Abstract
The blood–brain barrier poses major hurdles in the treatment of brain-related ailments. Over the past decade, interest in peptides-based therapeutics has thrived a lot because of their higher benefit to risk ratio. However, a complete knowledgebase providing a well-annotated picture of the peptide as a therapeutic molecule to cure brain-related ailments is lacking. We have built up a knowledgebase B3Pdb on blood–brain barrier (BBB)-penetrating peptides in the present study. The B3Pdb holds clinically relevant experimental information on 1225 BBB-penetrating peptides, including mode of delivery, animal model, in vitro/in vivo experiments, chemical modifications, length. Hoping that drug delivery systems can improve central nervous system disorder-related therapeutics. In this regard, B3Pdb is an important resource to support the rational design of therapeutics peptides for CNS-related disorders. The complete ready-to-use and updated database with a user-friendly web interface is available to the scientific community at https://webs.iiitd.edu.in/raghava/b3pdb/.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the population statistics, the world population is shifting towards the aging pyramid. Age-related disorders are prevalent that includes brain cancer and other central nervous system disorders (Gustavsson et al. 2011). Despite the rapid development and advancement in medical technologies, the cure for central nervous system disorders is far beyond the scope of currently used conventional therapeutics (Neuwelt et al. 2008). The delivery of therapeutics to the brain is a major challenge in the drug development process. This deadlock in central nervous system disorder is due to the fact that almost 98% of small molecule-based drugs and nearly 100% of large molecules-based drugs fail to cross the blood–brain barrier (BBB) (Pardridge 1998). Several strategies are put forward in literature to circumvent the BBB, but few have shown satisfactory results in terms of efficiency to safety ratio. One of the spectra is marked by the direct delivery of therapeutic molecules into the brain tissue or in subarachnoid space, which poses discomfort and often shows local effects in terms of clinical efficacy (Dong 2018). Other includes the modification in therapeutics molecule, which is done to improve their efficacy. But this process has proven to be effective only for small molecules-based drugs (Oller-Salvia et al. 2016). In the literature, several techniques are put forward to deliver the therapeutic molecule for treating CNS disorders, but each technique has its own risk and benefits. Considering the high benefit to risk ratio, the most feasible non-invasive approach for delivering the therapeutic molecules to the brain tissue is BBB shuttles or BBB-crossing peptides. The BBB-crossing peptides or BBB shuttles have proven their efficacy in pre-clinical studies to deliver therapeutic molecules into the brain tissue, and several are in clinical trials for the same (Malakoutikhah et al. 2011). Over the past decade, the research into the peptide as shuttle/carrier to deliver the drug molecule to the brain tissue has thrived significantly because of their high benefit to risk ratio. The peptide as a shuttle can also overcome the limitations of conventional protein-based carriers such as complex derivatization, immunogenicity, and high cost of production (Oller-Salvia et al. 2016).
The central nervous system disorders account for nearly 1/4th of the global disease burden in Europe and other developed countries. Across the translational neuroscience areas, drug discovery and development is a risky, time-consuming, and costly process. The researchers and industrial partners are experimenting with the way of making drug discovery more effective and efficient. The researchers should focus on the advancement in drug discovery and drug delivery for brain-related disorders (Pankevich et al. 2014). Unfortunately, the pharmaceutical industry is more focused on developing a drug molecule to cure brain-related disorders. As a result of this, there are fewer biologics approved by the Food and Drug Administration that can effectively carry the cargo molecule to the brain tissue without altering the integrity of BBB. This situation is less expected to change if the pharmaceutical industries continue their conventional practices of discovering drug molecules without focusing on the delivery vehicles. Considering this scenario, the present study focuses on the in-depth review analysis of BBB-crossing peptides, mechanism of BBB-crossing peptides to deliver the cargo, and a curated list of peptides along with modifications, in vivo/in vitro results of pre-clinical and clinical studies. The available information related to BBB-crossing peptides in literature is shown in the form of a web server https://webs.iiitd.edu.in/raghava/b3pdb/. We consider that the BBB-crossing peptides present in our web server will be highly beneficial for the pharmaceutical industry and researchers for the development of a novel cargo that can effectively cross the BBB.
