Abstract
Laminar architecture of primary auditory cortex (A1) has long been investigated by traditional histochemical techniques such as Nissl staining, retrograde and anterograde tracings. Uncertainty still remains, however, about laminar boundaries in mice. Here we investigated the cortical lamina structure by combining neuronal tracing and immunofluorochemistry for laminar specific markers. Most retrogradely labeled corticothalamic neurons expressed Forkhead box protein P2 (Foxp2) and distributed within the laminar band of Foxp2-expressing cells, identifying layer 6. Cut-like homeobox 1 (Cux1) expression in layer 2–4 neurons divided the upper layers into low expression layers 2/3 and high expression layers 3/4, which overlapped with the dense terminals of vesicular glutamate transporter 2 (vGluT2) and anterogradely labeled lemniscal thalamocortical axons. In layer 5, between Cux1-expressing layers 2–4 and Foxp2-defined layer 6, retrogradely labeled corticocollicular projection neurons mostly expressed COUP-TF interacting protein 2 (Ctip2). Ctip2-expressing neurons formed a laminar band in the middle of layer 5 distant from layer 6, creating a laminar gap between the two laminas. This gap contained a high population of commissural neurons projecting to contralateral A1 compared to other layers and received vGluT2-immunopositive, presumptive thalamocortical axon collateral inputs. Our study shows that layer 5 is much wider than layer 6, and layer 5 can be divided into at least three sublayers. The thalamorecipient layers 3/4 may be separated from layers 2/3 using Cux1 and can be also divided into layer 4 and layer 3 based on the neuronal soma size. These data provide a new insight for the laminar structure of mouse A1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The laminar distribution of excitatory neurons in rodent primary auditory cortex (A1) has been traditionally determined by identifying the location of the retrogradely labeled projection neurons or assigning cell types based on the morphology of Golgi stained neurons, referring to laminar boundaries determined by Nissl staining (for example, mice: Petrof et al. 2012; rats: Beyerl 1978; Games and Winer 1988; Doucet et al. 2003). The use of these gold standard techniques remains effective for architectonic characterization of the cortex. The determination of laminar boundaries using Nissl staining, however, has led to different conclusions about the laminar distribution of excitatory neurons. In rodent A1, there are contrasting reports indicating that the laminar thicknesses of layer 5 and layer 6 have different proportions or are nearly equal. An earlier work based on Nissl staining showed that layer 6 is approximately half the thickness of layer 5 (Heumann et al. 1977). In contrast, later studies reported an approximate equal proportion in the two lower layers (Games and Winer 1988; Doucet et al. 2003; Anderson et al. 2009). How the laminar boundaries are defined will influence conclusions on anatomical distributions of neurons and their function. For example, assuming the near-equal proportion in the lower layers, it was concluded that corticocollicular projection neurons were present in lower layer 5 adjacent to layer 6. A more recent study, however, showed retrogradely labeled corticocollicular neurons distributed to layer 5, leaving an unlabeled area in the deep layer (presumptive layer 6) narrower than expected (Slater et al. 2013). If the equal lower layer proportion was assumed, the labeled cells would have occupied the upper part of layer 6. Recent retrograde tracer injection studies showed that corticothalamic projection neurons were distributed to layer 6, whose laminar thickness was much narrower than layer 5 thickness (Smith et al. 2012). These studies raise a question as to true laminar boundaries for the lower layers.
Laminar boundaries of the upper layers, except layer 1, are also obscure. It is generally accepted that primary thalamocortical axons terminate deep layer 3 and layer 4 (layers 3/4) in A1 (Morel and Kaas 1992; Morel et al. 1993; Romanski and LeDoux 1993; Jones et al. 1995; Huang and Winer 2000; Hackett et al. 2001; Smith and Populin 2001; Kimura et al. 2003; Rose and Metherate 2005; de la Mothe et al. 2006; Llano and Sherman 2008; Smith et al. 2012). Layer 3/4 neurons are considered as mostly pyramidal as spiny stellate neurons are rare in auditory cortex (Meyer et al. 1989; Fitzpatrick and Henson 1994; Smith and Populin 2001; Barbour and Callaway 2008; Richardson et al. 2009). However, to what extent those neurons are spread within layers 2–4 and whether layers 2/3 and layers 3/4 can be anatomically distinguished are unclear.
The difficulties in determining laminar boundaries may be overcome by the use of laminar specific transcription factors or fate-determining genes that are developmentally regulated to specify neuronal subtypes and cortical laminar distributions (Bertrand et al. 2002; Molyneaux et al. 2007). Some of the factors are persistently expressed in a specific layer throughout life to maintain neuronal identity (Holmberg and Perlmann 2012), allowing the identification of layers. For example, corticospinal motor neurons identified by a retrograde tracer expressed COUP-TF interacting protein 2 (Ctip2) in layer 5 of sensorimotor cortex (Arlotta et al. 2005; Tantirigama et al. 2014). Ctip2 was also expressed by a subset of retrogradely labeled corticostriatal projection neurons in layer 5 of motor cortex (Sohur et al. 2014). Meanwhile, developmental studies indicated that Forkhead box protein P2 (Foxp2) is mainly expressed by layer 6 neurons (Hisaoka et al. 2010). Foxp2 expression in corticothalamic projection neurons of layer 6 has been reported in somatosensory and motor cortices (Sorensen et al. 2015). For the upper layers, cells in the subventricular zone during embryonic development express Cut-like homeobox 1 (Cux1) and become layers 2/3 and layer 4 neurons (Nieto et al. 2004). Cux1 identifies layers 2/3 in motor cortex (Sohur et al. 2014) and layers 2–4 in sensory cortex (Xing et al. 2016). These “laminar specific” transcription factors may allow us to determine laminar boundaries and more precise sublayer determination for corticocollicular, corticothalamic, and corticocortical projection neurons in A1.
Here, combining the immunofluorescence staining of Foxp2 and Ctip2 with the retrograde identification of projection neurons, we attempted to define the laminar boundaries for the lower layers of A1 in 7-week old FVB mice. The laminar boundaries of the upper layers are also examined using immunostaining against neuronal nuclei (NeuN), Cux1 and vesicular glutamate transporter 2 (vGluT2). We propose laminar boundaries for thin layer 6, three sublayers of layer 5, thalamorecipient layers 3/4, and layers 2/3 in mouse A1.
Materials and methods
Animals
All animal procedures were performed in accordance with the Declaration of Helsinki and the Guidelines of Animal Use Committee at Soka University, and were approved by the Soka Bioethics Committee for Life Sciences. Animals were housed in a vivarium with a 12/12 h light/dark cycle. FVB strain mice aged from 55 to 65 days were mated in house. The day the pups were born were termed postnatal day (P) 0.
Retrograde and anterograde tracer labeling
Mice were anesthetized with gas mixture containing N2O:O2 (1.5:1) and isofluorane (2.5%). Body temperature was maintained around 35 °C using a heat pad. Deeply anaesthetized mice were prepared for aseptic surgery and placed in the stereotaxic apparatus (Narishige, Japan). Following anesthesia, 1.0% lidocaine (Wako, Japan) was locally applied on the head, the skin was incised, and the dorsal surface of the brain skull was exposed. Bupivacaine (0.25%; LKT Laboratories, USA) was applied to the incision area. A small hole in the skull was made using a mini router drill above an injection site as described briefly below. A borosilicate glass injection pipette (GD-1.5, Narishige) was pulled to a taper length of ~ 10 mm and a tip diameter of 30–50 μm using a puller (P-97, Sutter), and it was connected to a 1.0-μl Hamilton syringe to make a tracer injector. This injector was stereotaxically positioned above the injection site and inserted into the brain to the injection target area. In each injection, ~ 50 nl of either Alexa488-conjugated cholera toxin β-subunit (CTB, 0.2%, C34775, Life technologies, Japan) or Red Retrobeads (Lumafluor Inc., USA), or ~ 60–80 nl of tetramethylrhodamine dextran (TMR-Dex: 3000 MW, D3308, Life technologies) was delivered over a time span of 10 min with ~ 10 nl/2 min steps by pressure ejection. The injector remained in place for an additional 10 min before being withdrawn. After injection, a small piece of a gel form (Pfizer) was placed in the hole of craniotomy, and the skins were sutured, followed by application of 0.25% bupivacaine to the sutured skin. The mouse was injected with buprenorphine (0.1 mg/kg, i.p.; Otsuka Pharmaceuticals) and was returned to a cage. The same dose was injected after ~ 4–6 h. For rearing at night, drinking water was spiked with buprenorphine at 0.01 mg/ml. For the following 2 days, the mouse received buprenorphine by i.p. injection at ~ 6-h intervals during the day and in the drinking water at night.
For retrograde labeling of corticothalamic projection neurons of A1, the injector was advanced toward the ventral division of medial geniculate nucleus (MGv) in the left hemisphere with the stereotaxic coordinate of 2.5 mm posterior from bregma, 2.4 mm lateral from the midline, and 3.0–3.3 mm from the surface of the brain. To label commissural projection neurons of A1, the injector was inserted into contralateral A1 in the left hemisphere with the following coordinate: 2.5 mm posterior from bregma, 4.0 mm lateral from the midline, and 0.6–0.8 mm from the surface of the brain. For the corticocollicular projection neurons, the injector was targeted to the central nucleus of inferior colliculus (IC) with the following coordinate: 0.5 mm posterior from lambda, 0.8–1.0 mm lateral from the midline, and 0.5–0.8 mm from the surface of the brain. For the MGv injections, only CTB was used, while for IC and A1 injections, either CTB or the Retrobeads was used. The animals were injected with the retrograde tracers at P20 and were returned to cages for 4-week recovery after injection. For anterograde tracer injection aiming to label thalamocortical fibers, the MGv of the right hemisphere was injected with 2% TMR-Dex with a stereotaxic coordinate of 2.5–2.6 mm posterior from bregma, 2.4–2.5 mm lateral from the midline, and 2.7–2.9 mm from the brain surface. The mice received injections at P30–35 and were kept 10–15 days before sacrificing.
Brain section preparation
Mice at P45–48 were deeply anesthetized with an intraperitoneal injection of a mixture of urethane (1.3 g/kg) and xylazine (13.3 mg/kg). Transcardial perfusion was performed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4. Fixed brains were removed from the skull and immersed in the fixative overnight. Brains were sectioned coronally at 50 µm steps using a vibratome (DTK-1000, Dosaka, Japan) and collected in 24-well plates containing 0.1 M phosphate buffer with 0.9% NaCl (PBS), and stored at 4 °C or at − 20 °C in 30% ethylene glycol and 30% glycerol mixture in PBS until use. To determine whether injections were confined to designated locations, all sections near the injection sites were examined under the epi-fluorescent microscope (5× Zeiss Plan-NEOFLUAR lens, Axioskop 2, Zeiss). Only those that have successful injections were further used for immunofluorescence staining and analysis.
Immunofluorochemistry
Immunofluorochemistry was performed using standard protocols. Briefly, a blocking solution was prepared with 5% sera (donkey or goat sera, Sigma) and 0.3% Triton-X 100. For sections that were labeled with the Retrobeads, a blocking solution was prepared with 3% sera (goat sera, Sigma), 2% dimethyl sulfoxide (DMSO, 041-29351, Wako), and 1 mg/ml digitonin (300410, Millipore). Selected brain sections were incubated in an appropriate blocking solution at room temperature for 90 min, followed by the application of primary antibodies (see Table 1) for either 90 min at room temperature or 20–24 h at 4 °C. After washing in PBS, secondary antibodies in the blocking solutions were applied at room temperature for 80 min. Secondary antibodies used for immunofluorescent identification of appropriate primary antibodies were as follows: Alexa Fluor 488-conjugated goat anti-rabbit IgG (111-545-144) and Alexa Fluor 488-conjugated goat anti-mouse IgG (115-545-146) from Jackson ImmunoResearch, Alexa Fluor 555-conjugated donkey anti-goat IgG (Ab150134) and Alexa Fluor 568-conjugated donkey anti-rat IgG (Ab175475) from Abcam, Alexa Fluor 488-conjugated goat anti-rat IgG (A11006), Alexa Fluor 633-conjugated goat anti-rat IgG (A21094), Alexa Fluor 633-conjugated goat anti-rabbit IgG (A21070), Alexa Fluor 647-conjugated goat anti-guinea pig IgG (A21450) from Thermo Fisher/ Life technologies. For mouse primary antibodies, Mouse-on-Mouse (M.O.M, BMK-2202, Vectashield) blocking reagent was used to reduce nonspecific mouse Ig staining. For pan-nuclear staining, sections were incubated with Hoescht-33342 (5 µg/ml, Sigma) for 30 min. For fluorescent microscopy, all sections were mounted with Vectashield antifade mounting medium (H1000, Vector).
