Abstract
A single-nucleotide polymorphism (SNP) in the promoter region of MDM2 (SNP309T>G, rs2279744) has been shown to increase the expression of the MDM2 protein in various cancer types. However, only one study has analyzed the role of the MDM2 polymorphism in the development of Kaposi’s sarcoma (KS). The association of MDM2 SNP309 with classic KS risk was evaluated in 79 Iranian patients with classic KS and 123 healthy controls. The MDM2 SNP309 was genotyped using PCR and restriction fragment length polymorphism methods. No significant correlation was found between the SNP309 polymorphism in MDM2 promoter and classic KS risk. There was no significant correlation between gender and disease stage. However, a significant association was found between SNP309 GG genotype and younger age (≤50 years) (odds ratio 9.5, 95% confidence intervals 1.5–60, p = 0.03). Our findings support no major role for the MDM2 SNP309 in KS development although it might influence the clinical outcome of KS in younger patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kaposi’s sarcoma (KS) is a rare tumor that occurs in four different clinico-epidemiological forms including classic, endemic, iatrogenic, and epidemic KS [1–3]. Human herpesvirus 8 (HHV-8) is shown to be the primary etiological cause of all KS forms [4, 5]. The seroprevalence rate of HHV-8 significantly varies in different geographical regions. Indeed, it is below 10% in northern Europe, North America, and most parts of Asia, from 10 to 30% in the Mediterranean area, and above 50% in sub-Saharan Africa and parts of South America. In general, the seroprevalence of HHV-8 infection is in parallel to Kaposi’s sarcoma incidence [6–9].
While the seroprevalence of HHV-8 is reported to be high in some populations, only a small percentage of HHV-8-seropositive subjects progress to KS, supporting a prerequisite role of virus infection although it appears not to be sufficient for KS development. While the role of immune suppression behind KS progression is well documented, both environmental and genetic factors together with ethnic origins are likely to play a major role in this scenario [2, 10, 11].
The prevalence of HHV-8 has been reported in 2–3.6% of general population [12, 13], 16.9% of haemodialysis patients [13], 25% of renal transplant recipients [14] and 45.7% of HIV-infected patients [13]. The incidence of KS was reported to be high among Iranian renal transplant recipients (ranging from 0.45 to 2.4%) [13] although KS is a rare cancer in general population (0.06–0.17 per 100,000) [15]. The high KS incidence in renal transplant subjects suggests that the virus might be prevalent in general population, but only a few infected people may have the chance to develop KS due to specific predisposing genetic or environmental factors.
MDM2 is a major negative regulator of p53 [16, 17] and its overexpression can inhibit the function of p53, allowing the damaged cells to escape the cell-cycle checkpoint control and finally progress to cancer [17–19]. In several human tumors, the overexpression of MDM2 protein has been shown, suggesting a main role for this protein in tumorigenesis via proteasome-mediated degradation of p53 and, consequently, inhibition of apoptosis [20, 21].
A polymorphism in the MDM2 promoter (SNP309T>G, rs2279744) has been linked to the increased binding of Sp1 transcription factor and subsequent higher MDM2 protein levels [18, 19, 22]. The MDM2 polymorphism has also been associated with the earlier onset of breast cancer in Li–Fraumeni patients, colorectal cancer, soft tissue sarcoma, cutaneous melanoma, diffuse large B-cell lymphoma, and non-small-cell lung cancer [23–27].
To investigate the association of the MDM2 SNP309 with the risk of KS, we hypothesized that MDM2 SNP309 may influence the risk of KS occurrence in Iranian patients. Using restriction fragment length polymorphism, MDM2 SNP309 was genotyped in 79 classic KS patients and 123 healthy controls.
Materials and methods
Patients and controls
Eighty-six formalin-fixed paraffin-embedded cutaneous KS biopsies were obtained from patients attending the Dermatology Department of Razi Hospital over a 14-year period (2000–2014). Seventy-nine samples from patients with classic KS were included and seven samples (three epidemic and four iatrogenic) were excluded from this study. Also, a total of 123 blood samples were obtained from healthy subjects, referring to the South Tehran Health Center. The study was approved by the local ethical committee of Tehran University of Medical Sciences and informed consent was obtained from all the study subjects.