BBB-crossing peptides: an ideal biological therapeutic for CNS disorders
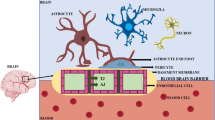
The BBB is a physical, metabolic barrier that ensures brain homeostasis by controlling the transfer of molecules from the blood to neural tissues. The BBB is characterized by non-fenestrated endothelial cells. Tight junctions mark the lining of the brain endothelial cells. The presence of tight junctions in the brain endothelial cells dramatically reduces the cell-to-cell communication and limits the transport of substances to the central nervous system (Wong et al. 2012). Despite the tight junction, the blood–brain barrier also consists of some characteristics features such as I) insufficient quantity of pinocytotic vesicles; II) presence of the high amount of astrocytes; and III) permeability reduction via ABC cassettes, multidrug resistance-associated trans-membrane proteins, which further makes it nearly impossible for any substance to cross the barrier (Ronaldson et al. 2008) (Fig. 1).
Despite as a barrier, the BBB is also the gateway to the brain as it allows necessary molecules, ions, nutrients to reach the brain tissue. Literature evidence reveals that several hydrophobic compounds of around < 500 Da and polar molecules like glucose, amino acids, and peptides can cross the BBB via specific transporters into the endothelial cytosol. These selective molecules can diffuse into the brain's extracellular space from the endothelial cytosol (Nagpal et al. 2013). Considering this scenario, a wide variety of protein shuttle vectors has been investigated by researchers, such as Apoplipoprotein A & E, leptin, metallotransferrin, and a non-toxic mutant of Diptheria toxin (Oller-Salvia et al. 2016). Unfortunately, the protein-based shuttle vectors to cross the BBB shows moderate efficacy and selectivity in clinical studies. Several virus-based shuttle vectors are also available in the literature to deliver the cargo with high specificity (Lathwal et al. 2020a). However, the use of the virus as a shuttle vector may pose a greater risk due to its high immunogenic and pathogenic nature (Lathwal et al. 2020b). To overcome the limitation of protein and virus-based shuttle vectors, researchers have designed antibody-based vectors to enhance the delivery of drug molecules across the BBB. Despite their overwhelming specificity, the production, purification, and immunogenicity are the major issues associated with the use of antibodies as delivery vehicles (Almeida et al. 2014). This is the primary reason that research in the past decade has focused mainly on peptides. Peptide offers several advantageous features over other vectors in terms of high specificity, low immunogenicity, small size, and low-cost production.
By utilizing the mechanism of this endogenous transport, several pre-clinical and clinical studies show that peptides as a shuttle molecule can cross and transport the cargo such as proteins, genetic material, nanoparticles, and small molecule across the BBB (Pardridge 2012). Few notable examples include—the use of RVG-9R peptide in delivering the FvE siRNA in the neuronal cells of mice to prevent viral encephalitis (Kumar et al. 2007), the use of synB3 peptide in delivering morphine glucuronide across the blood–brain barrier in mice model (De Boer and Gaillard 2007), and effective delivery of plasmid expressing Angiopep-2 peptide across the blood–brain barrier in the treatment of brain glial tumor (Ke et al. 2009). In addition, Angiopep-2 (Demeule et al. 2008b) and glutathione (Gaillard et al. 2012) peptides and their formulations are in clinical trials for the delivery of a wide variety of pharmaceuticals to the brain tissues. Several peptides as a BBB shuttle vector with high efficiency and selectivity were found in the literature (Table 1).
B3Pdb: a database of BBB peptides
The development of an effective brain targeting treatment strategy remains at the forefront of the research for the past several decades. Recent advances in peptide-based therapeutics have provided promising solutions to challenges faced by the conventional approaches in treating central nervous system disorders. Several clinical and pre-clinical studies highlight that cell-penetrating peptides can effectively deliver proteins, peptides, siRNA, AS-DNA, plasmid DNA, nucleic acids, and nanoparticles across the blood–brain barriers. Brain-penetrating peptides possess great therapeutic potential but scattered information in literature poses a significant challenge to the researchers in further improving their clinical efficacy in customized conditions. Only resource that maintain information about BBB-crossing peptides is Brainpeps (Van Dorpe et al. 2012). It provides chemical and functional information related to 259 BBB-penetrating peptides. This database does not provide any information regarding the in vivo/in vitro and therapeutic efficacy of peptides. Over the years, it needed to be updated with some additional experimental information. Keeping the necessity of a unified platform that provides all the available literature information related to the brain-penetrating peptide and their clinical efficacy, we have designed a web resource, B3Pdb. The present version of the database contains information related to animal models, chemical modifications, in vivo/in vitro activities, and therapeutic properties, which were absent in the previous version of the BBB-penetrating peptide database. As shown in Fig. 2, B3Pdb has nearly two times more entries and unique peptides in comparison to Brianpeps.