Image acquisition and analysis
Epifluorescence images were acquired from 4 to 5 coronal sections for each cortical area using an upright fluorescent microscope (5× Zeiss Plan-NEOFLUAR lens, Axioskop 2, Zeiss) with a CCD camera (AxioCam HRc, Zeiss), and were analyzed using Axiovision software. Coronal sections containing primary sensory cortices were selected as described previously (Chang et al. 2018). To define the analysis location of A1, we first constructed in coronal sections a horizontal line perpendicular to the midline through the center of MGv and constructed a second line through temporal cortex from the center of MGv at a 15°–20° angle to the first line. This second line was assumed to run through A1 and defined as the center line of A1 (cf. Cruikshank et al. 2002; Kawai et al. 2007). For cell counts, the region of interest (ROI) was a region delimited by pia, the border between layer 6 and the subcortical white matter (L6/WM), and two lines parallel to and ± 0.2 mm distant from the center line. For primary visual cortex (V1), the same coronal sections used for A1 were analyzed. We constructed a vertical line (parallel to the midline) that crosses the middle of MGv, and drawn a second line through the dorsal cortex from MGv at a 15° angle to the first line. This second line was assumed to run through V1 and defined as the center line of V1. For primary somatosensory cortex (S1), the barrel field in coronal sections was identified with Hoechst nuclear staining as previously reported (Ultanir et al. 2007).
For laminar thicknesses of presumptive layers 2–4 and layer 6, the distance between the edges of laminar bands formed by Cux1 and Foxp2 immunoreactive cells, respectively, was measured along the center line and the two equally distant parallel lines forming the ROI and averaged in each section. Layer 5 thickness was measured as the distance between the bottom edge of Cux1 immunoreactive cells and the top edge of Foxp2 immunoreactive cells. Layer 3/4 thickness was measured as the distance of laminar band formed by high intensity Cux1 immunoreactive cells. Laminar proportion was obtained by normalizing laminar thickness to the cortical thickness measured from pia to the L6/WM border for each section. The laminar thickness and the laminar proportion were averaged across 4–5 sections per animal.
Confocal fluorescence images were acquired with a Leica TCS SP8 confocal microscope. For cell quantification and distribution analysis, z-stack images were taken at 2048 × 2048 resolution with z-steps of 3.0 μm to obtain 40–50 µm-thick optical sections using 10× HC PL APO CS2 lens. Images were analyzed with NIH ImageJ or Fiji software. For the identification of positive fluorescence staining, a threshold intensity was first defined as the mean plus three times the standard deviation (SD) of the background intensity in randomly selected unstained areas, and then it was subtracted from the entire intensity values. Stained structures with more than 40 pixels (22.5 μm2) were counted as positively identified cells. For magnified images, z-stack images were taken at 0.5 μm steps using 63× HC PL APO GLYC CORR CS2 lens and were collapsed by summing optical sections in all images, except Fig. 7c, where one z-optical section was shown for three-dimensional localization of stains. Images shown in figures were adjusted for intensity using either Fiji or Las X (Leica) software for optimum visualization.
Laminar boundaries as defined by NeuN, Cux1 and Foxp2-expressing neurons. a Representative images of coronal sections with Hoechst (Ho), NeuN, Cux1, and Foxp2 co-staining in A1 (top), V1 (middle), and S1 (bottom). Scale bar, 100 μm. (right) Relative fluorescence intensities of NeuN+ cells with distance from pia (0%) to the L6/WM border (100%) for A1 (blue), V1 (red), and S1 (green: barrel hollow; yellow: septa). SEMs are indicated by lighter colored lines above and below the averages (n = 3). b Comparison of the relative fluorescence intensities of NeuN+ cells. The plots for A1 (blue) and V1 (red) are the same as a. The plot for S1 (green) is the average intensity that includes hollow and septa regions. a, b Colored arrows indicate the borders for L5/6 (arrows) and L4/5 (dotted arrows). Fluorescence intensities for NeuN+ cells in layer 1 were excluded from analysis due to the variability in its laminar thickness. Arrows in black indicate the upper edge of the “gap” in layer 5. c NeuN+ soma size analysis. (bottom plots) All the data points within 200 μm from the L4/5 border toward pia were plotted (black, dark gray, and light gray; n = 3) for A1 (top), V1 (middle) and S1 (bottom). (top plots) Cumulative distribution analysis of soma size. Distributions with the largest differences between two adjacent groups are shown. Red lines indicate the position of the largest statistical difference using Kolmogorov–Smirnov test (*p < 0.05, n = 3), suggesting the border of layer 3 and layer 4. d Comparison of average NeuN+ somatic size among A1 (blue), V1 (red) and S1 (green) at each location from L4/5 border. Horizontal bars with asterisk indicate the distance range with significance (ANOVA with Tukey–Kramer’s post hoc test, p < 0.05, n = 3)
For cell distribution analysis, the distance of CTB, Foxp2, or Ctip2 labeled cells present within ROI was measured from pia along a line parallel to the center line, and then proportional distance was obtained at 1% steps by normalizing the distance to the cortical thickness with pia and the L6/WM border being 0% and 100%, respectively. The number of cells at each step was averaged across animals and plotted against their respective proportional distance.
A laminar proportion of the Ctip2-immunoreactive cell band in layer 5 was determined as a proportional distance between the upper and lower edges of the band. The edges were defined as the proportional position at which the number of cells counted at 1% bin was less than 3% of the average of Ctip2 cell number in 4 sections per mouse for 3 mice.
For the fluorescence intensity analysis of NeuN, Cux1 and vGluT2, we divided a defined ROI equally into four equally sized, vertically rectangular areas, and the fluorescence intensity from the L6/WM border to the top edge of layer 2 was measured using the Plot Profile (pixel intensity counting) function of ImageJ. For the intensity measurement of the hollow and the septum of the barrel field of S1, the rectangular areas had a width of 50–100 μm and 30–50 μm, respectively. The background intensity of each section was subtracted from raw grayscale intensity profiles to minimize the differences in tissue staining among animals. The fluorescent intensities of the rectangular areas were converted from pixels to distances at 1% steps (90% being the vertical length of the area) and averaged. The averaged intensities of 3–4 sections in each animal were averaged across animals for plotting. Layer 1 was excluded because of the difficulty in measuring its intensity without including the area outside the brain section due to the inconsistent curvature of the pia.
For soma size measurement, confocal images containing presumptive layer 4 were captured using the 63× lens as described above. After thresholding of the images as above, analysis areas within an image of 100 µm horizontal width × 200 µm vertical width were selected, and the somatic area of NeuN-stained cells was manually drawn at the focus in the z-plane showing a maximum soma area as described previously (Sigl-Glöckner and Brecht 2017). Cells with partial structure that are located near the edge of the analysis area were excluded from analysis. To define the layer 3 and layer 4 border, the number of cells having the NeuN immunopositive (NeuN+) soma size from 50 to 180 μm2 was counted at 10 μm2 steps over the length of the ROI that begins from the layer 4 and layer 5 border to 200 μm from the border toward pia. For each soma size group, the cumulative distribution of cell counts was plotted. The distribution of the soma size was compared between two adjacent groups using the Kolmogorov–Smirnov test to determine the distance from the layer 4 and 5 (L4/5) border that gives the largest difference between the two cumulative frequency plots for the soma size. Since layer 4 is thought to contain granular neurons of smaller soma size compared to pyramidal neurons, it is expected that there will be a location from the L4/5 border that shows the largest difference in the distribution of the number of cells between two soma size groups.
To define the width of the gap from the layers 5 and 6 (L5/6) border, the number of Ctip2-immunopositive cells were counted at 1% steps from pia (0%) to the border of L6/WM (100%), and cell counts between two adjacent bins were compared for the statistical difference in the number of cells that exist toward the pia from the L5/6 border using Mann–Whitney’s U test.
Statistical analysis
Statistical comparisons were performed using installed functions or programmed macros on Microsoft Excel. Statistical significance for parametric distributions was determined using one-way ANOVA with post hoc tests. Averaged data were reported as mean ± standard error of the mean (SEM).
Results
Laminar boundaries in primary sensory cortices
We tried to re-define the laminar thickness and the laminar boundaries in A1 of male FVB mice at P48 (see “Materials and methods” for A1 definition). These laminar features were also compared to primary visual cortex (V1) and primary somatosensory cortex (S1). Staining of coronal sections for the three sensory cortices with the pan-nuclear marker Hoechst and the neuronal marker NeuN showed the typical distribution patterns of labeled cells (Fig. 1). The laminar border between layers 1 and 2 (L1/2) can be clearly defined using the sections immunolabeled against NeuN. The border between layers 4 and 5 (L4/5) was also distinguishable as both Hoechst positive (Ho+) and NeuN immunopositive (NeuN+) cells delineated the bottom edge of presumptive layer 4. The septa of S1 (between the hollows of the barrel fields) were also visible. To better define the borders of L1/2 and L4/5, we co-immunostained the NeuN+ sections with Cux1, the laminar marker that labels layers 2–4. Co-immunostained images showed a thick horizontal band of Cux1 immunopositive (Cux1+) cells, confirming the borders of L1/2 and L4/5 defined by NeuN. Meanwhile, the border between layers 5 and 6 (L5/6) in NeuN-stained sections could be estimated but with much less confidence. Therefore, we co-immunostained the sections against Foxp2, the laminar marker that is expressed in layer 6. Foxp2-immunopositive (Foxp2+) neurons were distributed to the bottom layer, matching with the NeuN-based estimate for the L5/6 border. The two bands of Cux1+ and Foxp2+ cells created a mostly unstained area between them, having less dense distributions of layer 5 neurons at the top and the bottom of the layer. NeuN+ cells in layer 6 were smaller in soma size and more densely distributed.
Next, we measured the laminar thickness of layer 1, the Cux1+ laminar band for layers 2–4, the unstained area between Cux1 and Foxp2 bands for layer 5, and the laminar thickness of Foxp2+ laminar band for layer 6 (Table 2). Layer 1 of the three cortical areas was similar in thickness, but layers 2–4 were narrower in A1 than in S1 and V1. In the lower layers, the thickness of layer 5 was much wider in A1 than in V1 and S1, while the Foxp2+ band thickness in A1 was similar to that in V1 but significantly narrower in S1. We also examined proportional laminar size in the three cortices. The laminar proportions for the upper layers (layer 1 and layers 2–4 combined) were less than 50% of the total cortical thickness in A1, but the proportions were more than 50% in V1 and S1. The laminar proportion of layers 2–4 was significantly smaller in A1 than in the other two cortices, while that of layer 5 in A1 was significantly larger than the other two cortices. Layer 6 proportions of A1 and V1 were similar to each other, but much smaller than the proportion in S1. Comparison between layer 5 and layer 6 in A1 shows that the layer 5 proportion was two times larger than the layer 6 proportion. These observations suggest that the three sensory cortices have distinct laminar proportions.