Detection of HHV-8 genome in samples
Genomic DNA for classic KS samples was extracted according to the previously published procedures [28, 29]. Peripheral blood mononuclear cells (PBMCs) were isolated from 123 fresh whole-blood samples containing EDTA by Ficoll-Paque centrifugation (GE Healthcare, Amersham, UK) and stored at −70 °C until use. Genomic DNA was extracted from peripheral blood leukocytes using a QIAamp DNA Blood Mini Kit (Qiagen, GmbH, Hildelberg, Germany) according to manufacturer’s instruction.
MDM2 SNP309 polymorphisms analysis
The MDM2 SNP309 analysis was carried out by PCR and restriction fragment length polymorphism (RFLP). A 174-base pairs (bp) amplicon size of the MDM2 intron 1 region, containing the MspA1I polymorphic site at nucleotide 309, was amplified with the B-MDM2-309F (5′-GGGAGTTCAGGGTAAAGG-3′) and the B-MDM2-309R (5′-GACCAGCTCAAGAGGAAA-3′) primers. PCR reactions were performed in a 50-μl reaction mixture containing 100–200 ng of DNA template, 2.5 mM MgCl2, 50 μM of each dNTP, 20 pmol of each primer and 1.25 U of HotMaster™ Taq DNA polymerase (five Prime GmbH, Hamburg, Germany), with the following PCR thermal conditions: an initial 1-min denaturation at 94 °C, followed by 40 cycles of 55 °C for 45 s, 68 °C for 1 min, 94 °C for 30 s and a final annealing at 55 °C for 30 s with 5 min of elongation at 68 °C. Digestion of MDM2 PCR products was performed by MspA1I restriction enzyme (Promega, Madison, WI, USA) for 3 h at 37 °C, and run on a 7% polyacrylamide gel.
Statistical analysis
The observed allele frequencies of study groups were evaluated by the Hardy–Weinberg equilibrium theory. A Fisher’s exact test or χ 2 test was applied to compare the proportions of MDM2 alleles between case and control groups by Epi Info 7 Statistical Analysis System Software (Centers for Disease Control and Prevention, USA). The association between MDM2 genotypes with the age of onset, gender, stage of disease and distribution at presentation of tumor was also analyzed. p values less than 0.05 were considered statistically significant.
Results
A total of 79 samples taken from cutaneous classic KS lesions (59 men and 20 women) were included in this study. The mean age of patients was 61.3 years (range 25–89) at the time of diagnosis (Table 1).
The MDM2 SNP309 allele frequencies were studied using a PCR–RFLP-based method (Fig. 1). The distribution of the MDM2 SNP309 genotypes in cases and controls are shown in Table 2. The frequencies of the MDM2 SNP309 polymorphisms were found in Hardy–Weinberg equilibrium among case (χ 2 = 3.88; df = 1; p = 0.05) and control group (χ 2 = 3.5; df = 1; p = 0.06). The frequency of the MDM2 SNP309 T/T, T/G and G/G alleles among 79 classic KS subjects were 31.6% (n = 25), 58.2% (n = 46), and 10.2% (n = 8), respectively. The corresponding figures among 123 healthy controls were also 26.9% (n = 33), 57.7% (n = 71) and 15.4% (n = 19). Crude odds ratios (OR) and 95% confidence intervals (CI) were used to assess the association between the MDM2 SNP309 genotypes and the risk of KS (Table 2). Regardless of being recessive and dominant models, no significant association was found between increased KS risk and the MDM2 SNP309 genotypes (OR 1.6, 95% CI 0.67–3.9 and OR 0.79, 95% CI 0.42–1.5, respectively).
The polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) result of MDM2 SNP309. MDM2 SNP309 T allele was not cleaved by MspAI endonuclease and had a single band of 174 bp. The MDM2 SNP309 G allele was cleaved by MspAI and had two small bands of 126 and 48 bp. The MDM2 SNP309 heterozygote had three fragments of 174, 126 and 48 bp
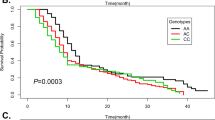
Stratification of classic KS cases was performed according to the age of onset, gender, and distribution at tumor presentation (Table 3). A statistically significant difference was found between the age of KS onset and GG genotype (odds ratio 9.5, 95% Confidence Intervals 1.5–60, p = 0.03). Indeed, an average age of KS onset was at 47.5 years for GG allele compared to 63.2 years for TT alleles. However, the association found between the gender and distribution at presentation with MDM2 SNP309 genotypes was not statistically significant. These results suggest that the G allele might be associated to the KS development in young people.