Data collection and curation
The repository specifically dedicated to BBB-crossing peptides was built up by curating the relevant research articles from PubMed and Patents. The PubMed was searched with different combinations of keywords such as ‘blood–brain barrier’ or ‘penetrating/crossing/permeating peptides’ till July 2020, as an advanced search query that should be included in the research articles' title/abstract. The freely available articles were downloaded and look for clinically relevant information. The relevant information of BBB-penetrating peptides includes—‘name, sequence, length, conformation, origin, nature, the cell line used, other information like in vivo/in vitro model systems, subcellular localization, uptake efficiency, and therapeutic properties, were manually extracted after a thorough reading of the research articles. The research articles that did not have such relevant information are excluded from the study. The complete architecture of the developed web resource is provided in Fig. 3.
B3Pdb: data content and analysis
All the curated information regarding the BBB-crossing peptides was cataloged in 29 fields. The B3Pdb holds 465 unique and 1225 peptides as a total number of records and several clinically experimental relevant information. Out of 1225, nearly 1107 peptides are in their native linear form, while 26 peptides are in cyclic form. While curating the research article, curators pay specific attention to the quality of the content. We have specifically included some additional information such as chemical modifications, model organisms for in vivo activities, as well as cell lines used for in vitro activities, which was otherwise unavailable in the previous version of the other published database. B3Pdb holds information regarding 110 C-terminal modifications and 115 N-terminal modifications in the peptides, which was done to improve their efficacy. Further data analysis reveals that out of 1225, 335 peptides record has bin size of peptide length up to 5, 235 have 6–10, 90 have 11–15, 137 have 16–20, 76 have 20–30, and 53 peptide entries have the length of more than 33 amino acids residues. Further data analysis reveals that the preferred mode of delivery of BBB peptide in the in vivo/in vitro condition is the intravenous (318 records) followed by in situ brain perfusion (65), intracarotid (24), intraperitoneal (18), and intranasal (08). The data analysis of B3Pdb reveals that the preferred choice of an experimental model to check the efficacy of BBB-crossing peptide was C57BL6 mice (64 records) followed by BALB/c mice (36) and adult Sparague-Dawley rats (8) and so on. The complete data analysis statistics is shown in Fig. 4.
Clinical aspects of the work and conclusion
Mounting evidence suggests that BBB-crossing peptides hold great potential in pre-clinical studies for delivering therapeutic molecules such as DNA, RNA, protein, small molecules into the cell. Literature analysis reveals that several BBB-crossing peptides already reached the clinical trials, with some are in the advanced stage of their pre-clinical testing. For example, the Angiopep-2 peptide was used to deliver drugs like Paclitaxel, Doxorubicin, Etoposide and showed promising efficacy in phase-I clinical trials (Bertrand et al. 2011). The conjugate of Angiopep-2 with Bevacizumab already reached phase-II for the treatment of high-grade glioma (NCT01480583). Also, the conjugate of Angiopep-2 with nanoprobe is used as a two-order brain imaging system to diagnose brain tumors (Yan et al. 2012). To further improve the efficacy of BBB-crossing, peptide chemical modifications and PEGylation are also done. GSH is one such peptide that is mainly applied as a PEGylated liposome loaded with drugs, commonly known as G-technology. G-technology loaded with doxorubicin has been investigated for the treatment of phase-I/IIa brain cancer (NCT01386580). The apolipoprotein-derived peptides have been successfully studied for the delivery of hydrophobic and enzymatic delivery across the BBB (Wang et al. 2013). The retro-enantiomer form of THR peptide has been investigated to disrupt B-amyloid in the mouse brain tissue, suggesting the improved therapeutic efficacy of chemically modified peptides (Prades et al. 2012).