In an attempt to demonstrate possible differences in neuronal distributions among the three cortices, we quantified the fluorescence intensity of NeuN+ cells, averaging the intensity within the ROI of 400 μm horizontal width from the L1/2 border to the L6/WM border (Fig. 1a, b, averages of four sections/mouse, n = 3 mice). The fluorescence intensity was plotted against the proportional distance from the L1/2 border to the L6/WM border. Layer 1 was not quantified because of its variable width. In A1, the intensity was relatively high in the upper layers with a lower level of intensity around the L4/5 border (indicated by a dotted arrow) (Fig. 1a, b). In V1 and S1, the upper layer intensity appeared elevated in the middle of layers 2–4 and lowered toward to the L4/5 border. No clear difference in the NeuN+ intensity for the hollow and the septa in S1 was detected (Fig. 1a). In the three cortices, a low peak of the NeuN+ intensity was observed under the L4/5 border in layer 5. The intensity then showed a positive peak in the middle of layer 5 at ~ 60% in A1 and at ~ 70% in V1, suggesting a comparatively dense neuronal distribution there. The positive peak in the middle layer 5 of S1 was less obvious. In layer 6, there was a positive peak at ~ 90% in A1 and V1 near the WM, but the peak was found at ~ 80% near the L5/6 border in S1. These data suggest different patterns of NeuN+ cell distributions among the three sensory cortices.
With the L1/2, L4/5, and L5/6 borders being established, we next wished to determine the border between layers 3 and 4 (L3/4). Given possible differences in soma size between layer 4 neurons and layer 3 neurons, we decided quantitatively measure, at the plane of confocal focus, the somatic area of NeuN+ cells located between the L4/5 border toward pia for 200 μm (Fig. 1c, d). For the three cortices, a total of 12 sections (4 sections/mouse in 3 mice) were analyzed. The range of soma size was 60–175 μm2 among the total cell number of 1288 neurons examined (average cell number = 107 ± 4 neuron/section) in A1, 47–169 μm2 (1810 total neurons; 151 ± 4 neurons/section) in V1, and 45–186 μm2 (1733 total neurons; 144 ± 5 neurons/section) in S1. To determine the location of the border, we sought to find two adjacent bins at the bin size of 10 μm2 in soma size that give the most difference in the distribution of neuron counts within 200 μm above the L4/5 border. The idea is that, since layer 4 would contain smaller size neurons and layer 3 would have comparatively larger size neurons, there will be a cortical location that separates the bins that give the most difference in the decrease of the number of neurons having the smaller soma size and the increase of the number of neurons having the larger soma size. To accomplish this, the cumulative distribution curve of cell counts in each bin of soma size was constructed as a function of the distance from L4/5 borders at 10 μm steps, and two curves of adjacent bins were compared using the Kolmogorov–Smirnov test to detect the most difference in the D statistical values (p < 0.001). The distribution of cells with the soma size of 100–110 μm2 (241 cells) was significantly different from that with the soma size of 110–120 μm2 (142 cells) in A1, and the largest difference was detected at 110 μm from the L4/5 border. In V1, the significant difference was detected between the soma size of 80–90 μm2 (503 cells) and 90–100 μm2 (289 cells), and the largest difference can be detected at 110 μm from the L4/5 border. In S1, the largest difference can be detected at 140–150 μm from the L4/5 border (the soma size of 90–100 μm2, 236 cells; the soma size of 100–110 μm2, 86 cells). The average soma sizes of layer 4 neurons were 95 ± 1 μm2 in A1 (0–110 μm), 79 ± 1 μm2 in V1 (0–110 μm), and 80 ± 1 μm2 in S1 (0–150 μm). The soma sizes of layer 4 neurons in A1 were significantly larger than those in V1 and in S1 (one-way ANOVA with Tukey–Kramer post hoc test, p < 0.05, n = 3). Interestingly, the layer 4 laminar proportion was similar in all the three sensory cortices with 13–14% of the total cortical thickness.
Corticothalamic projection neurons express Foxp2, defining layer 6 in A1
We next examined whether corticothalamic neurons projecting to the ventral division of medial geniculate nucleus (MGv) distribute within the Foxp2+ band using retrograde tracing in three mice (Fig. 2). A small amount of the cholera toxin β-subunits conjugated with Alexa Fluor 488 (CTB) was stereotaxically injected into medial geniculate nucleus (MGN) aiming at MGv at P20, and after 4 weeks, mouse brain sections were processed for immunofluorochemistry and confocal analysis. Figure 2a shows anterior to posterior coronal sections in one of the representative injections that centered within MGv, and CTB-labeled (CTB+) cells in the lower layers of the ipsilateral cortex. The CTB+ cells in A1 were located within the cortical proportion of 20% from the L6/WM border (i.e., 80–100% from pia), with a peak of the labeled cells found in the middle of the layer (85–95%, Fig. 2c). Quantitative analysis of CTB+ corticothalamic neurons indicated that 95.1 ± 1.1% of them expressed Foxp2 (Fig. 2d), and importantly the majority of the CTB+ neurons was distributed within the Foxp2+ laminar band (Fig. 2b, c; 1933 Foxp2+CTB+ neurons among the total of 2000 CTB+ neurons, 4 sections per mouse, n = 3 mice). Taken together, these data support the idea that the Foxp2+ laminar band represents layer 6, which is about half the thickness of layer 5 in A1.
Retrograde labeling of corticothalamic projection neurons in A1. a Coronal sections containing A1 with corticothalamic neurons retrogradely labeled with CTB. 50 μm between the sections. Yellow lines indicate the A1 area analyzed. Insert: CTB injection site in MGv (v). White and black scale bars, 500 μm. L lateral, D dorsal, A anterior. b Hoechst (Ho), Foxp2 and Ctip2 co-immunostaining of CTB-labeled sections in A1. White scale bar, 100 μm. Boxed areas are magnified below. Arrows indicate some of the CTB-labeled neurons expressing Foxp2, Ctip2, and Ho. Black scale bar, 50 μm. c Distribution of the average number of Foxp2-immunopositive cells (black line) and CTB-labeled cells (dotted line) with the distance from pia (0%) to the L6/WM border (100%). Shaded areas indicate SEM (n = 3). d Proportions of CTB-labeled neurons expressing (+) or not expressing (−) Foxp2 (top) or Ctip2 (bottom) in Foxp2-defined presumptive layer 6. e Venn diagram for the proportion of corticothalamic CTB-labeled neurons that express Foxp2 (purple), Ctip2 (yellow), both Foxp2 and Ctip2 (orange), or neither of them (blue)
We noticed that the corticothalamic neurons also expressed Ctip2. It was found that 85.0 ± 1.5% of CTB+ neurons expressed Ctip2 (Fig. 2d). Indeed, among the CTB+ corticothalamic neurons, 83.1 ± 1.2% expressed both Foxp2 and Ctip2 transcription factors, while about 12.0% expressed Foxp2 but not Ctip2 (Fig. 2e). These data suggest that most of MGv-projecting corticothalamic neurons in A1 co-express Foxp2 and Ctip2 in maturing mice.
Corticocollicular neurons express Ctip2 in the middle of layer 5 and at the bottom of layer 6
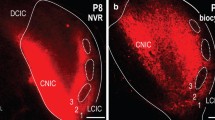
Ctip2 expression was found not only in layer 6 neurons but also in neurons located above them (Fig. 2b), consistent with the studies showing its expression in subcortical projection neurons in layer 5 of somatosensory cortex (McKenna et al. 2011; Harb et al. 2016). A previous study in A1 also indicated that corticocollicular neurons were distributed just above layer 6 in rats (Games and Winer 1988; Doucet et al. 2003). Therefore, we examined if the Ctip2-immunopositive (Ctip2+) neurons were corticocollicular neurons. Retrograde tracers (CTB, 2 mice; Red Retrobeads, 1 mouse) were injected into the inferior colliculus (IC), aiming at the central nucleus of IC, as corticofugal inputs target the entire IC (Saldaña et al. 1996). Figure 3a shows a series of brain sections with retrogradely labeled cells in auditory cortex, including A1. The labeled neurons were found mainly in the lower layers, forming a band that extended ventrally toward the rhinal fissure and dorsally beyond A1. There were also some labeled neurons near the subcortical white matter. The brain sections containing retrogradely labeled corticocollicular neurons were subjected to co-staining with Ctip2 as well as Foxp2 (Fig. 3b). In the co-stained sections, the distribution of the corticocollicular neurons matched with the distribution of Ctip2+ cells in presumptive layer 5 above the Foxp2+ laminar band (Fig. 3c). Indeed, 89.4 ± 4.5% of the corticocollicular neurons co-expressed Ctip2 (Fig. 3d; 378 Ctip2+ neurons out of 436 CTB+ or Retrobeads+ corticocollicular neurons). We also found some retrogradely labeled neurons within the cortical proportion of 95–100% from pia (i.e., ~ 40 μm from the L6/WM border, Fig. 3b, c). Among these neurons, 88.0 ± 6.0% expressed Ctip2 (63 Ctip2+ neurons out of 76 corticocollicular neurons). Since the majority of layer 6 corticothalamic neurons expressed Foxp2 (Fig. 2), we wondered if any of the corticocollicular neurons at the bottom of layer 6 also expressed Foxp2. We found about 89% of CTB+ neurons expressed Foxp2 (64 Foxp2+CTB+ neurons among 72 CTB+ neurons, n = 2 mice), and among those Foxp2+CTB+ neurons, 88% was Ctip2+ (56 out of 64). These results indicate that nearly 90% of corticocollicular neurons express Ctip2 in A1.
Retrograde labeling of corticocollicular projection neurons in the lower layers of A1. a Coronal sections with Retrobeads-labeled cells in auditory cortex arranged from anterior (top-left) to posterior (bottom-right) sections with 50 μm between sections. Yellow lines indicate the A1 area analyzed. (top-right insert) Injection site in IC with the center in the central nucleus of IC (c) and spread into the external (e) and dorsal (d) cortices. White and black scale bars, 500 μm. L lateral, D dorsal, A anterior. b Co-stained images of Hoechst (Ho), Ctip2 and Foxp2 with CTB-labeled cells in A1. Scale bar, 100 μm. Boxed areas were magnified below. Arrows indicate some of the CTB-labeled cells expressing Ctip2, but not Foxp2. Scale bar, 50 μm. c Distribution of the average number of Ctip2-immunopositive (Ctip2+, line) cells and retrogradely labeled cells (dotted line) over the distance from pia (0%) to the L6/WM border (100%). SEMs for averaged cell numbers are indicated by gray shaded area (n = 3). d Proportions of retrogradely labeled neurons that express or do not express Ctip2, Ctip2+ or Ctip2−, respectively, in layer 5 (left) and layer 6 (right)
Laminar gap between corticothalamic and corticocollicular neuron bands
When the brain sections containing retrogradely labeled corticothalamic neurons were co-immunostained with antibodies against Foxp2 and Ctip2, we noticed that there was a gap between two laminar bands: one consisting of Ctip2+ cells in layer 5 and the other consisting of Foxp2+ cells in the deep layer (see the merged image in Fig. 2b). We initially wondered if this gap belonged to layer 6. However, given that corticothalamic neurons contained within the Foxp2+ laminar band (see above), and the analysis of corticothalamic projection neurons (Fig. 2) did not indicate any labeled neurons in the gap, we speculated that the gap is not a part of layer 6, but probably belongs to layer 5 instead. In the experiments with corticocollicular projection neurons (Fig. 3), there were a few CTB+ neurons near the bottom of the Ctip2+ laminar band, but not to the extent that they fill the gap in every section. Thus, unlike the previous notion of neighboring existence of corticocollicular neurons next to layer 6 in rats, corticocollicular neurons are distantly located above layer 6 corticothalamic neurons.