Discussion
While some epidemiological studies have evaluated the association of MDM2 SNP309 polymorphism and risk of several cancers [18, 19, 30–36], only one study has evaluated the association of the MDM2 SNP309 polymorphism in KS patients [37]. In the present study, the MDM2 nucleotide 309 polymorphism was investigated in 79 classic KS cases and 123 healthy controls to investigate the impact of these alleles on the risk of KS development. Although this study supports no association between genetic polymorphisms in MDM2 and the KS risk, an association was found between the increased frequency of the MDM2 SNP309 G/G genotype and younger age of KS patients.
While the correlation between MDM2 SNP309 and different types of malignancies has been studied in previous studies, the results were shown to be inconsistent [18, 22, 38–41]. Our results are in line with the previous studies reporting no correlation between MDM2 SNP309 and some types of cancers [32, 38–40]. In this regard, Wilkening et al. (2007) performed a combined analysis on breast, colorectal, and lung cancers to assess the consistency of the associations for the MDM2 SNP309 G allele. The results obtained from this study indicate no impact for MDM2 SNP309 alleles and the risk of breast or colorectal cancers (OR = 0.97, 95% CI = 0.87–1.08 and OR = 0.97, 95% CI = 0.76–1.25), respectively [41]. However, the combined estimation of the ORs showed a higher risk for GG opposed to TT (OR = 1.27, 95% CI = 1.12–1.44) in lung cancer. The data also revealed that MDM2 SNP309 has slight or no impact on the risk of several cancers although it might have an effect on the time of tumor onset and prognosis [41].
The potential role of the MDM2 SNP309 polymorphism in KS progression has been investigated in few geographical regions in the world [37]. In Africa, MDM2 SNP309 G genotype has not been associated with the risk of KS [37]. However, in Caucasian KS patients, MDM2 SNP309 G genotype has been associated with the increased risk of KS (OR 1.99, 95% CI 0.95–4.2) [37].
It should be considered that HHV-8 has evolved its own strategies to manipulate and inhibit the p53 activity. HHV-8 expresses several viral proteins that inhibit p53 functions at several levels and thereby mediate viral oncogenesis: (1) the latency-associated nuclear antigen (LANA) binds to both p53 and MDM2, thereby inhibiting the ability of p53 to induce cell death [42, 43]. LANA interacts with the p53 and represses its transcriptional activity [42]. (2) the viral interferon regulatory factors 1, 3 and 4 interfere with p53 signaling through different mechanisms [44]. vIRF1 and vIRF3 directed ubiquitination and proteasome-mediated degradation of p53 [45, 46]. vIRF3 also antagonizes p53 oligomerization and the DNA-binding affinity due to inhibition of p53 phosphorylation [45]. vIRF4 led to decrease in total p53 levels, due to interaction with and stabilization of MDM2 [47].
According to the age of KS onset, the G allele tended to have an increased risk at a young age, suggesting that the G allele might be associated with susceptibility to KS risk in younger age. To support this view, an association between the G allele and increased risk at a young age has been observed for cutaneous melanoma, soft tissue sarcomas, breast cancer, and renal cell carcinoma [26, 27, 48, 49]. In a study conducted on sporadic soft tissue sarcomas, the average age of onset was 45 years and 57 years for those patients who had the G/G and T/T genotype, respectively [48]. It has also been shown that the G allele of SNP309 increases the basal level of MDM2 in cells, as a result of the creation of an enhanced SP1 transcription factor-binding site in the MDM2 promoter. The higher levels of MDM2 in cells diminish the p53 apoptotic responses. The lower frequency of cells undergoing apoptosis and the propagation of mutated cells in individuals harboring G/G genotype at SNP30 of MDM2 have been suggested to permit cancers to arise at younger ages over a lifetime [48].
In agreement with some previous studies showing no association between the MDM2 alleles and gender or distribution at presentation in cutaneous melanoma, lung cancer and pancreatic cancer, we found no association between the gender and stage of disease [26, 50, 51].
While modest sample size of the KS cases can be considered as a limiting factor in the present study, by matching on age and sex in both, we have tried to minimize the potential confounding factors.
In conclusion, our findings partially support no association between the MDM2 SNP309 genotypes and the risk of KS development. An explanation of this finding is the fact that other genes and SNPs can modify the MDM2 SNP309 GG phenotype and enhance p53 function in a cell. Although MDM2 SNP309 is not a risk factor in KS development, it might influence the age of classic KS onset. To confirm the role of MDM2 polymorphism in KS development, the findings further need to be verified in large studies.