These pieces of evidence suggest that BBB-crossing peptides hold great therapeutic potential. Despite the considerable achievement in drug delivery, new peptide-based shuttle vectors with enhanced selectivity and transport capacity are still required. Thus, to improve the existing BBB shuttle vectors or to design the new shuttle vectors, complete knowledge of the existing available shuttle vectors is required. The information regarding BBB peptides is scattered in the literature, which poses a problem in studying the versatile nature of these peptides. This lacuna in literature motivates us to develop a web resource that focuses primarily on manually curated information regarding the BBB-crossing peptides. The peptide modification information is highly valuable for experimental scientists and genetic engineers who wish to design new peptides or modify existing peptides for a better therapeutics regimen. In this regard, B3Pdb holds great promise as it provides information on chemical modifications done to improve BBB-crossing peptides' efficacy. B3Pdb includes information on chemical modifications done in peptides in order to increase their efficacy, such as N-terminal (110 records), C-terminal (115 records), and cyclic modifications (30 records). Second, the B3Pdb helps genetic engineers and experimental scientists in designing experimental protocols, as it stores data on 35 model organisms and 21 cell lines along with the other relevant experimental details. Third, the data stored in the B3Pdb also facilitate clinicians and researchers in guiding the route of administration of B3PPs. This will help in achieving the greater benefits with minimum side effects. One of the major limitations of the present version is that it lacks the structural information related to BBB-penetrating peptides besides the SMILES format. We have also not incorporated clinical trial-related information due to the lack of data. Such information can improve the quality of the database. We have incorporated the data submission page for the users to submit the experimental data to improve and up-gradate the database. The curator of B3Pdb has developed the web resource, keeping in mind that the scientific research community will get the maximum benefit from it. We foresee that the developed unified single platform for blood–brain barrier-penetrating peptides will be highly informative for clinicians and genetic engineers.
Availability of data and materials
B3Pdb is freely available at https://webs.iiitd.edu.in/raghava/b3pdb/ and can be easily accessed using desktop, tablet, or smartphone.
References
Almeida CR, Serra T, Oliveira MI et al (2014) Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater 10:613–622. https://doi.org/10.1016/j.actbio.2013.10.035
Alvarez-Erviti L, Seow Y, Yin H et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Bertrand Y, Currie JC, Poirier J et al (2011) Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer 105:1697–1707. https://doi.org/10.1038/bjc.2011.427
Caillé I, Allinquant B, Dupont E et al (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131:2173–2181. https://doi.org/10.1242/dev.01103
Ché C, Yang G, Thiot C et al (2010) New angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem 53:2814–2824. https://doi.org/10.1021/jm9016637
De Boer AG, Gaillard PJ (2007) Drug targeting to the brain. Annu Rev Pharmacol Toxicol 47:323–355
Demeule M, Currie JC, Bertrand Y et al (2008a) Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J Neurochem 106:1534–1544. https://doi.org/10.1111/j.1471-4159.2008.05492.x
Demeule M, Regina A, Ché C et al (2008b) Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 324:1064–1072. https://doi.org/10.1124/jpet.107.131318
Dong X (2018) Current strategies for brain drug delivery. Theranostics 8:1481–1493
Drin G, Cottin S, Blanc E, Rees AR, Temsamani J (2003) Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem 278(33):31192–31201. https://doi.org/10.1074/jbc.M303938200
Gaillard PJ, Appeldoorn CCM, Rip J et al (2012) Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. In: Journal of Controlled Release. pp 364–369
Gustavsson A, Svensson M, Jacobi F et al (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:718–779. https://doi.org/10.1016/j.euroneuro.2011.08.008
Hwang DW, Son S, Jang J, Youn H, Lee S, Lee D, Lee Y-S, Jeong JM, Kim WJ, Lee DS (2011) A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials 32(21):4968–4975. https://doi.org/10.1016/j.biomaterials.2011.03.047