Commissural corticocortical neurons distribute widely in A1, populating highly in the laminar gap
Commissural projection neurons are known to be distributed throughout the cortex, except layer 1, in the rat (Jacobson and Trojanowski 1974; Rüttgers et al. 1990). We wondered if and how they are distributed in and outside the gap in mice. We injected retrograde tracers into the contralateral auditory cortex, aiming at A1 (Fig. 4a). In all the injections, the tip of the injector was placed in the lower layers of A1, and tracers were spread centering the tip but also along the injector across the cortex. As found previously, CTB+ cells were distributed throughout cortical layers except layer 1 (Fig. 4a, b). Almost all, except 1.6 ± 0.5%, of the CTB+ corticocortical projection neurons did not express Ctip2 in layer 5 (Fig. 4c; 815 Ctip2-immunonegative cells out of 827 total CTB+ cells counted, 4 sections per mouse, n = 3 mice). Meanwhile 16.0 ± 3.2% of the CTB+ corticocortical neurons expressed Ctip2 in the Foxp2+ cell band of layer 6 (Fig. 4d; 35 Ctip2+ cells out of 224 total CTB+ cells). Thus, there was about ten times higher proportion of Ctip2-expressing corticocortical neurons in layer 6 than in layer 5. The proportion of Ctip2+ corticocortical neurons in layer 6 was about fivefold higher than the proportion of Foxp2-expressing CTB+ corticocortical neurons (3.6 ± 0.5%), where the majority expressed Ctip2 (7 out of 8 cells) (Fig. 4e). Taken together, these data suggest that commissural projection neurons mostly lack Ctip2 in the lower layers and that the expression of Ctip2 in commissural neurons depends on their laminar location.
Characterization of retrogradely labeled commissural corticocortical neurons. a Coronal sections with the injection in the contralateral A1 (left) and retrogradely CTB-labeled cells (right). Images are from anterior to posterior direction from the top to the bottom with 50 μm distance between sections. Yellow lines indicate A1. Scale bar, 500 μm. b Co-stained images of Hoechst (Ho), Ctip2, and Foxp2 in CTB-injected brain sections. Scale bar, 100 μm. Boxed areas are magnified below to show the laminar gap between Ctip2+ cells in layer 5 and Foxp2+ cells in layer 6. Scale bar, 50 μm. c Proportions of CTB-labeled neurons with or without Ctip2 expression in layer 5 (Ctip2+ and Ctip2−, respectively). d Proportions of CTB-labeled neurons with (+) or without (−) Ctip2 (left) or Foxp2 (right) expression in layer 6. e Venn diagram for the proportion of CTB-labeled commissural neurons that express Foxp2 (purple), Ctip2 (yellow), both Foxp2 and Ctip2 (orange), or neither of them (blue) in layer 6. n = 3 for c–e
Importantly, in the lower layers, many of the retrogradely labeled neurons were found within the gap between the Ctip2+ and Foxp2+ laminar bands (Fig. 4b). To compare the distribution of the corticocortical neurons to that of the corticocollicular projection neurons in different animals, we normalized the cortical thickness of stained sections and compared the number of the two types of retrogradely labeled cells at 10% bin steps from pia to the L6/WM border in A1 (Fig. 5; corticocortical, n = 3 mice; corticocollicular, n = 3 mice). We found an elevated number of corticocortical neurons within 20–30% from the border of L6/WM, which is the location of the gap exactly. Thus, we hypothesize that the gap is an important source of commissural corticocortical projection neurons in A1. The distribution plot also shows the lack of corticocollicular neurons in the upper part of the Foxp2+ layer 6. Taken together, these data further support the idea that the gap most likely belongs to layer 5, not layer 6, in A1.
Distribution of retrogradely labeled corticocortical and corticocollicular neurons. a CTB-labeled corticocortical (CCort, left) and corticocollicular (CCol, right) cells in A1. Scale bar, 100 μm. b Distribution of retrogradely labeled corticocortical cells (green line) and corticocollicular cells (purple line) overlaid with estimated laminar thicknesses and locations of Foxp2+, Ctip2+, and Cux1+ cell bands. The number of retrogradely labeled cells was averaged at 1% bins of proportional distance (four sections per mouse, n = 3 mice). SEMs for the averaged cell counts are indicated by lighter green (corticocortical) and purple (corticocollicular) lines shown only above the averages. The higher and lower fluorescent intensity differences of Cux1 labeled cells were assumed to divide layers 2–4 at layer 3. The laminar proportion is indicated above as short vertical lines as defined in Table 2. The border between layers 2/3 and layer 4 is based on the proportion of layer 4 as defined by the NeuN+ soma size (Fig. 1)
vGluT2 immunopositive fibers terminate in the laminar gap in layer 5
With the gap likely belonging to layer 5 and the laminar thickness of layer 5 being much wider than previously thought (Fig. 2; Table 2), these findings may solve the uncertainty as to which layer the thalamocortical axon collaterals mainly terminate. To explore this, we triple-stained coronal sections with Foxp2, Ctip2, and vGluT2 in three mice (Fig. 6). As predicted from previous reports (Herkenham 1980; Kaneko and Fujiyama 2002; Smith et al. 2012; Hackett et al. 2016), we observed high intensity vGluT2 immunopositive (vGluT2+) stains in three laminar bands—layer 1, the thalamocortical recipient lower layer 3 and layer 4, and the lower layers—and a diffuse, lower intensity staining throughout A1 (Fig. 6a). Interestingly, the vGluT2+ laminar band in the lower layers was found mostly in the gap at the bottom of layer 5. A magnified image around the gap shows the high intensity vGluT2+ fibers/puncta filling the gap created by Ctip2+ cells in layers 5 and 6, and slightly overlapping with the bottom edge of layer 5 Ctip2+ cells and the upper edge of the Foxp2+ cells. A quantitative analysis of vGluT2+ fluorescence intensity also demonstrated a peak of the intensity in the laminar gap (see Fig. 7). Further, to confirm that vGluT2+ puncta are actually expressed in the thalamocortical axon collaterals in A1, presumptive thalamocortical axons were anterogradely labeled with trimethylrhodamine-conjugated dextrans (TMR-Dex) injected into MGv and co-stained with antibodies against vGluT2 in three mice (see Fig. 7). vGluT2+ puncta were detected within some of the TMR-dextran-labeled axon fibers. These data suggest that the thalamocortical axon collaterals in the lower layers mainly terminate at the bottom of layer 5, where the commissural neurons highly populate.
Distribution of vGluT2, Ctip2, and Foxp2 immunoreactivities in A1, V1 and S1. Co-stained images of Hoechst (Ho), vGluT2, Ctip2, and Foxp2 in A1 (a), V1 (b), and S1 (c). White scale bars, 100 μm. Boxed areas were magnified below for layer 5/6 borders. Black scale bars, 50 μm. d Distribution of the average number of Ctip2+ cells per coronal section for A1 (blue), V1 (red), and S1 (green) (n = 3). e The distribution of the averaged Ctip2+ cell counts in d was aligned at the L5/6 border (0%) for the three cortices and magnified with the proportional distance measured from the border toward pia. Dotted lines indicate the distance from the L5/6 border at which significant increase of Ctip2+ cells was detected compared to an adjacent bin (*p < 0.05, Mann–Whitney U test, n = 12 sections, 4 sections/mouse, 3 mice)
Anterogradely labeled thalamocortical fibers overlap with vGluT2+ bands and Cux1+ high intensity laminar band, and contain vGluT2+ puncta in A1. a, b Representative images with Hoechst (Ho), vGluT2, TMR-Dex-labeled thalamocortical axons, and Cux1 co-staining in coronal sections. a A TMR-Dex injection site in MG with an injector injury. Scale bar, 200 μm. b (top) Low magnification images in A1 of the same brain as a showing the overlap of vGluT2+ upper layer band, TMR-Dex thalamocortical axon terminals, and high-intensity Cux1 band. White scale bar, 100 μm. b (bottom) Magnified images of white boxes for (i) the main thalamorecipient layers 3 and 4 and (ii) the thalamocortical axon collaterals in layers 5. vGluT2+ lower layer band partially overlapped with TMR-Dex labeled axon terminals in layer 5. Black scale bar, 50 μm. c Single confocal z-plane images of yellow boxed areas in (i) and (ii). Orthogonal views of the z-plane images at central x- and y-positions (vertical and horizontal lines) are shown on the left and the top of the magnified images to demonstrate co-localization of vGluT2 and TMR-Dex-labeled thalamocortical fibers in layers 3/4 (top) and layer 5 (bottom). Arrowheads point to other colocalizations identified by the orthogonal image analysis. Yellow scale bar, 5 μm. d Relative fluorescence intensities of vGluT2 (blue) and Cux1 (purple) across the proportional distance from pia (0%) to the L6/WM border (100%) were averaged (n = 3 mice) and overlaid with estimated laminar proportions of Foxp2+, Ctip2+, and Cux1+ cell bands (see Table 2 for values). SEMs were indicated above the averaged lines with lighter colors. The L3/4 border was estimated by the NeuN soma size analysis (see Fig. 1). Layer 1 intensity was not quantified due to the variability in its laminar thickness. Layer 1 thickness was assumed to be 10% based on measured values (see Table 2)
We then tested if this staining pattern of the vGluT2+ lower layer band in A1 can be similarly observed in S1 and V1 (Fig. 6, brains were from the same three mice as for A1). Ctip2+ cells in layer 5 and Foxp2+ cells in layer 6 formed two laminar bands that appeared to form a narrower gap between them in both S1 and V1 compared to the gap in A1. To quantitatively demonstrate the difference in the size of the gap, the Ctip2+ cell counts were plotted at proportional positions of the cortical width at 1% steps (Fig. 6d, 4 sections/mouse, n = 3 mice). The average number of cells shows a valley between the two peaks of Ctip2+ cell counts in the lower layers. The lowest cell counts in the valley (negative peak) were found at the proportional position of 79% for A1, 73% for S1, and 78% for V1. With an arbitrary criterion of < 3 cells/section, the width of the gap was 10% (72–82% from pia) for A1, 3% (71–74%) for S1, and 4% (77–81%) for V1, indicating that the gap is about threefold wider in A1 than in S1 or V1. We also quantitatively analyzed the width of the gap from the L5/6 border (Fig. 6e). The Ctip2+ cell count distributions were aligned at the L5/6 border, and the number of Ctip2+ cells within 1% bins was compared between adjacent bins to detect a significant difference. In A1, the cell counts between 11–12% and 12–13% from the L5/6 border (71–72% and 70–71%, respectively, in the proportional position of the cortex) showed significant statistical difference using the one-tailed Mann–Whitney U test (U = 40, n = 12 for both bins, p < 0.05), indicating a gap of 12% from the border. Similar analyses in V1 and S1 resulted in the gap of 8% and 4%, respectively (V1: 7–8% (74–75%) and 8–9% (73–74%), U = 32; S1: 3–4% (72–73%) and 4–5% (71–72%), U = 40, n = 12 for both bins in V1 and S1, p < 0.05). These analyses indicate that A1 possesses a wider distance from the L5/6 border to the cluster of Ctip2+ cells in layer 5. Importantly, when we aligned the distance to the NeuN intensity plots (Fig. 1a, black arrows), the gap could be detected as a region of the lower fluorescence intensities in layer 5, suggesting that the gap is not simply due to the reduced expression of Ctip2 but due to the lower neuronal density.
