Abstract
Cortical area Te1 in the rat commonly is associated with primary auditory cortex. It is the source of direct projections to the inferior colliculus (IC), superior olivary complex (SOC), and the cochlear nucleus (CN). A question that arises is whether these descending pathways derive from a common source or separate populations of cortical neurons. We addressed this question in seven rats by injecting either Diamidino yellow (DiY) or Fast blue (FB) into the IC and injecting the other tracer into the CN (n=4) or SOC (n=3). All injections were made on the left side of the brain. In a sample of sections through area Te1 in both hemispheres, we counted single- and double-labeled cells. We estimate that IC-projecting cells outnumber those projecting to the CN or SOC by at least a factor of ten. The source of corticofugal pathways to the left IC was heavily biased towards the same side of the brain (ipsi/contra ratio 8±2.5), whereas it was more equally distributed between the two hemispheres for the left CN and SOC (ipsi/contra ratios ranged from 0.7–2.3). Finally, we observed that only 10–20% of those cells filled with a tracer injection in the CN or SOC also contained the tracer injected into the IC. In a previous study, we observed a similarly small percentage of double labeled cells when FB and DiY were injected into the CN and SOC, respectively. Combined with the distinct laminar distribution of IC-, SOC-, and CN-projecting neurons within layer V, the results suggest that these three pathways largely derive from different populations of cortical neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Auditory cortex is regarded as the terminus of the ascending auditory pathway, but it also represents the origin of a large descending system of projections. The axons of cortical neurons target cells in the inferior colliculus (IC; Anderson et al. 1980; Faye-Lund 1985; Herbert et al. 1991) and IC neurons project to the nuclei of the lateral lemniscus, the superior olivary complex (SOC), and the cochlear nucleus (CN; Caicedo and Herbert 1993; Saldaña 1993; Malmierca et al. 1996). Historically, the IC was considered an obligatory synapse in the ascending and descending pathways (Ramón y Cajal 1909; Geniec and Morest 1971) but recent application of sensitive tracers have shown that some cortical cells bypass the IC and directly innervate auditory brain stem nuclei (Feliciano et al. 1995; Weedman and Ryugo 1996a, 1996b). These monosynaptic projections suggest that auditory cortex exerts powerful descending influences at the earliest stages of sound processing.
The nature of these descending influences is mysterious at this point. In the bat, corticofugal pathways have been shown to influence plastic changes in the functional organization of the IC. For example, the frequency map in the IC can be modified by repeatedly presenting a pure tone or pairing a tone with an electrical shock to the leg (Gao and Suga 1998; Yan and Suga 1998). The changes in the frequency map required corticofugal input. We wondered whether the cortical neurons responsible for such plastic changes in the IC also project their axons to the SOC and CN. This result might suggest a similar functional role for corticofugal pathways in these lower brain stem nuclei.
We addressed this issue in rats by using a double-labeling protocol. In several animals, the retrograde tracer Diamidino yellow (DiY) or Fast blue (FB) was injected into the IC and paired with an injection of the other tracer into the CN or the SOC. We then counted the number of single- and double-labeled cells within area Te1 of auditory cortex. In each experiment, only 10–20% of those neurons filled by the tracer injection in the SOC or CN were double labeled. Furthermore, we confirmed our prior suggestion that cortical neurons projecting to the IC, CN, and SOC have distinctly different distributions within layer V. Collectively, these results suggest that cortical axons terminating in the CN and SOC are not simply collaterals of those fibers innervating the IC. Rather, it appears that cortical neurons projecting to each structure are largely independent.
Methods
Tracer injections and tissue processing
Seven Sprague-Dawley rats were the experimental animals. The rats were male and weighed between 250–400 g. All animals and procedures used were in accordance with NIH guidelines and with the approval of the Johns Hopkins Medical School Animal Care and Use Committee.