Ke W, Shao K, Huang R et al (2009) Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 30:6976–6985. https://doi.org/10.1016/j.biomaterials.2009.08.049
Kumar P, Wu H, McBride JL et al (2007) Transvascular delivery of small interfering RNA to the central nervous system. Nature 448:39–43. https://doi.org/10.1038/nature05901
Kurzrock R, Gabrail N, Chandhasin C et al (2012) Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther 11:308–316. https://doi.org/10.1158/1535-7163.MCT-11-0566
Lathwal A, Kumar R, Raghava GPS (2020a) OvirusTdb: A database of oncolytic viruses for the advancement of therapeutics in cancer. Virology 548:109–116. https://doi.org/10.1016/j.virol.2020.05.016
Lathwal A, Kumar R, Raghava GPS (2020b) Computer-aided designing of oncolytic viruses for overcoming translational challenges of cancer immunotherapy. Drug Discov Today 25:1198–1205
Lo SL, Wang S (2008) An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials 29:2408–2414. https://doi.org/10.1016/j.biomaterials.2008.01.031
Malakoutikhah M, Teixidó M, Giralt E (2011) Shuttle-mediated drug delivery to the brain. Angew Chemie Int Ed 50:7998–8014
Nagpal K, Singh SK, Mishra DN (2013) Drug targeting to brain: A systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opin Drug Deliv 10:927–955
Neuwelt E, Abbott NJ, Abrey L et al (2008) Strategies to advance translational research into brain barriers. Lancet Neurol 7:84–96
Oller-Salvia B, Sánchez-Navarro M, Giralt E, Teixidó M (2016) Blood-brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem Soc Rev 45:4690–4707
Pankevich DE, Altevogt BM, Dunlop J et al (2014) Improving and accelerating drug development for nervous system disorders. Neuron 84:546–553
Pardridge WM (1998) CNS drug design based on principles of blood-brain barrier transport. J Neurochem 70:1781–1792
Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32:1959–1972
Prades R, Guerrero S, Araya E et al (2012) Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 33:7194–7205. https://doi.org/10.1016/j.biomaterials.2012.06.063
Ren J, Shen S, Wang D et al (2012) The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 33:3324–3333. https://doi.org/10.1016/j.biomaterials.2012.01.025
Ronaldson PT, Persidsky Y, Bendayan R (2008) Regulation of ABC membrane transporters in glial cells: Relevance to the pharmacotherapy of brain HIV-1 infection. Glia 56:1711–1735. https://doi.org/10.1002/glia.20725
Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 285:1569–1572. https://doi.org/10.1126/science.285.5433.1569
Van Dorpe S, Bronselaer A, Nielandt J et al (2012) Brainpeps: The blood-brain barrier peptide database. Brain Struct Funct 217:687–718
Vizioli NM, Rusell ML, Carbajal ML et al (2005) On-line affinity selection of histidine-containing peptides using a polymeric monolithic support for capillary electrophoresis. Electrophoresis 26:2942–2948. https://doi.org/10.1002/elps.200410416
Wang D, El-Amouri SS, Dai M et al (2013) Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc Natl Acad Sci U S A 110:2999–3004. https://doi.org/10.1073/pnas.1222742110
Wong HL, Wu XY, Bendayan R (2012) Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev 64:686–700
Xiang L, Zhou R, Fu A et al (2011) Targeted delivery of large fusion protein into hippocampal neurons by systemic administration. J Drug Target 19:632–636. https://doi.org/10.3109/1061186X.2010.523788
Yan H, Wang L, Wang J et al (2012) Two-order targeted brain tumor imaging by using an optical/paramagnetic nanoprobe across the blood brain barrier. ACS Nano 6:410–420. https://doi.org/10.1021/nn203749v
Acknowledgements
Authors are thankful to funding agencies, Council of Scientific and Industrial Research (CSIR), University Grant Commission (UGC), and Department of Biotechnology, Govt. of India for financial support and fellowships.
Author information
Authors and Affiliations
Contributions
Vinod Kumar, Sumeet Patiyal, Rajesh Kumar, and Sukriti Sahai manually collected and curated all the data. Vinod Kumar, Sumeet Patiyal, and Dilraj Kaur developed the web interface. Vinod Kumar, Rajesh Kumar, Anjali Lathwal, and Gajendra P.S. Raghava prepared the manuscript. Gajendra P.S. Raghava conceived the idea, planned and coordinated the entire project.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, V., Patiyal, S., Kumar, R. et al. B3Pdb: an archive of blood–brain barrier-penetrating peptides. Brain Struct Funct 226, 2489–2495 (2021). https://doi.org/10.1007/s00429-021-02341-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02341-5