Having demonstrated the narrower gap in V1 and S1, we shall return to the question of vGlut2+ terminations in the gap. Qualitatively, with low magnification, the vGluT2+ fibers appeared to exist over the small gap and beyond in S1 and V1. Higher magnification images revealed that unlike A1, the high density vGluT2+ fibers/puncta existed over this gap and extended into the Ctip2+ cells in the bottom part of layer 5 and the Foxp2+ (and Ctip2+) cells in the top part of layer 6. Additionally, it was noted that the small gap contained low intensity Ctip2+ cells as well as low intensity Foxp2+ cells, suggesting the formation of a layer 5/6 interface in the gap. This is consistent with a previous observation that thalamocortical axon collaterals terminated in layers 5 and 6 in S1 (Watakabe et al. 2014). Overall, demonstrating the anatomical differences in the distribution of Ctip2+ and Foxp2+ neurons in the lower layers and the differences in how the vGluT2+ fibers/puncta terminate around the L5/6 border in A1 in comparison to S1 and V1, these results suggest a functional difference in thalamocortical axon collaterals in the primary sensory cortices.
vGluT2 and Cux1 co-expression patterns identify the main thalamorecipient layers
With the Cux1+ staining pattern in Fig. 1, we noticed that the fluorescent intensity of the layer 2–4 neurons was dichotic, where cells with higher intensity were distributed to the lower part, while those with lower intensity were distributed to the upper part of layers 2–4. The laminar thicknesses of the lower part and the upper part were 17.4 ± 1.3% and 19.5 ± 0.3% of the cortical thickness, respectively, in A1 (n = 3). Given the nearly equal separation of layers 2–4, we speculated that the lower part and the upper part represented layers 2/3 and the main thalamocortical input layers of layers 3/4, respectively. To examine this, we anterogradely labeled the thalamocortical axons and co-stained the sections for vGluT2 and Cux1 (Fig. 7). In three successful TMR-dextran injections into MGv, we found a relatively dense axonal plexus in the thalamorecipient layers (presumably layers 3/4) of A1 and some low-density fibers above and below the layers. Co-staining of the labeled axons with vGluT2 revealed that the vertical spread of the anterogradely labeled terminals mostly matched the width of vGluT2+ band. In magnified images, the labeled thalamocortical axons appeared to contain vGlut2+ puncta, consistent with the notion that vGluT2 immunoreactivity in the thalamorecipient layers represented primary thalamocortical axon terminals in layers 3/4 of A1 (Hackett et al. 2016). It was also noted that the terminated vertical width of the labeled thalamocortical axons overlapped with the high intensity Cux1+ cell band, suggesting that the Cux1+ band represents the primary thalamorecipient layers. Further, the vGluT2+ middle band completely overlapped with the high intensity Cux1+ band as well as the thalamocortical axon terminals. Indeed, whereas some of the thalamocortical axons terminated above and below the high intensity Cux1+ band, vGluT2+ band demarked the Cux1+ band more clearly identifying the laminar borders as compared to the thalamocortical axon terminations. Higher magnification images show that the vGluT2+ fibers/puncta terminate around the Cux1 expressing somata. Further, a quantitative analysis of Cux1 and vGluT2 relative intensities demonstrates that the peak intensities of vGluT2 and Cux1 immunoreactivities matched in the upper layers (Fig. 7d, n = 3 mice). These data support the idea that the cells highly expressing Cux1 represents the thalamorecipient layer 3/4 neurons and that those with lower Cux1 intensity may form layer 2/3 neurons in A1. We should also point out that Cux1+ cells are somewhat populated in the laminar gap though much smaller in number compared to layers 3/4. Based on the matching intensity curves of Cux1 and vGluT2, we speculate a correlation between primary thalamocortical axon terminals and Cux1 expression.
Discussion
In this report, we attempted to re-define the laminar boundaries of maturing mouse A1 using retrograde tracing of projection neurons, vGluT2 staining of thalamocortical axon terminals, and immunofluorescent staining of transcription factors. We demonstrated that (1) the majority of the corticothalamic neurons were Foxp2+, forming a horizontal band to define layer 6 and identifying the border between layer 5 and layer 6, (2) the majority of corticocollicular neurons were Ctip2+ and were populated in the middle, not the bottom, of layer 5 (layer 5b), (3) there was a sublayer of layer 5, named layer 5c, between layer 5b and the Foxp2+ layer 6, having highly populated contralateral A1-projecting commissural neurons and the relatively high density termination of vGluT2+ thalamocortical axon collateral fibers, (4) there was a higher intensity Cux1+ horizontal band along with the vGluT2+ main thalamocortical axon terminals in the upper layers, forming the primary thalamorecipient band of layers 3/4 and concurrently identifying the boundary between layer 4 and layer 5, (5) there were NeuN+ neurons within the thalamocortical recipient layers that were smaller in soma size compared to the neighboring cells in the layer above, possibly separating the high intensity Cux1+ band into layer 4 and lower layer 3, (6) there was a lower intensity Cux1+ band, possibly representing layer 2 and upper layer 3, and (7) there was a sublayer of layer 5 sandwiched by the major thalamorecipient band and layer 5b, we call layer 5a, having commissural projection neurons. We also found that, compared to S1 and V1, A1 had larger NeuN+ soma size in presumptive layer 4, smaller layer 2–4 thickness and larger layer 5 thickness, and a wider gap between Ctip2+ cells in layer 5 and Foxp2+ cells in layer 6, where a high level of vGlut2+ puncta/fibers, including thalamocortical axon collaterals, were found.
Characterization of layer 6 in A1 and other sensory cortices
Traditionally, layer 6 is known as the source of corticothalamic neurons that project to the core thalamic nucleus. Given that layer 6 contains both corticothalamic and corticocortical neurons (Kelly and Wong 1981; Imig et al. 1982; Code and Winer 1986; Rüttgers et al. 1990; Llano and Sherman 2008; Petrof et al. 2012), however, how they are distributed within layer 6 may determine whether each type of neuron can be used to define layer 6 and to identify the boundary between layers 5 and 6. In S1, primary thalamus projecting neurons are located in the upper part of layer 6, while secondary thalamus projecting neurons are located in the lower part of layer 6 (Bourassa et al. 1995; Zhang and Deschênes 1997). Thus, fully labeling the former will provide the evidence for the boundary of layers 5/6. In A1, although there appears to be some corticothalamic neurons projecting to the secondary thalamus (i.e., the dorsal division of MGN, MGd) in layer 6 (Ojima 1994; Llano and Sherman 2008), the majority of MGd projecting neurons are located outside of A1 in the dorsal and ventral areas of auditory cortex, while the majority of primary thalamus (i.e., MGv) projecting neurons are distributed to layer 6 of A1 (Llano and Sherman 2008; Smith et al. 2012). Thus, if corticothalamic MGv projecting neurons were fully labeled, we will be able to define layer 6. However, because it is probably unlikely for retrograde tracers to label all corticothalamic neurons, the uncertainty still remains.
An alternative approach to label layer 6 corticothalamic neurons will be to use laminar-specific genetic markers (laminar markers) that are specific to them. Identifying the expression of a laminar marker in cortical neurons will be useful in determining the laminar distribution of the identified neurons and possibly the laminar boundaries if the labeled neurons were distributed widely throughout the layer. In this study, we utilized Foxp2, whose specificity as the genetic marker of corticothalamic neurons in A1 has been lacking. Combining retrograde tracing and immunofluorochemistry, we demonstrated that the majority of corticothalamic neurons expressed Foxp2, suggesting that the laminar band created by Foxp2+ neurons defines layer 6 in A1.
Foxp2 has been previously demonstrated as a laminar marker of corticothalamic neurons via various approaches in S1 and V1. Laminar-specific transcriptome analysis provided important and useful information regarding laminar and cell type specific genes in the neocortex. Micro-dissection of layers in S1 and transcript analysis demonstrated the expression of Foxp2 gene in layer 6 (Belgard et al. 2011). In the transgenic animal models generated by crossing the neurotensin receptor 1 (Ntsr1)-Cre mouse line (Gong et al. 2007) with the Ai14 or Ai3 reporter line (Madisen et al. 2010), the fluorescence-labeled neurons projected to first and higher order thalamic nuclei in the visual system (Olsen et al. 2012; Grant et al. 2016) and in the somatosensory system (Chevée et al. 2018), indicating that the Ntsr1 gene is expressed in corticothalamic neurons. Using the Ntsr1-Cre::Ai14 mice, cell-specific transcriptome analysis confirmed the expression of Foxp2 in the corticothalamic neurons in V1 (Tasic et al. 2016) and S1 (Chevée et al. 2018). Foxp2 expression in corticothalamic neurons was also demonstrated by co-labeling of retrogradely traced corticothalamic neurons and fluorescence in situ hybridization against Foxp2 transcripts, where ~ 30% of the retrogradely labeled corticothalamic neurons expressed them (Sorensen et al. 2015). In the report, Foxp2 transcripts were distributed in presumptive layer 6 (see also Ferland et al. 2003), where the labeled cells were distributed sparsely compared to our results of relatively dense Foxp2+ cell distribution in A1. Also, a gene expression analysis of transcription factors which regulates the development of corticothalamic neurons showed that Foxp2 was regulated by a transcription factor T-Box brain protein 1 (Tbr1, Deriziotis et al. 2014) to promote the identity of corticothalamic projecting neurons in S1 (Bedogni et al. 2010; McKenna et al. 2011; Kwan et al. 2012). In V1, a retrograde tracing study using the Ntsr1-Cre::Ai14 mice demonstrated that ~ 98% of the tdTomato-expressing neurons were Foxp2 positive (Sundberg et al. 2018). Since ~ 87% of retrogradely traced corticothalamic neurons were tdTomato-positive in this mouse line, the majority of corticothalamic neurons is expected to express Foxp2. Further support comes from immunohistochemical studies against dopamine- and adenosine 3′, 5′-monophosphate-regulated phosphoprotein-32,000 (DARPP-32) in adult mouse sensory cortices (Hisaoka et al. 2010). Foxp2 expression has been found reportedly in all of the dopaminoceptive, pyramidal neurons, which express DARPP-32 specifically in layer 6 (Ouimet et al. 1984; Wang et al. 2004; Rajput et al. 2009). Thus, these data strongly support Foxp2 as a specific laminar marker of layer 6 corticothalamic neurons in primary sensory cortices. Our study combining Foxp2 immunolabeling and CTB retrograde tracing in A1 will add to this notion.
Foxp2 was also expressed in most retrogradely labeled corticocollicular neurons at the bottom of layer 6 in A1. This layer is often referred to as layer 6b, a thin layer populating with multiple types of neurons (reviewed in Kanold and Luhmann 2010; Hoerder-Suabedissen and Molnár 2015). Layer 6b neurons are considered as surviving subplate neurons. Genetic analysis of subplate neurons indicated the different degree of expression of subplate neuron markers, including complexin 3 (Cplx3) and connective tissue growth factor (CTGF) (Hoerder-Suabedissen et al. 2009). The expression of these subplate neuron markers has been corroborated with their co-labeling in the Lpar1-eGFP mice that express the fluorescent protein off the regulatory elements of the G-protein-coupled lysophosphatidic acid receptor 1 (Lpar1) gene in layer 6b (Hoerder-Suabedissen and Molnár 2013). These cells were largely distributed within ~ 50 μm from the WM at P8. The retrogradely labeled corticocollicular neurons also existed within this distance (see Fig. 3c). Thus, the corticocollicular neurons in A1 are likely co-distributed along with the remnants of subplate neurons. Whether subplate neurons project to IC needs further investigation.
Recent studies of cell type-specific gene expression in layer 6b neurons indicated that A1 is different from S1 or V1. In a transgenic mouse line generated by crossing dopamine receptor D1a (Drd1a)-Cre and the Ai14 reporter (Drd1a-Cre::Ai14-tdTomato mouse), Cre-expressing tdTomato-expressing neurons appeared in the subplate of somatosensory cortex at P8 and progressively increased during postnatal development, where fluorescently labeled neurons were clearly located at the bottom layer of S1 at P35 (Hoerder-Suabedissen et al. 2018). Similarly labeled neurons were detected in V1. The tdTomato-labeled axon fibers were found in secondary thalamic nuclei, and layer 1 and the upper part of layer 5 under the barrel field in S1 or presumptive layer 4 in V1. These patterns of subplate neuron projections were also observed previously in S1 of the Golli-τ−eGFP mouse (Piñon et al. 2009) and in the wild-type mouse brain immunochemically labeled against Cplx3 (Viswanathan et al. 2017). Meanwhile, unlike S1 and V1, Cre-expressing neurons in the Drd1a-Cre::Ai14-tdTomato mouse were few in A1, and little tdTomato-labeled axonal fibers were visible in auditory thalamus and A1. Further, Cplx3 immunopositive (Cplx3+) fibers were apparently undetected in A1 despite the somatic expression of Cplx3. These data point out possible differences in gene expression in subplate/layer 6b neurons between A1 and S1 or V1. In addition, gene expression may be age-dependent as found previously for Foxp2, Ctip2, ER81 and Cux1 (Chang et al. 2018).