We employed the retrograde tracers Fast blue (FB; Sigma, St. Louis, MO, USA; 1.5–3% aqueous solution) and Diamidino yellow (DiY; Sigma; 1.5–3% aqueous solution) in a series of double-labeling experiments. DiY was injected into the IC in six rats and paired with a FB injection into the CN (n=4) or SOC (n=2). In one rat, FB was injected into the IC and DiY into the SOC. All injections were made on the same side of the brain. Our procedures for anesthetizing the animal, surgically exposing the CN, and injecting tracer into the CN or the SOC have been described previously (Weedman and Ryugo 1996b; Doucet et al. 2002). We used two procedures to inject tracer into the IC. For two animals, stereotaxic coordinates (Paxinos and Watson 1998) were used to make a small hole in the skull dorsal to the IC. We then advanced a 10-µl Hamilton syringe filled with DiY into the opening, through occipital cortex, and into the IC. We injected approximately 300 nl DiY solution along each of two dorsal/ventral tracks separated by 0.5 mm along the medial/lateral axis (e.g., Fig. 1). For the remaining animals, we removed a portion of the skull and aspirated those regions of temporal and occipital cortex overlying the lateral surface of the IC. A Drummond oocyte injector and glass pipette (40-µm inner tip diameter) were advanced into the IC at three or four dorsal/ventral locations and the tracer was injected at various depths. We injected 300 nl of the tracer solution. A clear difference between the two groups of rats was the large number of DiY-labeled neurons observed in nonauditory regions of cortex after using the first procedure. These labeled cells most likely were filled because of the DiY left along the path of the syringe. However, there was no obvious difference between the cases in terms of the laminar distribution of labeled cells or the number of double-labeled cells within area Te1. Therefore, results from the two groups of rats were pooled.
After allowing the animals to survive between 8 and 11 days, they were given a lethal dose of sodium pentobarbital (100 mg/kg). When the animal was areflexic to a paw pinch, it was perfused with 25–50 ml 1% sodium nitrite in 0.1 M phosphate-buffered saline (pH 7.4), followed by 10% formalin in 0.1 M phosphate buffer (PB, pH 7.4). The brains were postfixed for 1 h in the same fixative and then cryoprotected overnight with a solution of 30% sucrose (in PB). The next day, the brain was sectioned in the coronal plane (75-µm thick sections) using a freezing microtome. Half the sections were mounted on subbed slides, allowed to air dry for 1 h, and then coverslipped with Krystalon (Harleco, EM Science, Gibbstown, NJ, USA). The remaining sections were mounted on subbed slides, allowed to dry overnight, stained using Cresyl violet, and coverslipped with Permount.
Data analysis
The distance of each coronal section from bregma along the rostral/caudal axis was defined in the following way. A digitized atlas (Swanson 1998) provided drawings of coronal sections through the rat brain. For each case, we chose the coronal section that best matched the drawing at 5.65 mm caudal to bregma (level 38). Knowledge of the distance between each section was then used to order the remaining sections.
Retrogradely labeled cells were viewed with a fluorescent microscope (Zeiss Axioscope) and a high magnification objective (×20 or ×40) in order to classify them as single or double labeled. This distinction can be made fairly easily because (1) FB and DiY accumulate in different regions of the cell—FB in the cytoplasm and dendrites, DiY in the nucleus—and (2) they fluoresce with different colors when irradiated with the same wavelength of light (Keizer et al. 1983). For the CN/IC experiments, the number of cells filled with FB and those with FB/DiY were tallied for all cortical sections between 4 and 7 mm posterior to bregma. The totals for each group were multiplied by two in order to estimate the number of cortical neurons that project to the CN and those that project to both the CN and IC. The same procedure was used to estimate the total number of SOC- and IC/SOC-projecting cortical neurons. Our analysis was confined to sections between 4 and 7 mm posterior to bregma because, for most cases, more caudal sections were damaged due to our method of exposing and injecting the IC (see above). As for the anterior border, we noticed in a prior study (and this study) that more rostral regions of cortex contained many labeled cells after injecting FB or DiY into the SOC (Doucet et al. 2002). However, also in this prior study, we demonstrated that most of these cells were labeled due to tracer unintentionally deposited in the brainstem dorsal to the SOC. Since we used the same method for injecting the SOC in these present experiments, we did not count labeled cells closer than 4 mm to bregma. The region of temporal cortex between 4 and 7 mm posterior to bregma includes nearly all of area Te1 (Doucet et al. 2002; Doron et al. 2002). However, our estimates of the total number of cells projecting to each structure should be considered lower bounds.
The laminar distribution of DiY-, FB-, and double-labeled cells was documented for five animals. In each case, five or six equally-spaced sections through cortex were chosen between 4 and 7 mm posterior to bregma. For each section, several pictures were taken (×10 objective) that collectively circumscribed the cortical regions that contained labeled neurons. Photographs were obtained and stored using a cooled, 3-chip RGB digital CCD camera (C5810 Hamamatsu) interfaced with a Macintosh G3 computer. Image processing software (Adobe Photoshop v5.0) then was used to build montages, to draw the boundaries of gray and white matter, and to plot the position of each filled cell. For three of these five cases, we counted DiY-labeled (IC-projecting) neurons within area Te1. In order to define the dorsal and ventral borders of area Te1, we used the distribution of cortical cells that project to the CN as we explained and justified in a prior study (Doucet et al. 2002). For each animal, the number of DiY-labeled cells recorded within each section was plotted versus its distance from bregma. The area under this curve was used to estimate the total number of cortical cells in area Te1 that project their axons to the IC. These estimates should be considered lower bounds (see above). Means and standard deviations are provided when appropriate.
