prescribed for treatment of migraines, are contraindicated in sporadic hemiplegic migraine. Triptans should be avoided in patients with hemiplegic migraine because of the vasoconstrictive properties of this class of drugs and concerns regarding stoke.
ASL has several advantages compared with DSC PWI. ASL requires no gadolinium-based contrast, which is an advantage in the case of young patients and in those who may have renal impairment.
In addition, ASL provides information on absolute CBF and allows repeated measurements that may be used to evaluate multiple interventions. Because of its unique advantages compared with conventional perfusion studies, this perfusion sequence has significant potential for evaluation of patients with acutely symptomatic migraines. Although ASL can provide absolute quantification of CBF, there are some issues of concern, such as underestimation of CBF because of delayed blood flow, insensitivity toward white matter, and poor signal-to-noise ratio [6]. These flaws may lead to the inaccuracy of ASL in comparison to DSC; therefore, improvements in the ASL technique are necessary.
Conclusion
When patients with acute migraine symptoms and neurologic deficits undergo imaging examination, conventional imaging sequences may not reveal any abnormalities. However, hypoperfusion may be visualized in the affected cerebral hemisphere on PWI.
With unique advantages compared with DSC PWI, the ASL technique has significant potential for evaluation of patients with sporadic hemiplegic migraine.
Abbreviations
- ASL:
-
Arterial spin labeling
- CBF:
-
Cerebral blood flow
- DSC:
-
Dynamic susceptibility contrast
- PWI:
-
Perfusion-weighted magnetic resonance imaging
- Tmax:
-
Time-to max
References
Aurora SK, Welch KM (2000) Migraine: imaging the aura. Curr Opin Neurol 13(3):273–276
Förster A, Wenz H, Kerl HU, Brockmann MA, Groden C (2014) Perfusion patterns in migraine with aura. Cephalalgia 34(11):870–876
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 98(8):4687–4692
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33(9):629–808
Hsu DA, Stafstrom CE, Rowley HA, Kiff JE, Dulli DA (2008) Hemiplegic migraine: hyperperfusion and abortive therapy with intravenous verapamil. Brain Dev 30(1):86–90
Huang YC, Liu HL, Lee JD, Yang JT, Weng HH, Lee M, Yeh MY, Tsai YH (2013) Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MRI in patients with acute stroke. PLoS One 8(7):e69085
Jacob A, Mahavish K, Bowden A, Smith ET, Enevoldson P, White RP (2006) Imaging abnormalities in sporadic hemiplegic migraine on conventional MRI, diffusion and perfusion MRI and MRS. Cephalalgia 26(8):1004–1009
Lindahl AJ, Allder S, Jefferson D, Allder S, Moody A, Martel A (2002) Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J Neurol Neurosurg Psychiatry 73(2):202–203
Oberndorfer S, Wöber C, Nasel C, Asenbaum S, Lahrmann H, Fueger B, Grisold W (2004) Familial hemiplegic migraine: follow- up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 24(7):533–539
Thomsen LL, Olesen J (2004) Sporadic hemiplegic migraine. Cephalalgia 24(12):1016–1023
Conflict of interest
The authors declare that they have no (financial) conflict of interest.
Ethical approval
The case report was approved by the local ethical committee.
Informed consent
The requirement of informed consent was waived for this retrospective study.
Source of support
No sources of financial and material support to be declared.
Author’s contributions
SH Kim and MJ Kang contributed in the conception, analysis, and interpretation of the study. SS Choi drafted the manuscript for important intellectual content.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mario Bianchetti
Rights and permissions
About this article
Cite this article
Kim, S., Kang, M. & Choi, S. A case report of sporadic hemiplegic migraine associated cerebral hypoperfusion: comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MR imaging. Eur J Pediatr 175, 295–298 (2016). https://doi.org/10.1007/s00431-015-2609-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2609-2