Comparison to previous retrograde tracing studies in the lower layers of A1
A comparison of our study with previous retrograde tracing studies of corticothalamic neurons in the auditory system supports our contention that the narrow band in the deep layer of A1 represents the full thickness of layer 6. In the previous study of retrograde labeling using the Retrobeads injected into mouse MGN, the labeled neurons were found in layer 6 as well as in the upper layer 5 (Petrof et al. 2012). The injection site image reveals the Retrobeads spreading in MGv as well as in MGd. Although some of the layer 6 neurons might be MGd projecting corticothalamic neurons, it is most likely that the majority of MGv projecting neurons were in layer 6, while MGd projecting neurons were in layer 5 (Llano and Sherman 2008). Laminar boundaries drawn based on these results indicate narrow layer 6 and wide layer 5, consistent with our results.
In another study, biotinylated dextran amine (BDA) injection into rat MGv retrogradely labeled the somata of corticothalamic neurons mostly at the bottom portion of A1 (Smith et al. 2012). Because the laminar thickness of their layer 6 was assumed to be proportionately similar to that of layer 5, the location of the labeled corticothalamic neurons was determined to be at the lower part of layer 6. However, with the assumption of disproportionate lower layers with wide layer 5 and narrow layer 6 as we propose here, the distribution of their labeled neurons will be consistent with our study.
To further confirm that the Foxp2+ band represents layer 6 will require characterization of layer 6 corticothalamic neurons with respect to their somatic and dendritic morphology and functional properties, including their projections. It would be also important to characterize neurons in the layer immediately above the Foxp2+ laminar band in presumptive layer 5 so as to distinguish their morphological and functional properties.
Characterization of layer 5 sublayers in A1
Our study using both laminar markers and retrograde tracers indicated that layer 5 can be divided into three sublayers in mice: (1) layer 5a in the upper part of layer 5, located under the Cux1+ layer 4, containing corticocollicular projection neurons and commissural corticocortical projection neurons that lack Ctip2 expression, (2) layer 5b in the middle part of layer 5 with many corticocollicular projection neurons that mostly express Ctip2 and commissural corticocortical projection neurons with little Ctip2 expression, and (3) layer 5c in the lower part of layer 5 located above Foxp2+ presumptive layer 6, consisting of commissural projection neurons that lack Ctip2 expression and having thalamocortical axon collateral terminals.
Previous reports support these laminar assignments at least for the lower two sublayers. The retrograde labeling of layer 5 and layer 6 corticothalamic neurons showed an unlabeled gap between them (layer 5b in Petrof et al. 2012), which might be the same as our layer 5c. Meanwhile, the retrograde labeling of corticocollicular projection neurons resulted in an elevated density of labeled neurons in the middle of layer 5 (Slater et al. 2013), which we call layer 5b. Layer 5a has not yet been well characterized in any previous work in mice, to our knowledge, perhaps due to the lack of a defining marker for the laminar boundary between layers 4 and 5. To this end, Cux1 will be helpful in the future studies as it defines the layer 4/5 border in examining layer 5a neurons and afferent/efferent projections.
Layer 5a neurons in A1 possibly include those expressing RAR-related orphan receptor beta subunit (RORβ) (Takeuchi et al. 2007) and ETS-Variant 1 (Etv1 a.k.a., Ets-Related Protein 81, ER81) (Chang et al. 2018; see also; Hirokawa et al. 2008). In V1 and S1 as well, neurons in the upper part of layer 5 also expressed those two genes (Takeuchi et al. 2007; Belgard et al. 2011; Tasic et al. 2016; Chevée et al. 2018). The candidate source of layer 5a projection would be secondary thalamic nuclei as found previously in S1, where the paralemniscal, medial posterior thalamic nuclei project there just under the barrel of S1 (Koralek et al. 1988; Chmielowska et al. 1989; Lu and Lin 1993). In A1, thalamocortical axonal boutons from MGv and MGd were detected in the upper part of layer 5 in the rat (Romanski and LeDoux 1993; Smith et al. 2012). Another possible source of layer 5a terminating axons will be layer 6b neurons that developed from subplate neurons. Layer 6b neurons express Cplx3 gene in A1 as well as in S1 in young postnatal mice (Viswanathan et al. 2017). Although Cplx3+ fibers were not detected at P7 in A1, they projected to the upper part of layer 5 in S1. Further anatomical characterization of afferent and efferent projections in layer 5a will be necessary to identify the properties of the neurons in A1.
Layer 5b will contain heterogenous populations of excitatory neurons, and our retrograde tracing identified Ctip2 positive and negative corticocollicular neurons, suggesting the presence of the heterogenous populations of corticocollicular neurons in layer 5. Using retinol binding protein 4 (Rbp4)-Cre mice, which express Cre recombinase mostly in layer 5 (Gong et al. 2007; Grant et al. 2016), and the injection of an adeno-associated virus (AAV) vector encoding fluorescent protein (EYFP)-fused channelrhodopsin 2 (ChR2), Rbp4-expressing corticocollicular neurons mainly projected to the shell region of IC (Xiong et al. 2015). Since a great majority of retrogradely labeled corticocollicular neurons in layer 5 project to the central nucleus of IC (ICc; Saldaña et al. 1996; Schofield 2009), it is likely that there are corticocollicular neurons in layer 5 that do not express Rbp4 genes. (It is of note that Rbp4 gene is also expressed in layer 6b and may project to ICc. However, since layer 6 corticocollicular neurons project much less to ICc than to the shell of IC (Schofield 2009), the Rbp4 neurons in layer 6b may be the main ICc projecting cells). Since single cell transcriptome analyses showed the co-expression of Rbp4 and Bcl11b (Ctip2) genes in layer 5 neurons in V1 (Tasic et al. 2016), we speculate the presence of Ctip2- and Rbp4-negative corticocollicular neurons albeit small in their proportion. Of note also is our previous finding that ~ 30% in A1 and ~ 40% in V1 and S1 of ER81 (Etv1) expressing neurons co-expressed Ctip2 in layer 5 of mature mice (Chang et al. 2018). It can be speculated that there are Ctip2+ corticocollicular neurons that either express or do not express ER81. The single cell transcriptome analysis also indicated a small population of layer 5 Rbp4 neurons that expressed Bcl11b (Ctip2) but not Etv1 (ER81). Therefore, in addition to the difference in the expression of Ctip2 and Rbp4, there may be additional differences in the expression of ER81 that differentiate corticocollicular neurons.
The properties of layer 5c neurons are largely unclear. We find that the “gap” contains NeuN+ neurons with low density and a populated Cux1+ neurons, whose significance remains to be investigated. The single cell transcriptome analysis in V1 indicated the expression of Cux1 in a subpopulation of layer 5 neurons expressing the α6 subunit of nicotinic acetylcholine receptors (nAChRs) in the Rbp4-Cre::Ki14-tdTomato mouse line (Allen Brain Atlas, Seattle, WA, USA: Allen Institute for Brain Science. Available from http://casestudies.brain-map.org, last accessed July 13, 2018). Little is known about the functional role of the nAChRs containing α6 subunits (α6∗−nAChRs) in V1 (Quik et al. 2011). In addition, whether α6∗−nAChRs are expressed in layer 5c of A1 is not known. Further studies of layer 5c neurons, including their regulation via α6∗−nAChRs, will be of interest in understanding of sensory information processing.
Laminar boundaries in the lower layers
The definition of laminar boundaries will be important for our understanding of laminar functions. Although we observed that commissural corticocortical projection neurons distributed throughout layer 5 (and other layers) as reported previously in rodents (Games and Winer 1988; Rock and Apicella 2015), there were contrasting reports regarding the location of corticocollicular projection neurons. The middle layer 5 distribution of corticocollicular projection neurons in our study is consistent with the studies in mice (Slater et al. 2013), but contrasts with previous reports in rats that showed their distribution of mostly lower three-fourths of layer 5 (Games and Winer 1988) or throughout layer 5 (Doucet et al. 2003). These contrasting results may be simply due to species difference. Another possibility would be the difference between the two species regarding the definition of the boundary between layers 5 and 6. The use of Foxp2 in combination with retrograde labeling in mice suggested disproportionate lower layers with wide layer 5 and narrow layer 6, occupying ~ 35% and ~ 18% of the cortical thickness, respectively. These values of laminar proportions are quite consistent with the study by Heumann et al. (1977), who reported ~ 37% and ~ 20% for layer 5 and layer 6, respectively, at P60. In contrast, based on the Nissl and Golgi staining in rats, Games and Winer (1988) reported 26% and 22% for layer 5 and layer 6, respectively. Similar proportions were reported for mice (Anderson et al. 2009). Thus, the estimates of laminar proportions based on Nissl staining resulted in different conclusions even within the same species. In addition, although the Golgi staining can provide clear cell morphology, not all cells can be stained, and the detailed cell types of the labeled cells are mostly unclear. Therefore, the definition of laminar proportion in A1 may need to be carefully considered in the lower layers. Further support for this comes from the studies of lemniscal thalamocortical axon collaterals as described below.
Thalamocortical axon collaterals
Laminar termination sites of lemniscal thalamocortical axon collaterals had been debated for many years with layer 5 (Mitani et al. 1984; Romanski and LeDoux 1993), layer 6 (Llano and Sherman 2008) or the layer 5/6 border (Smith et al. 2012) as their recipient layer(s) in A1. It is possible that the termination layers differed because how the laminar boundary was defined. Smith et al. (2012) reported BDA-labeled lemniscal thalamocortical boutons terminating in presumptive layer 5/6 border in rat A1. Based on the distribution of retrogradely labeled corticothalamic neurons in layer 6, however, the densely terminating boutons might be at the bottom layer 5 if the narrow layer 6 could be assumed as we described here. Based on the analysis of layer 6, our data support the notion that the thalamocortical axon collaterals mainly terminate at the bottom of layer 5. In this sublayer, it can be speculated that not only layer 5 neurons but also layer 6 neurons would receive relatively strong thalamocortical collateral inputs as recent functional evidence indicates (layer 5: Sun et al. 2013; layer 6: Zhou et al. 2010; both: Viaene et al. 2011; Ji et al. 2016). Since we also found vGluT2+ neuropils in both layers 5 and 6 outside the dense layer 5c-terminals, functional significance of the different terminal densities will need to be explored. Interestingly, unlike A1, our studies indicated much narrower gap between Ctip2+ laminar band and Foxp2+ laminar band (presumptive layer 6) in V1 and S1 (Fig. 6). The dense vGluT2+ fibers/puncta like A1 also occupied the gap and partially overlapped both the Ctip2+ cells in layer 5 and Foxp2+ cells in layer 6. The functional significance of these contrasting observations between A1 and V1 or S1 needs to be further investigated.
Laminar boundaries in the upper layers of A1
Laminar boundaries within layers 2–4 in the upper layers in A1 could not be easily distinguished earlier. In this study, we report Cux1 staining as a possible marker to differentiate the upper layers into two: the main thalamorecipient layers (lower layer 3 and layer 4) and layers 2/3. Previously, enzyme expression patterns such as cytochrome oxidase (Anderson et al. 2009; Hackett et al. 2011, 2016) and acetylcholinesterase (Robertson et al. 1991; Anderson et al. 2009) have been utilized to define cortical lamina in the upper layers. Due to the diffuse nature of the staining, however, laminar boundaries could only be estimated. A more direct way to visualize axons utilized anterograde tracers such as BDA (Llano and Sherman 2008; Smith et al. 2012) or fluorophore-conjugated dextran amines (Rose and Metherate 2005) to label cytosolic milieu of thalamocortical axons. This anterograde tracing method identified thick axon plexus in putative layers 3/4. However, the laminar boundary was still unclear because labeled axons were spread above and below the thalamorecipient layers. Similar results were obtained by the immunohistochemical detection of vGluT2 (Kaneko et al. 2002; Nakamura et al. 2005; Graziano et al. 2008; Hackett et al. 2016), despite more axons being labeled compared to the anterograde tracing.