Results
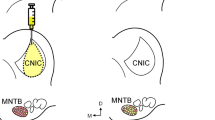
The data from one experiment are summarized in Fig. 1. The regions of the CN, SOC, and IC innervated by cortical axons have been described in prior studies (e.g., Feliciano et al. 1995; Saldaña et al. 1996). The tracer injections were centered in these regions in an attempt to label as many cortical neurons as possible. Corticofugal pathways ending in the CN form synapses primarily in the granule cell domain (Feliciano et al. 1995; Weedman and Ryugo 1996a). Accordingly, the injection sites in the CN were centered in the granule cell lamina that separates the ventral and dorsal divisions (Fig. 1). Injection sites in the SOC were located between the lateral borders of the lateral superior olive (LSO) and the medial nucleus of the trapezoid body (MNTB; data not shown). In all cases, the tracer was deposited in the dorsal half of the SOC, i.e., dorsal to the ventral nucleus of the trapezoid body (VNTB). Along the rostral/caudal axis, the injection sites spanned 200–300 µm and were centered near the midpoint of the LSO. In each experiment, the IC injection was deposited in each of the three main divisions: the external cortex (EC), the dorsal cortex (DC), and the central nucleus (see Fig. 1). The injection site typically spread 300 µm along the rostral/caudal axis. The center of the injections varied across experiments such that, collectively, they covered the rostral/caudal extent of the IC.
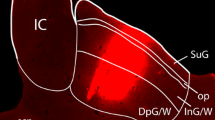
Retrograde labeling in area Te1 for a rat that received an injection of Fast blue (FB) into the cochlear nucleus (CN) and Diamidino yellow (DiY) into the inferior colliculus (IC). The top of the figure displays a photomicrograph and drawing of a single coronal section through each injection site (CeN central nucleus, DCN dorsal cochlear nucleus, DC dorsal cortex, EC external cortex, GCL granule cell lamina, ICP inferior cerebellar peduncle, V spinal track of the trigeminal nerve). A Display of the location of labeled cortical cells shown in panels B–E. In the sagittal view of the brain, the gray line through auditory cortex (temporal areas Te1, Te2, and Te3) indicates the approximate position of the cells along the rostral/caudal axis. The gray rectangle in the coronal view of the section ipsilateral to the injection sites indicates the region shown in the subsequent panels. B Photomontage of layer V. The cortical surface is to the left. Notice that the few FB-labeled cells are located in deep layer V whereas the DiY-labeled neurons are distributed more broadly. Scale bar 100 µm. C Higher magnification photomontage illustrating the laminar organization of cortical cells projecting to the CN (blue) vs those targeting the IC (yellow) within layer V. For this panel, the cortical surface is towards the top of the figure. Scale bar 50 µm. D,E Examples of labeled cortical cells. In these experiments, most labeled cells contained either FB or DiY (blue or yellow arrows). Cortical cells that contained both dyes (blue and yellow arrow in D) were observed much less frequently
The pattern of retrograde labeling in temporal cortex was consistent with prior studies that injected a single tracer into the CN (Weedman and Ryugo 1996b), SOC (Mulders and Robertson 2000; Doucet et al. 2002), or IC (Games and Winer 1988). Cortical neurons labeled with an injection in the IC were distributed over a much larger area than those labeled by injections in the CN or SOC. This result confirms prior observations that both primary and secondary areas of the auditory cortex are robust sources of input to the IC, whereas corticofugal pathways to the CN and SOC predominantly derive from primary regions. Retrogradely labeled neurons were confined to layer V, except for a thin layer of IC-projecting cells in layer VI (see also Games and Winer 1988). Occasionally, one or two CN-projecting cells in an experiment also appeared to be located in upper layer VI. IC-projecting neurons had the broadest distribution within layer V, being scattered in both the middle and deep regions. In contrast, CN-projecting neurons had a narrow laminar distribution and abutted the border of layer V and layer VI. These differences are observed quite clearly in Fig. 1B,C. The distribution of SOC-projecting neurons is slightly broader and centered in more superficial regions of layer V when compared to CN-projecting cells (Doucet et al. 2002). However, SOC-projecting cells are located in deeper regions of layer V than most IC-projecting neurons (data not shown). To summarize, within layer V of area Te1, the set of neurons projecting to the CN, SOC, and IC each had a distinct laminar distribution.
