Abstract
Ovarian clear cell carcinoma (OCCC) is a subtype of ovarian carcinoma characterized by unique biological features and highly malignant characteristics including low chemosensitivity. Therefore, new therapeutic targets are needed. These could include the downstream pathways of receptor tyrosine kinases, especially the human epidermal growth factor receptor 2 (HER2). Our main objective was to characterize the HER2 status using immunohistochemistry (IHC) and FISH on 118 OCCCs, also considering the novel paradigm of HER2-zero and HER2-low status. Other aims included determination of the association between HER2 status and survival, HER2 gene DNA and RNA NGS analysis, HER2 gene expression analysis, and correlation between IHC and gene expression in HER2-zero and HER2-low cases. Cases with HER2 overexpression/amplification accounted for 5.1% (6/118), with additional 3% harbouring HER2 gene mutation. The remaining 112 (94.9%) cases were HER2-negative. Of these, 75% were classified as HER2-zero and 25% as HER2-low. This percentage of HER2 aberrations is significant concerning their possible therapeutic influence. Cases from the HER2-zero group showed significantly better survival. Although this relationship lost statistical significance in multivariate analysis, the results have potential therapeutic significance. HER2 gene expression analysis showed a significant correlation with HER2 IHC status in the entire cohort (HER2-positive vs. HER2-negative), while in the cohort of only HER2-negative cases, the results did not reach statistical significance, suggesting that gene expression analysis would not be suitable to confirm the subdivision into HER2-low and HER2-zero. Our results also emphasize the need for standardized HER2 testing in OCCC to determine the best predictor of clinical response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary ovarian carcinoma continues to be the most lethal malignancy of the female genital tract. Ovarian clear cell carcinoma (OCCC) is a histological subtype of ovarian carcinoma characterized by unique biological features and highly malignant characteristics, the development of which has been closely linked to endometriosis [10]. While in Eastern Asian countries the incidence of OCCC is relatively high (reaching up to 30%), among the European and North American population it represents a rarer malignancy [15, 26, 53]. In comparison to high grade serous carcinoma, patients with OCCC have a lower stage-for-stage survival [46, 54]. Contributing to the adverse outcome is the very low sensitivity of OCCC to standard chemotherapeutic regimes based on platinum derivates and taxanes [46, 47] which, however, even now represent the mainstay of OCCC treatment. Therefore, new therapeutic targets are needed, but until recently, the research into targeted therapy has been limited by the rarity of the tumour.
The main possible molecular targets for OCCC include the downstream pathways of receptor tyrosine kinases, especially the human epidermal growth factor receptor 2 (HER2) [2]. HER2 is encoded by the ERBB2 oncogene (Erb-B2 Receptor Tyrosine Kinase 2; OMIM#164870) located at the long arm of chromosome 17q12. The evaluation of HER2 immunohistochemical status for predictive purposes has become the standard of care for early and advanced breast cancer, bringing about a significant decrease in recurrence and mortality [8, 19, 40, 41, 44, 45]. While in the past, HER2-positive breast cancer was associated with a worse prognosis; the emergence of targeted anti-HER2 therapy (such as trastuzumab and pertuzumab) has turned this adverse factor into an advantage, leading to a remarkable improvement in the prognosis of these patients. In the past decade, the use of HER2 targeted therapy has also become a major therapeutic tool for advanced stage gastroesophageal cancer, where the addition of trastuzumab to systemic chemotherapy leads to prolonged survival [3, 4, 21, 27, 28]. Recently, it has also been confirmed that trastuzumab provides both longer progression-free and overall survival in patients with advanced and recurrent HER2-positive uterine serous carcinoma [11, 12].
Recently, the introduction of the new antibody–drug conjugate trastuzumab deruxtecan (T-DXd) showed that not only HER2-positive patients but also HER2-low expressing patients were responsive to this therapy [1, 36,37,38]. This discovery has sparked new interest in exploring the proportion of patients who can be classified as HER2-low (IHC score 1 + , or 2 + without amplification on FISH), who could also benefit from targeted therapies [9, 35].
For OCCC, the need for novel therapeutic approaches is undeniable. The evaluation of HER2 status has so far been reported in a handful of studies, which are mostly performed on limited cohorts (often with less than 20 included patients) [13, 16, 24, 29, 33, 42, 50, 52]. The reported rate of HER2 overexpression covers a wide range from 0 to 45.6% (with the largest study comprising 95 cases), which clearly indicates that the current data is not entirely reliable [33].
The main objectives of this study were to (i) characterize the HER2 status using immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) on a large, well-defined cohort of 118 OCCCs, taking into consideration the novel paradigm of HER2-zero and HER2-low status; (ii) analyse the relationship between HER2 status and selected clinico-pathological parameters; (iii) determine the association between HER2 status and patient survival; (iv) perform DNA and RNA NGS analysis of the HER2 gene; (v) perform HER2 gene expression analysis and examine the correlation between IHC and gene expression in HER2-zero and HER2-low cases.