We therefore sought another way, the laminar marker, for better definition of boundaries. In previous studies of S1, Cux1+ cells showed the clear nuclear staining in layers 2–4 (Nieto et al. 2004) and a laminar gradient of the upper layer neurons (Ferrere et al. 2006). We extended these studies by combining Cux1 and vGluT2 immunostaining and found a correlated distribution of the main vGluT2+ thalamocortical terminals with the high intensity Cux1+ neurons in the lower part of layers 2–4 in A1. Our data lead us to propose that the horizontal band of the high intensity Cux1+ neurons represent the thalamorecipient layers 3/4, defining the boundary of layer 4 and layer 5 at the bottom edge of the Cux1+ band and the boundary of the upper and lower layer 3 at the top of the high intensity Cux1+ band.
The analysis of soma size based on NeuN+ neurons allowed us to tentatively define a laminar band for putative layer 4 in A1 as well as in V1 and S1. Whether the laminar bands represent true layer 4 will require further tests. A useful transgenic mouse line would be a sodium channel epithelial 1 alpha subunit (Scnn1a)-Cre mouse line (Madisen et al. 2010). Crossing this line with a reporter mouse line previously demonstrated a thin band in the middle of A1 (Li et al. 2014) and the barrel field of S1 (Guy et al. 2015; Wagener et al. 2016). Cux1 genes are also expressed in layer 4 neurons along with Scnn1a and RORβ (Tasic et al. 2016). Combining these markers along with morphological and functional tests will further our understanding of the nature of layer 4 and layer 3 neurons in A1.
Consideration for structure and function studies in rodents
Laminar architecture of the neocortex has traditionally been the basis on which our understanding of the complex brain structure was interpreted. Proper laminar definition and correct assignment of the location of neuronal somata will be therefore important in interpreting the identified functional properties of neurons and their functional roles in neural circuits that they are embedded. In single cell electrophysiological recording techniques such as whole cell patch-clamp recordings, we often identify somatic locations and cell morphology after recordings by staining for biocytin or equivalent molecules incorporated into the recorded cells via internal solutions. In such experiments, the knowledge of laminar proportion in the same age of animals will be needed for the proper assignment of the location of recorded neurons. Even when the brain slices prepared from the transgenic mice that express fluorescent proteins in certain types of neurons are used for the recordings, the determination of laminar proportion at the age used will be necessary, unless the genetic promoter used to encode fluorescent proteins is proven to be (sub)layer specific. In addition, optogenetic techniques to examine neuronal circuit function will also rely on the layer- and cell type-specificity of light-sensitive channel expression in relation to proper laminar definitions. These techniques of the fluorescent visualization and the functional characterization of genetically modified neurons are indeed powerful and will provide us a great deal of information. However, the cell type specificity of gene expression, the extent of the anatomical or functional reporter expression among the transduced cell types, and the developmental changes in gene expression need to be carefully considered along with the proper definition of cytoarchitecture and myeloarchitecture of the mouse brain. We decided to re-examine laminar definition in mouse because of the seemingly unstandardized use of laminar definitions in the literature. Our efforts to combine adequate anatomical determination of cortical layers, neuronal distribution and cell types combined with proper characterization of genetic composition in individual neurons would be important to establish neuronal function in cortical circuits.
Abbreviations
- AAV:
-
Adeno-associated virus
- A1:
-
Primary auditory cortex
- Bcl11b:
-
B-cell lymphoma/leukaemia 11B
- BDA:
-
Biotinylated dextran amine
- ChR2:
-
Channelrhodopsin 2
- Cplx3:
-
Complexin 3
- CTB:
-
Chorela toxin β-subunit conjugated with Alexa Fluor 488
- Ctip2:
-
COUP-TF interacting protein 2
- Cux1:
-
Cut-like homeobox 1
- DARPP-32:
-
Dopamine- and adenosine 3′, 5′-monophosphate-regulated phosphoprotein-32,000
- DMSO:
-
Dimethyl sulfoxide
- Drd1a:
-
Dopamine receptor D1a
- ER81:
-
Ets-related protein 81
- Etv1:
-
ETS-variant 1
- Foxp2:
-
Forkhead box protein P2
- IC:
-
Inferior colliculus
- ICc:
-
Central nucleus of IC
- L6/WM:
-
Layer 6 and the subcortical white matter
- Lpar1:
-
Lysophosphatidic acid receptor 1
- MGd:
-
The dorsal division of medial geniculate nucleus
- MGN:
-
Medial geniculate nucleus
- MGv:
-
The ventral division of medial geniculate nucleus
- nAChRs:
-
Nicotinic acetylcholine receptors
- NeuN:
-
Neuronal nuclei
- Ntsr1:
-
Neurotensin receptor 1
- P:
-
Postnatal day
- Rbp4:
-
Retinol binding protein 4
- ROI:
-
Region of interest
- RORβ:
-
RAR-related orphan receptor beta subunit
- S1:
-
Primary somatosensory cortex
- Scnn1a:
-
Sodium channel epithelial 1 alpha subunit
- Tbr1:
-
T-box brain protein 1
- TMR-Dex:
-
Tetramethylrhodamine dextran
- V1:
-
Primary visual cortex
- vGluT2:
-
Vesicular glutamate transporter 2
References
Anderson LA, Christianson GB, Linden JF (2009) Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res 1252:130–142. https://doi.org/10.1016/j.brainres.2008.11.037
Arlotta P, Molyneaux BJ, Chen J et al (2005) Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221. https://doi.org/10.1016/j.neuron.2004.12.036
Barbour DL, Callaway EM (2008) Excitatory local connections of superficial neurons in rat auditory cortex. J Neurosci 28:11174–11185. https://doi.org/10.1523/JNEUROSCI.2093-08.2008
Bedogni F, Hodge RD, Elsen GE et al (2010) Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA 107:13129–13134. https://doi.org/10.1073/pnas.1002285107
Belgard TG, Marques AC, Oliver PL et al (2011) A transcriptomic atlas of mouse neocortical layers. Neuron 71:605–616. https://doi.org/10.1016/J.NEURON.2011.06.039
Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3:517–530. https://doi.org/10.1038/nrn874
Beyerl BD (1978) Afferent projections to the central nucleus of the inferior colliculus in the rat. Brain Res 145:209–223. https://doi.org/10.1016/0006-8993(78)90858-2
Bourassa J, Pinault D, Deschênes M (1995) Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci 7:19–30. https://doi.org/10.1111/j.1460-9568.1995.tb01016.x
Chang M, Suzuki N, Kawai HD (2018) Laminar specific gene expression reveals differences in postnatal laminar maturation in mouse auditory, visual, and somatosensory cortex. J Comp Neurol. https://doi.org/10.1002/cne.24481
Chevée M, Robertson JDJ, Cannon GH et al (2018) Variation in activity state, axonal projection, and position define the transcriptional identity of individual neocortical projection neurons. Cell Rep 22:441–455. https://doi.org/10.1016/J.CELREP.2017.12.046
Chmielowska J, Carvell GE, Simons DJ (1989) Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285:325–338. https://doi.org/10.1002/cne.902850304
Code RA, Winer JA (1986) Columnar organization and reciprocity of commissural connections in cat primary auditory cortex (AI). Hear Res 23:205–222. https://doi.org/10.1016/0378-5955(86)90110-3
Cruikshank SJ, Rose HJ, Metherate R (2002) Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol 87:361–384. https://doi.org/10.1152/jn.00549.2001
de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA (2006) Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol 496:72–96. https://doi.org/10.1002/cne.20924
Deriziotis P, O’Roak BJ, Graham SA et al (2014) De novo TBR1 mutations in sporadic autism disrupt protein functions. Nat Commun 5:4954. https://doi.org/10.1038/ncomms5954
Doucet JR, Molavi DL, Ryugo DK (2003) The source of corticocollicular and corticobulbar projections in area Te1 of the rat. Exp Brain Res 153:461–466. https://doi.org/10.1007/s00221-003-1604-4
Ferland RJ, Cherry TJ, Preware PO et al (2003) Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol 460:266–279. https://doi.org/10.1002/cne.10654
Ferrere A, Vitalis T, Gingras H et al (2006) Expression of Cux-1 and Cux-2 in the developing somatosensory cortex of normal and barrel-defective mice. Anat Rec Part A Discov Mol Cell Evol Biol 288:158–165. https://doi.org/10.1002/ar.a.20284
Fitzpatrick DC, Henson OW (1994) Cell types in the mustached bat auditory cortex. Brain Behav Evol 43:79–91. https://doi.org/10.1159/000113626
Games KD, Winer JA (1988) Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res 34:1–25. https://doi.org/10.1016/0378-5955(88)90047-0
Gong S, Doughty M, Harbaugh CR et al (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27:9817–9823. https://doi.org/10.1523/JNEUROSCI.2707-07.2007
Grant E, Hoerder-Suabedissen A, Molnár Z (2016) The regulation of corticofugal fiber targeting by retinal inputs. Cereb Cortex 26:1336–1348. https://doi.org/10.1093/cercor/bhv315
Graziano A, Liu XB, Murray KD, Jones EG (2008) Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol 507:1258–1276. https://doi.org/10.1002/cne.21592
Guy J, Wagener RJ, Möck M, Staiger JF (2015) Persistence of functional sensory maps in the absence of cortical layers in the somsatosensory cortex of Reeler mice. Cereb Cortex 25:2517–2528. https://doi.org/10.1093/cercor/bhu052
Hackett TA, Preuss TM, Kaas JH (2001) Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol 441:197–222. https://doi.org/10.1002/cne.1407
Hackett TA, Takahata T, Balaram P (2011) VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hear Res 274:129–141. https://doi.org/10.1016/j.heares.2010.11.001
Hackett TA, Clause AR, Takahata T et al (2016) Differential maturation of vesicular glutamate and GABA transporter expression in the mouse auditory forebrain during the first weeks of hearing. Brain Struct Funct 221:2619–2673. https://doi.org/10.1007/s00429-015-1062-3
Harb K, Magrinelli E, Nicolas CS et al (2016) Area-specific development of distinct projection neuron subclasses is regulated by postnatal epigenetic modifications. Elife 5:e09531. https://doi.org/10.7554/eLife.09531
Herkenham M (1980) Laminar organization of thalamic projections to the rat neocortex. Science 207:532–535. https://doi.org/10.1126/science.7352263
Heumann D, Leuba G, Rabinowicz T (1977) Postnatal development of the mouse cerebral neocortex. II. Quantitative cytoarchitectonics of visual and auditory areas. J Hirnforsch 18:483–500
Hirokawa J, Watakabe A, Ohsawa S, Yamamori T (2008) Analysis of area-specific expression patterns of RORbeta, ER81 and Nurr1 mRNAs in rat neocortex by double In Situ hybridization and cortical box method. PLoS One 3:e3266. https://doi.org/10.1371/journal.pone.0003266
Hisaoka T, Nakamura Y, Senba E, Morikawa Y (2010) The forkhead transcription factors, Foxp1 and Foxp2, identify different subpopulations of projection neurons in the mouse cerebral cortex. Neuroscience 166:551–563. https://doi.org/10.1016/j.neuroscience.2009.12.055
Hoerder-Suabedissen A, Molnár Z (2013) Molecular diversity of early-born subplate neurons. Cereb Cortex 23:1473–1483. https://doi.org/10.1093/cercor/bhs137
Hoerder-Suabedissen A, Molnár Z (2015) Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci 16:133–146. https://doi.org/10.1038/nrn3915
Hoerder-Suabedissen A, Wang WZ, Lee S et al (2009) Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex 19:1738–1750. https://doi.org/10.1093/cercor/bhn195
Hoerder-Suabedissen A, Hayashi S, Upton L et al (2018) Subset of cortical layer 6b neurons selectively innervates higher order thalamic nuclei in mice. Cereb Cortex 28:1882–1897. https://doi.org/10.1093/cercor/bhy036