IC-projecting neurons far outnumbered those targeting the CN or the SOC. Our estimate of the average number of cortical cells (both hemispheres) in area Te1 that project to one IC is 14,502±3,065. Most labeled neurons were located in the cortical hemisphere ipsilateral to the injected IC. The average ratio of ipsilateral to contralateral neurons equaled 8±2.5. For CN and SOC injections, the average number of labeled cortical cells was 952±253 and 1161±368, respectively. Neurons that project to these two lower brainstem structures were more equally distributed between the two hemispheres—ipsi/contra ratios ranged from 0.7 to 2.3. These results for CN and SOC injections are within the range of those reported previously (Weedman and Ryugo 1996b; Doucet et al. 2002). In the double-labeling experiments described here, most cells were filled with only one tracer (Fig. 1D,E). Across all seven cases, the percentage of CN- or SOC-projecting neurons that also contained the tracer injected into the IC (Fig. 1D) varied between 10–20%. We did not observe any systematic difference between the laminar position, rostral/caudal location, or cortical hemisphere of single- versus double-labeled cells.
Discussion
The main finding of this study is that most cortical neurons projecting to the IC, SOC, or CN appear to target only one of these structures. The source of all three pathways are pyramidal neurons within layer V, except for corticofugal pathways ending in the IC, which also derive from neurons in layer VI. Layer V cells also project to the contralateral auditory cortex (Games and Winer 1988), striatum (Moriizumi and Hattori 1991), and pontine nuclei (Wiesendanger and Wiesendanger 1982). A previous double-labeling study found little overlap between the set of pyramidal neurons projecting to the IC and to the striatum (Moriizumi and Hattori 1991). In a prior study, we concluded that few pyramidal cells project to both the CN and SOC (Doucet et al. 2002). A criticism that can be levied against all of these studies is that the tracer injections did not completely fill each structure and therefore larger injections would produce more double-labeled cells. This criticism is valid, particularly for a large structure such as the IC, and our quantitative estimates of collateralization should be considered lower bounds. However, we did not observe any obvious correlation between the total number of labeled cortical neurons and those that were double labeled. Furthermore, in every experiment, tracer was deposited in each region of the IC known to receive cortical input, and the location of the injection sites varied across experiments such that they collectively covered the IC. These results make it likely that we filled every corticocollicular neuron in at least one experiment. Based on these results, we propose that the corticofugal pathways ending in the IC, SOC, and CN are largely independent. In the cat and the rat, layer V pyramidal neurons in the primary auditory cortex can be partitioned into different classes on the basis of cell size and dendritic morphology (Games and Winer 1988; Winer and Prieto 2001). The picture emerging is that several distinct classes of pyramidal neurons can be distinguished on the basis of their structural target. It will be interesting to determine how dendritic and somatic morphology correlate with axonal morphology.
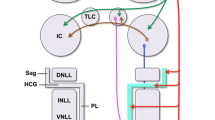
Additional evidence in favor of separate sources of cortical input to the IC, SOC, and CN is their distinct distributions within layer V. Layer V in cats has been partitioned into three sublaminae (Va, Vb, and Vc) based on cytoarchitecture and connectional data (Winer and Prieto 2001). We used a similar scheme to summarize the distribution of pyramidal cells projecting to the auditory midbrain and brain stem in rats (Fig. 2). Superficial regions of layer V (Va) project to the opposite auditory cortex, and a small number of these neurons project to the IC (Games and Winer 1988). Most of the corticocollicular pathway derives from cells distributed in the middle (Vb) and deep (Vc) regions of layer V. Layer Vc is the source of corticobulbar projections to both the CN and SOC, but clear differences in their distributions suggest even finer distinctions with layer V. Since the laminar distribution of corticostriatal and corticopontine pyramidal cells has not been described in detail, it is likely that we are only beginning to elucidate the structural organization of this region of cortex.