Materials and methods
Samples
The study was performed on formalin-fixed, paraffin-embedded (FFPE) tissue blocks, which were sourced from the archives of the participating co-authors from departments from the Czech Republic and Hungary. The selection criteria were set to include all patients diagnosed with a clear cell carcinoma of the ovary available in the participating department’s archive. All cases were reviewed by two experienced pathologists (PD, MKB) and only those cases fulfilling the diagnostic criteria of clear cell carcinoma were included in the study (n = 121). These criteria included immunohistochemical profile compatible with the diagnosis—positivity of PAX8 and at least one marker of “clear cell” differentiation (HNF1B, AMACR, or napsin A) together with the negativity of WT1. During the study, additional 3 cases were excluded due to unsuccessful FISH, which yielded a study cohort of 118 cases in total.
The clinicopathologic characteristics of the cohort are summarized in Table 1. The main parameters evaluated include tumour stage (TNM and FIGO) and age at the time of diagnosis. The most common disease stage was T1c (34%) and T1a (33%). The median follow-up time was 37 months. During the follow-up period, 21 patients had a local recurrence, 13 developed distant metastases, and 24 patients died (14 of those due to diagnosis).
The haematoxylin and eosin-stained slides of all the selected tumour samples were reviewed, and suitable tumour areas were marked for the removal of individual tissue cores, which were used for the construction of tissue microarrays (TMAs). From each tumour donor block two tissue cores (each 2.0 mm in diameter) were taken using the tissue microarray instrument TMA Master (3DHISTECH Ltd., Budapest, Hungary) and the evaluation of the studied cases was performed with the use of TMAs.
Immunohistochemical analysis
Using the constructed TMAs, the immunohistochemical (IHC) analysis was performed on 4-µm thick sections of FFPE tissue. In cases where the TMA approach was not suitable due to technical difficulties with the sample processing (n = 12) whole-tissue sections were used.
The IHC evaluation of the HER2 status was performed using the antibody PATHWAY anti-HER-2/neu (clone 4B5, Roche, Basel, Switzerland). The heat induced epitope retrieval using diluted EnVision FLEX Target Retrieval Solution was used for the pre-treatment of HER2. The detection of the primary antibody was performed using the Ventana BenchMark ULTRA instrument (Roche, Basel, Switzerland) with the Ultraview Universal DAB Detection Kit. HER2 scoring was performed in accordance with the 2018 ASCO Guidelines for breast carcinoma [51]. HER2-positive tumours were defined as tumours with a score of 2 + with FISH-confirmed amplification, or tumours with a score of 3 + (complete, strong circumferential immunoreactivity of > 10% of tumour cells). The group of HER2-negative tumours was further divided into two sub-groups: HER2-zero (score 0) and HER2-low (score 1 + , score 2 + without amplification), as previously suggested by other works [1, 36]. The IHC expression was double-blindly evaluated by 2 independent pathologists (MKB, KN).
For the assessment of the clinicopathological characteristics and survival outcomes the cases were categorized as HER2-zero, HER2-low, and HER2-positive.
Fluorescent in situ hybridization (FISH) analysis for HER2
All cases with HER2 IHC 2 + were tested for amplification by FISH using 4-µm thick whole tissue sections of FFPE tumour tissue and ZytoLight ® SPEC ERBB2/CEN 17 Dual Color Probe (cat. No. Z-2077, ZytoVision GmbH, Bremerhaven, Germany) according to the manufacturer’s protocol. The scoring of ISH results was performed in accordance with the 2018 ASCO Guidelines for breast carcinoma [51]. A HER2/CEP17 ratio ≥ 2.0 was considered as amplification (positive, group 1). The assessment was carried out by a pathologist experienced with FISH analysis (KN).
Next generation sequencing (NGS) analysis of DNA and RNA
The isolation of nucleic acids from FFPE tumour tissue and the following capture DNA and RNA NGS analyses were performed as described previously, with the focus on ERBB2 (HER2) [7]. The sequence capture NGS analysis of DNA (DNA NGS) and/or RNA (RNA-Seq) was performed for all qualitatively sufficient cases (DNA NGS: 100/118, 84.7%, and RNA-seq: 103/118, 87.3%). Complete DNA and RNA NGS analysis could be carried out for 92 samples had (same sample set was used for comprehensive genomic and transcriptomic analyses, which is a subject of another study from our group, currently under review).
The demultiplexed RNA-Seq data were analysed using the CLC GW by an in-house pipeline which includes targeted RNA-Seq expression analysis (RNA-Seq Analysis module). The bioinformatics pipeline and module settings are available upon request.
Normalization of the mRNA expression was evaluated as RPKM (reads per kilobase of transcript per million reads mapped) and VCP, SF3B1, and ATP5F1B genes were used as reference.