References
Buonaguro FM, Tornesello ML, Buonaguro L, Satriano RA, Ruocco E, Castello G, Ruocco V (2003) Kaposi’s sarcoma: aetiopathogenesis, histology and clinical features. J Eur Acad Dermatol Venereol 17(2):138–154
Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R (2000) Classic kaposi sarcoma: epidemiology and risk factors. Cancer 88(3):500–517
Kaposi M (1872) Idiopathisches multiples pigmentsarkom her haut. Arch Dermatol Shypilol 4:265–273
Buonaguro FM, Tornesello ML, Beth-Giraldo E, Hatzakis A, Mueller N, Downing R, Biryamwaho B, Sempala SD, Giraldo G (1996) Herpesvirus-like DNA sequences detected in endemic, classic, iatrogenic and epidemic Kaposi’s sarcoma (KS) biopsies. Int J Cancer 65(1):25–28
Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266(5192):1865–1869
Mesri EA, Cesarman E, Boshoff C (2010) Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer 10(10):707–719
Reinheimer C, Allwinn R, Sturmer M (2011) Do fewer cases of Kaposi’s sarcoma in HIV-infected patients reflect a decrease in HHV8 seroprevalence? Med Microbiol Immunol 200(3):161–164
Simpson GR, Schulz TF, Whitby D, Cook PM, Boshoff C, Rainbow L, Howard MR, Gao SJ, Bohenzky RA, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder RS, Weller IV, Weiss RA, Moore PS (1996) Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. The Lancet 348(9035):1133–1138
Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo CR, Saah A, Phair J, Detels R, Chang Y, Moore PS (1996) KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med 2(8):925–928
de Souza V, Sumita LM, Nascimento MC, Oliveira J, Mascheretti M, Quiroga M, Freire WS, Tateno A, Boulos M, Mayaud P, Pannuti CS (2007) Human herpesvirus-8 infection and oral shedding in Amerindian and non-Amerindian populations in the Brazilian Amazon region. J Infect Dis 196(6):844–852
Jalilvand S, Shoja Z, Marashi SM, Shahmahmoodi S, Safaie-Naraghi Z, Nourijelyani K, Nesheli AB, Mokhtari-Azad T (2015) Mitochondrial haplogroups and control region polymorphisms in Kaposi’s sarcoma patients. J Med Virol 87(9):1608–1615
Kakavand-Ghalehnoei R, Shoja Z, Najafi A, Mollahoseini MH, Shahmahmoodi S, Marashi SM, Nejati A, Jalilvand S (2016) Prevalence of human herpesvirus-8 among HIV-infected patients, intravenous drug users and the general population in Iran. Sex Health 13(3):295–298
Jalilvand S, Shoja Z, Mokhtari-Azad T, Nategh R, Gharehbaghian A (2011) Seroprevalence of Human herpesvirus 8 (HHV-8) and incidence of Kaposi’s sarcoma in Iran. Infect Agent Cancer 6:5
Ahmadpoor P, Ilkhanizadeh B, Sharifzadeh P, Makhdoomi K, Ghafari A, Nahali A, Yekta Z, Noroozinia F (2007) Seroprevalence of human herpes virus-8 in renal transplant recipients: a single center study from Iran. Transplant Proc 39(4):1000–1002. doi:10.1016/j.transproceed.2007.02.037
Mousavi SM, Mohagheghi MA, Jerrahi AM (2007) Epidemiology of Kaposi’s sarcoma in Iran: 1984–2006. Asian Pac J Cancer Prev 8(4):557–560
Wang X, Jiang X (2012) Mdm2 and MdmX partner to regulate p53. FEBS Lett 586(10):1390–1396
Freedman DA, Wu L, Levine AJ (1999) Functions of the MDM2 oncoprotein. Cell Mol Life Sci 55(1):96–107
Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, Onel K, Yip L, Hwang SJ, Strong LC, Lozano G, Levine AJ (2004) A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119(5):591–602
Yoon YJ, Chang HY, Ahn SH, Kim JK, Park YK, Kang DR, Park JY, Myoung SM, Kim dY, Chon CY, Han KH (2008) MDM2 and p53 polymorphisms are associated with the development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Carcinogenesis 29(6):1192–1196
Freedman DA, Levine AJ (1999) Regulation of the p53 protein by the MDM2 oncoprotein–thirty-eighth G.H.A. Clowes Memorial Award Lecture. Cancer Res 59(1):1–7
Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B (1993) p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res 53(10 Suppl):2231–2234
Hu Z, Jin G, Wang L, Chen F, Wang X, Shen H (2007) MDM2 promoter polymorphism SNP309 contributes to tumor susceptibility: evidence from 21 case-control studies. Cancer Epidemiol Biomarkers Prev 16(12):2717–2723
Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, Robins H, Bartel F, Taubert H, Wuerl P, Hait W, Toppmeyer D, Offit K, Levine AJ (2006) MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res 66(10):5104–5110
Lind H, Zienolddiny S, Ekstrom PO, Skaug V, Haugen A (2006) Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer 119(3):718–721
Menin C, Scaini MC, De Salvo GL, Biscuola M, Quaggio M, Esposito G, Belluco C, Montagna M, Agata S, D’Andrea E, Nitti D, Amadori A, Bertorelle R (2006) Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst 98(4):285–288
Firoz EF, Warycha M, Zakrzewski J, Pollens D, Wang G, Shapiro R, Berman R, Pavlick A, Manga P, Ostrer H, Celebi JT, Kamino H, Darvishian F, Rolnitzky L, Goldberg JD, Osman I, Polsky D (2009) Association of MDM2 SNP309, age of onset, and gender in cutaneous melanoma. Clin Cancer Res 15(7):2573–2580
Taubert H, Bartel F, Greither T, Bache M, Kappler M, Kohler T, Bohnke A, Lautenschlager C, Schmidt H, Holzhausen HJ, Hauptmann S, Wurl P (2008) Association of HDM2 transcript levels with age of onset and prognosis in soft tissue sarcomas. Mol Cancer Res 6(10):1575–1581
Tornesello ML, Biryahwaho B, Downing R, Hatzakis A, Alessi E, Cusini M, Ruocco V, Katongole-Mbidde E, Loquercio G, Buonaguro L, Buonaguro FM (2010) Human herpesvirus type 8 variants circulating in Europe, Africa and North America in classic, endemic and epidemic Kaposi’s sarcoma lesions during pre-AIDS and AIDS era. Virology 398(2):280–289
Jalilvand S, Tornesello ML, Buonaguro FM, Buonaguro L, Naraghi ZS, Shoja Z, Ziaee AA, Hamkar R, Shahmahmoodi S, Nategh R, Mokhtari-Azad T (2012) Molecular epidemiology of human herpesvirus 8 variants in Kaposi’s sarcoma from Iranian patients. Virus Res 163(2):644–649
Di V V, Buonaguro L, Izzo F, Losito S, Botti G, Buonaguro FM, Tornesello ML (2011) TP53 and MDM2 gene polymorphisms and risk of hepatocellular carcinoma among Italian patients. Infect Agent Cancer 6:13
Alhopuro P, Ylisaukko-Oja SK, Koskinen WJ, Bono P, Arola J, Jarvinen HJ, Mecklin JP, Atula T, Kontio R, Makitie AA, Suominen S, Leivo I, Vahteristo P, Aaltonen LM, Aaltonen LA (2005) The MDM2 promoter polymorphism SNP309T–>G and the risk of uterine leiomyosarcoma, colorectal cancer, and squamous cell carcinoma of the head and neck. J Med Genet 42(9):694–698
Horikawa Y, Nadaoka J, Saito M, Kumazawa T, Inoue T, Yuasa T, Tsuchiya N, Nishiyama H, Ogawa O, Habuchi T (2008) Clinical implications of the MDM2 SNP309 and p53 Arg72Pro polymorphisms in transitional cell carcinoma of the bladder. Oncol Rep 20(1):49–55
Li G, Zhai X, Zhang Z, Chamberlain RM, Spitz MR, Wei Q (2006) MDM2 gene promoter polymorphisms and risk of lung cancer: a case-control analysis. Carcinogenesis 27(10):2028–2033
Phillips CL, Gerbing R, Alonzo T, Perentesis JP, Harley IT, Meshinchi S, Bhatla D, Radloff G, Davies SM (2010) MDM2 polymorphism increases susceptibility to childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer 55(2):248–253
Onat OE, Tez M, Ozcelik T, Toruner GA (2006) MDM2 T309G polymorphism is associated with bladder cancer. Anticancer Res 26(5A):3473–3475
Terry K, McGrath M, Lee IM, Buring J, De VI (2008) MDM2 SNP309 is associated with endometrial cancer risk. Cancer Epidemiol Biomark Prev 17(4):983–986