Summary of our findings with respect to the laminar organization of cortical cells projecting to the inferior colliculus (IC), superior olivary complex (SOC), and cochlear nucleus (CN). On the left is a photomicrograph displaying a Nissl-stained section through area Te1 of the rat. The surface of cortex is at the top of the figure. On the right is a schematic of layers V and VI that illustrates the distribution of neurons that project to the IC, SOC, and CN. A small number of corticocollicular cells are located near the border of layer VI and white matter (WM), but most are distributed within layer V. All three distributions overlap. However, notice that the cortical neurons projecting to more distant targets are more narrowly distributed and centered in deeper regions of layer V
Some general principles governing the organization of corticofugal pathways to subthalamic auditory structures are beginning to emerge. The set of cortical cells projecting to more distant targets is smaller, more evenly distributed between the two hemispheres, and centered in deeper regions of layer V. Finally, the majority of pyramidal neurons in layer V of area Te1 appear to project to a single subthalamic target.
References
Anderson RA, Snyder RL, Merzenich MM (1980) The topographic organization of corticocollicular projections from physiologically identified loci in the AI, AII, and anterior auditory cortical of the cat. J Comp Neurol 191:479–494
Caicedo A, Herbert H (1993) Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J Comp Neurol 328:377–392
Doron NN, Ledoux JE, Semple MN (2002) Redefining the tonotopic core of rat auditory cortex: physiological evidence for a posterior auditory field. J Comp Neurol 453:345–360
Doucet JR, Rose L, Ryugo DK (2002) The cellular origin of corticofugal projections to the superior olivary complex in the rat. Brain Res 925:28–41
Faye-Lund H (1985) The neocortical projection to the inferior colliculus in the albino rat. Anat Embryol 173:53–70
Feliciano M, Saldaña E, Mugnaini E (1995) Direct projections from the rat primary auditory neocortex to nucleus sagulum, paralemniscal regions, superior olivary complex and cochlear nuclei. Aud Neurosci 1:287–308
Games KD, Winer JA (1988) Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res 34:1–25
Gao E, Suga N (1998) Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA 95:12663–12670
Geniec P, Morest DK (1971) The neuronal architecture of the human posterior colliculus: A study with the Golgi method. Acta Otolaryngol [Suppl] 295:1–33
Herbert H, Aschoff A, Ostwald J (1991) Topography of projections from the auditory cortex to the inferior colliculus in the rat. J Comp Neurol 304:103–122
Keizer K, Kuypers HG, Huisman AM, and Dann O (1983) Diamidino yellow dihydrochloride (DY-2HCl): a new fluorescent retrograde neuronal tracer, which migrates only very slowly out of the cell. Exp Brain Res 51:179–191
Malmierca MS, Lebeau FEN, Rees A (1996) The topographical organization of descending projections from the central nucleus of the inferior colliculus in guinea pig. Hear Res 93:167–180
Moriizumi T, Hattori T (1991) Pyramidal cells in rat temporoauditory cortex project to both striatum and inferior colliculus. Brain Res Bull 27:141–144
Mulders WH, Robertson D (2000) Evidence for direct cortical innervation of medial olivocochlear neurones in rats. Hear Res 144:65–72
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Ramón y Cajal R (1909) Histologie du Système Nerveux de l'Homme et des Vertébrés. Instituto Ramón y Cajal, Madrid
Saldaña E (1993) Descending projections from the inferior colliculus to the cochlear nuclei in mammals. In: Merchan MA, Juiz JM, Godfrey DA, Mugnaini E (eds), The mammalian cochlear nuclei: organization and function. Plenum, New York, pp 153–165
Saldaña E, Feliciano M, Mugnaini E (1996) Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol 371:15–40
Swanson LW (ed) (1998) Brain maps: structure of the rat brain, 2nd edn. Elsevier, Amsterdam
Weedman DL, Ryugo DK (1996a) Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol 371:311–324
Weedman DL, Ryugo DK (1996b) Pyramidal cells in primary auditory cortex project to cochlear nucleus in rat. Brain Res 706:97–102
Wiesendanger R, Wiesendanger M (1982) The corticopontine system in the rat. I. Mapping of corticopontine neurons. J Comp Neurol 208:215–226
Winer JA, Prieto JJ (2001) Layer V in cat primary auditory cortex (AI): Cellular architecture and identification of projection neurons. J Comp Neurol 434:379–412
Yan W, Suga N (1998) Corticofual modulation of the midbrain frequency map in the bat auditory system. Nat Neurosci 1:54–58
Acknowledgements
Support was provided by grants DC04395, DC00232, and DC04505 from US National Institutes of Health/National Institute on Deafness and Other Communication Diseases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doucet, J.R., Molavi, D.L. & Ryugo, D.K. The source of corticocollicular and corticobulbar projections in area Te1 of the rat. Exp Brain Res 153, 461–466 (2003). https://doi.org/10.1007/s00221-003-1604-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1604-4