Holmberg J, Perlmann T (2012) Maintaining differentiated cellular identity. Nat Rev Genet 13:429–439. https://doi.org/10.1038/nrg3209
Huang CL, Winer JA (2000) Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol 427:302–331. https://doi.org/10.1002/1096-9861(20001113)427:2%3C302::AID-CNE10%3E3.0.CO;2-J
Imig TJ, Morel A, Kauer CD (1982) Covariation of distributions of callosal cell bodies and callosal axon terminals in layer III of cat primary auditory cortex. Brain Res 251:157–159. https://doi.org/10.1016/0006-8993(82)91283-5
Jacobson S, Trojanowski JQ (1974) The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res 74:149–155. https://doi.org/10.1016/0006-8993(74)90118-8
Ji X-Y, Zingg B, Mesik L et al (2016) Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb Cortex 26:2612–2625. https://doi.org/10.1093/cercor/bhv099
Jones EG, Dell’Anna ME, Molinari M et al (1995) Subdivisions of macaque monkey auditory cortex revealed by calcium-binding protein immunoreactivity. J Comp Neurol 362:153–170. https://doi.org/10.1002/cne.903620202
Kaneko T, Fujiyama F (2002) Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res 42:243–250. https://doi.org/10.1016/S0168-0102(02)00009-3
Kaneko T, Fujiyama F, Hioki H (2002) Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444:39–62. https://doi.org/10.1002/cne.10129
Kanold PO, Luhmann HJ (2010) The subplate and early cortical circuits. Annu Rev Neurosci 33:23–48. https://doi.org/10.1146/annurev-neuro-060909-153244
Kawai H, Lazar R, Metherate R (2007) Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci 10:1168–1175. https://doi.org/10.1038/nn1956
Kelly JP, Wong D (1981) Laminar connections of the cat’s auditory cortex. Brain Res 212:1–15. https://doi.org/10.1016/0006-8993(81)90027-5
Kimura A, Donishi T, Sakoda T et al (2003) Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience 117:1003–1016. https://doi.org/10.1016/S0306-4522(02)00949-1
Koralek K-A, Jensen KF, Killackey HP (1988) Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res 463:346–351. https://doi.org/10.1016/0006-8993(88)90408-8
Kwan KY, Sestan N, Anton ES (2012) Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 139:1535–1546. https://doi.org/10.1242/dev.069963
Li L, Ji X, Liang F et al (2014) A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci 34:13670–13683. https://doi.org/10.1523/JNEUROSCI.1516-14.2014
Llano DA, Sherman SM (2008) Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. J Comp Neurol 507:1209–1227. https://doi.org/10.1002/cne.21602
Lu SM, Lin RC-S (1993) Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10:1–16. https://doi.org/10.3109/08990229309028819
Madisen L, Zwingman TA, Sunkin SM et al (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140. https://doi.org/10.1038/nn.2467
McKenna WL, Betancourt J, Larkin KA et al (2011) Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci 31:549–564. https://doi.org/10.1523/JNEUROSCI.4131-10.2011
Meyer G, González-Hernández TH, Ferres-Torres R (1989) The spiny stellate neurons in layer IV of the human auditory cortex. A Golgi study. Neuroscience 33:489–498. https://doi.org/10.1016/0306-4522(89)90401-6
Mitani A, Itoh K, Nomura S et al (1984) Thalamocortical projections to layer I of the primary auditory cortex in the cat: a horseradish peroxidase study. Brain Res 310:347–350. https://doi.org/10.1016/0006-8993(84)90157-4
Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD (2007) Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8:427–437. https://doi.org/10.1038/nrn2151
Morel A, Kaas JH (1992) Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol 318:27–63. https://doi.org/10.1002/cne.903180104
Morel A, Garraghty PE, Kaas JH (1993) Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol 335:437–459. https://doi.org/10.1002/cne.903350312
Nakamura K, Hioki H, Fujiyama F, Kaneko T (2005) Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol 492:263–288. https://doi.org/10.1002/cne.20705
Nieto M, Monuki ES, Tang H et al (2004) Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol 479:168–180. https://doi.org/10.1002/cne.20322
Ojima H (1994) Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex 4:646–663. https://doi.org/10.1093/cercor/4.6.646
Olsen SR, Bortone DS, Adesnik H, Scanziani M (2012) Gain control by layer six in cortical circuits of vision. Nature 483:47–52. https://doi.org/10.1038/nature10835
Ouimet C, Miller P, Hemmings HC et al (1984) DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci 4:111–124
Petrof I, Viaene AN, Sherman SM (2012) Two populations of corticothalamic and interareal corticocortical cells in the subgranular layers of the mouse primary sensory cortices. J Comp Neurol 520:1678–1686. https://doi.org/10.1002/cne.23006
Piñon MC, Jethwa A, Jacobs E et al (2009) Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol 587:1903–1915. https://doi.org/10.1113/jphysiol.2008.167767
Quik M, Perez XA, Grady SR (2011) Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol 82:873–882. https://doi.org/10.1016/j.bcp.2011.06.001
Rajput PS, Kharmate G, Somvanshi RK, Kumar U (2009) Colocalization of dopamine receptor subtypes with dopamine and cAMP-regulated phosphoprotein (DARPP-32) in rat brain. Neurosci Res 65:53–63. https://doi.org/10.1016/j.neures.2009.05.005
Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS (2009) Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci 29:6406–6417. https://doi.org/10.1523/JNEUROSCI.0258-09.2009
Robertson RT, Mostamand F, Kageyama GH et al (1991) Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Brain Res Dev Brain Res 58:81–95. https://doi.org/10.1016/0165-3806(91)90240-J
Rock C, Apicella AJ (2015) Callosal projections drive neuronal-specific responses in the mouse auditory cortex. J Neurosci 35:6703–6713. https://doi.org/10.1523/JNEUROSCI.5049-14.2015
Romanski LM, LeDoux JE (1993) Organization of rodent auditory cortex: anterograde transport of pha-l from mgv to temporal neocortex. Cereb Cortex 3:499–514. https://doi.org/10.1093/cercor/3.6.499
Rose HJ, Metherate R (2005) Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol 94:2019–2030. https://doi.org/10.1152/jn.00860.2004
Rüttgers K, Aschoff A, Friauf E (1990) Commissural connections between the auditory cortices of the rat. Brain Res 509:71–79. https://doi.org/10.1016/0006-8993(90)90310-8
Saldaña E, Feliciano M, Mugnaini E (1996) Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol 371(19960715):15–40. https://doi.org/10.1002/(SICI)1096-9861(19960715)371:1%3C15::AID-CNE2%3E3.0.CO;2-O
Schofield BR (2009) Projections to the inferior colliculus from layer VI cells of auditory cortex. Neuroscience 159:246–258. https://doi.org/10.1016/j.neuroscience.2008.11.013
Sigl-Glöckner J, Brecht M (2017) Polyploidy and the cellular and areal diversity of rat cortical layer 5 pyramidal neurons. Cell Rep 20:2575–2583. https://doi.org/10.1016/j.celrep.2017.08.069
Slater BJ, Willis AM, Llano DA (2013) Evidence for layer-specific differences in auditory corticocollicular neurons. Neuroscience 229:144–154. https://doi.org/10.1016/j.neuroscience.2012.10.053
Smith PH, Populin LC (2001) Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol 436:508–519. https://doi.org/10.1002/cne.1084
Smith PH, Uhlrich DJ, Manning KA, Banks MI (2012) Thalamocortical projections to rat auditory cortex from the ventral and dorsal divisions of the medial geniculate nucleus. J Comp Neurol 520:34–51. https://doi.org/10.1002/cne.22682
Sohur US, Padmanabhan HK, Kotchetkov IS et al (2014) Anatomic and molecular development of corticostriatal projection neurons in mice. Cereb Cortex 24:293–303. https://doi.org/10.1093/cercor/bhs342
Sorensen SA, Bernard A, Menon V et al (2015) Correlated gene expression and target specificity demonstrate excitatory projection neuron diversity. Cereb Cortex 25:433–449. https://doi.org/10.1093/cercor/bht243
Sun YJ, Kim Y-J, Ibrahim LA et al (2013) Synaptic mechanisms underlying functional dichotomy between intrinsic-bursting and regular-spiking neurons in auditory cortical layer 5. J Neurosci 33:5326–5339. https://doi.org/10.1523/JNEUROSCI.4810-12.2013
Sundberg SC, Lindström SH, Sanchez GM, Granseth B (2018) Cre-expressing neurons in visual cortex of Ntsr1-Cre GN220 mice are corticothalamic and are depolarized by acetylcholine. J Comp Neurol 526:120–132. https://doi.org/10.1002/cne.24323
Takeuchi A, Hamasaki T, Litwack ED, O’Leary DDM (2007) Novel IgCAM, MDGA1, expressed in unique cortical area- and layer-specific patterns and transiently by distinct forebrain populations of cajal-retzius neurons. Cereb Cortex 17:1531–1541. https://doi.org/10.1093/cercor/bhl064
Tantirigama MLS, Oswald MJ, Duynstee C et al (2014) Expression of the developmental transcription factor Fezf2 identifies a distinct subpopulation of layer 5 intratelencephalic-projection neurons in mature mouse motor cortex. J Neurosci 34:4303–4308. https://doi.org/10.1523/JNEUROSCI.3111-13.2014
Tasic B, Menon V, Nguyen TN et al (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19:335–346. https://doi.org/10.1038/nn.4216
Ultanir SK, Kim J, Hall BJ et al (2007) Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA 104:19553–19558. https://doi.org/10.1073/pnas.0704031104
Viaene AN, Petrof I, Sherman SM (2011) Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci 31:12738–12747. https://doi.org/10.1523/JNEUROSCI.1565-11.2011
Viswanathan S, Sheikh A, Looger LL, Kanold PO (2017) Molecularly defined subplate neurons project both to thalamocortical recipient layers and thalamus. Cereb Cortex 27:4759–4768. https://doi.org/10.1093/cercor/bhw271
Wagener RJ, Witte M, Guy J et al (2016) Thalamocortical connections drive intracortical activation of functional columns in the mislaminated Reeler somatosensory cortex. Cereb Cortex 26:820–837. https://doi.org/10.1093/cercor/bhv257
Wang WW, Cao R, Rao ZR, Chen LW (2004) Differential expression of NMDA and AMPA receptor subunits in DARPP-32-containing neurons of the cerebral cortex, hippocampus and neostriatum of rats. Brain Res 998:174–183. https://doi.org/10.1016/j.brainres.2003.11.034
Watakabe A, Takaji M, Kato S et al (2014) Simultaneous visualization of extrinsic and intrinsic axon collaterals in Golgi-like detail for mouse corticothalamic and corticocortical cells: a double viral infection method. Front Neural Circuits 8:110. https://doi.org/10.3389/fncir.2014.00110
Xing L, Larsen RS, Bjorklund GR et al (2016) Layer specific and general requirements for ERK/MAPK signaling in the developing neocortex. Elife 5:1–29. https://doi.org/10.7554/eLife.11123
Xiong XR, Liang F, Zingg B et al (2015) Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat Commun 6:7224. https://doi.org/10.1038/ncomms8224
Zhang ZW, Deschênes M (1997) Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci 17:6365–6379. https://doi.org/10.1523/JNEUROSCI.17-16-06365.1997
Zhou Y, Liu B, Wu GK et al (2010) Preceding inhibition silences layer 6 neurons in auditory cortex. Neuron 65:706–717. https://doi.org/10.1016/j.neuron.2010.02.021
Acknowledgements
This work was supported by JSPS Grants-in-Aid for Scientific Research (C) 26430025 (H.D.K), Otsuka Toshimi Scholarship Foundation (M.C.), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Scholarship (M.C.).
Author information
Authors and Affiliations
Contributions
MC and HDK designed the research; MC performed the research; MC and HDK wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Chang, M., Kawai, H.D. A characterization of laminar architecture in mouse primary auditory cortex. Brain Struct Funct 223, 4187–4209 (2018). https://doi.org/10.1007/s00429-018-1744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1744-8