Tornesello ML, Buonaguro L, Cristillo M, Biryahwaho B, Downing R, Hatzakis A, Alessi E, Cusini M, Ruocco V, Viviano E, Romano N, Katongole-Mbidde E, Buonaguro FM (2011) MDM2 and CDKN1A gene polymorphisms and risk of Kaposi’s sarcoma in African and Caucasian patients. Biomarkers 16(1):42–50
Liu G, Cescon DW, Zhai R, Zhou W, Kulke MH, Ma C, Xu W, Su L, Asomaning K, Heist RS, Wain JC, Lynch TJ, Christiani DC (2010) p53 Arg72Pro, MDM2 T309G and CCND1 G870A polymorphisms are not associated with susceptibility to esophageal adenocarcinoma. Dis Esophagus 23(1):36–39
Ma H, Hu Z, Zhai X, Wang S, Wang X, Qin J, Jin G, Liu J, Wang X, Wei Q, Shen H (2006) Polymorphisms in the MDM2 promoter and risk of breast cancer: a case-control analysis in a Chinese population. Cancer Lett 240(2):261–267
Pine SR, Mechanic LE, Bowman ED, Welsh JA, Chanock SC, Shields PG, Harris CC (2006) MDM2 SNP309 and SNP354 are not associated with lung cancer risk. Cancer Epidemiol Biomarkers Prev 15(8):1559–1561
Wilkening S, Bermejo JL, Hemminki K (2007) MDM2 SNP309 and cancer risk: a combined analysis. Carcinogenesis 28(11):2262–2267
Friborg J Jr, Kong W, Hottiger MO, Nabel GJ (1999) p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402(6764):889–894. doi:10.1038/47266
Sarek G, Ojala PM (2007) p53 reactivation kills KSHV lymphomas efficiently in vitro and in vivo: new hope for treating aggressive viral lymphomas. Cell Cycle 6(18):2205–2209. doi:10.4161/cc.6.18.4730
Jacobs SR, Damania B (2011) The viral interferon regulatory factors of KSHV: immunosuppressors or oncogenes? Front Immunol 2:19
Baresova P, Musilova J, Pitha PM, Lubyova B (2014) p53 tumor suppressor protein stability and transcriptional activity are targeted by Kaposi’s sarcoma-associated herpesvirus-encoded viral interferon regulatory factor 3. Mol Cell Biol 34(3):386–399
Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU (2006) Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol 80(5):2257–2266
Lee HR, Toth Z, Shin YC, Lee JS, Chang H, Gu W, Oh TK, Kim MH, Jung JU (2009) Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J Virol 83(13):6739–6747
Bond GL, Hu W, Levine A (2005) A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res 65(13):5481–5484
Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Suehiro Y, Tanaka Y, Dahiya R (2007) MDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinoma. Clin Cancer Res 13(14):4123–4129
Asomaning K, Reid AE, Zhou W, Heist RS, Zhai R, Su L, Kwak EL, Blaszkowsky L, Zhu AX, Ryan DP, Christiani DC, Liu G (2008) MDM2 promoter polymorphism and pancreatic cancer risk and prognosis. Clin Cancer Res 14(12):4010–4015
Han JY, Lee GK, Jang DH, Lee SY, Lee JS (2008) Association of p53 codon 72 polymorphism and MDM2 SNP309 with clinical outcome of advanced nonsmall cell lung cancer. Cancer 113(4):799–807
Acknowledgements
We thank the Pathology Laboratory of Razi Hospital in Tehran for providing clinical samples. This study has been funded and supported by Tehran University of Medical Sciences (TUMS), Grant No. 17715 and 28039. It has also been a part of an MSc thesis supported by Tehran University of Medical Sciences; Grant No. 240/1222.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The study was approved by the Ethical Committee of Tehran University of Medical Sciences (Grant No. 17715).
Rights and permissions
About this article
Cite this article
Varmazyar, S., Marashi, S.M., Shoja, Z. et al. MDM2 gene polymorphisms and risk of classic Kaposi’s sarcoma among Iranian patients. Med Microbiol Immunol 206, 157–163 (2017). https://doi.org/10.1007/s00430-016-0491-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0491-9