Statistical analyses
All statistical analyses were performed using the program R (version 4.0.2, https://www.r-project.org/), Statistica (TIBCO), and/or CLC (Qiagen; CLC GW). The cases were divided according to the IHC expression of HER2 into 3 groups: (1) HER2-zero (score 0), (2) HER2-low (score 1 + , or score 2 + without amplification on FISH), (3) HER2 positive (score 3 + , or score 2 + with confirmed amplification on FISH). Correlations between the HER2 status and the clinicopathological characteristics were analysed using the Pearson chi-squared test or Fisher exact test according to the expected values.
Survival analyses were performed with four outcomes—overall survival (OS: the period from the date of diagnosis to the date of recorded death), relapse-free survival (RFS: the period from the date of diagnosis to the date of recurrence/death from diagnosis), local recurrence-free survival (LFS: the period from primary diagnosis till the first local recurrence), and distant metastasis-free survival (MFS: the period from primary diagnosis till the first distant metastasis). The date of diagnosis was the date of the surgical procedure. Survival analyses were plotted using the Kaplan–Meier model, and the differences between curves were tested for significance using the log-rank test. If a patient did not have an event, the case was censored in each analysis to the date of the last known follow-up.
To determinate whether HER2 status is an independent prognostic factor, the multivariate Cox’s Proportional Hazard Ratio Model involving age and FIGO stage as covariates was performed. Using the backward elimination of non-significant effects, a minimal adequate model was achieved.
All tests were two-sided and a p-value of less than 0.05 was considered as significant.
Differential expression in two-group module which is implemented in CLC GW was used for differential expression of ERBB2 in group HER2-positive versus HER2-low/HER2-zero and HER2-low versus HER2-zero. This module is a multi-factorial statistics test based on a negative binomial Generalized Linear Model (the statistical model for this module is thoroughly described in the CLC GW manual—https://digitalinsights.qiagen.com/technical-support/manuals/).
Results
Immunohistochemical and FISH findings
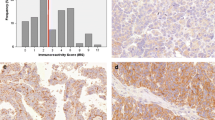
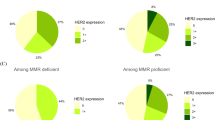
The representative examples of the immunostaining results are provided in Fig. 1, while the overview of the HER2 immunostaining results is described in Fig. 2. HER2 immunoreactivity was scored as 0 in 84 cases, as 1 + in 7 cases, as 2 + in 23 cases, and as 3 + in 4 cases. The expression of the HER2 protein was categorized into HER2-positive (6/118, 5%) and HER2-negative (comprising HER2-zero and HER2-low cases, total of 112/118, 95%). In the HER2-negative group, 84/112 (75%) cases were HER2-zero and 28/112 (25%) cases HER2-low.
The representative examples of the immunostaining results of 118 ovarian clear cell carcinomas using the HER2 antibody. All microphotographs are taken at × 200 magnification. A Negative result with complete absence of staining or incomplete membrane staining which is faint or barely perceptible and within ≤ 10% of the tumour cells (score 0), B Negative result with incomplete membrane staining which is faint or barely perceptible and within > 10% of the tumour cells (score 1 +). C Equivocal result showing weak to moderate complete membrane staining in > 10% of tumour cells (score 2 +). D Positive result with circumferential membrane staining which is complete, intense, and in > 10% of tumour cells
All 23 cases with an IHC score of 2 + were subsequently tested using FISH. Of these, 2 cases (2/23; 9%) were evaluated as positive, belonging to the group of classic HER2 amplified cancer (group 1). The remaining 21 cases were classified as negative (classic non-HER2 amplified cancer, group 5). There were no cases showing monosomy, co-amplification (previously polysomy 17), or borderline/equivocal results.
Prognostic significance of HER2 IHC status
The main evaluated clinicopathological parameters are summarized in Table 2 and included age, FIGO, TNM stage, and local and distant recurrence. None of the observed parameters showed any significant correlation with the HER2 IHC status.
To investigate the prognostic value of the HER2 IHC status, we performed a time to event analysis (OS, RFS, LFS, and MFS) in a 3-tier model (using all three categories HER2-zero, HER2-low, and HER2-positive), and also in a 2-tier model excluding the HER2-positive cases (using only the categories of HER2-zero and HER2-low, given the limitations of the small number of cases in the HER2-positive group).
There was no significant difference in any of the outcomes when applying all three categories (OS: χ2 = 4.48, p = 0.106, RFS: χ2 = 5.93, p = 0.051, LFS: χ2 = 2.75, p = 0.252, MFS: χ2 = 4.99, p = 0.082), although the HER2-zero cases showed a better probability of survival in all outcomes of interest when compared with a group including both the HER2-low and HER2-positive categories (there is a limitation in the number of complete cases, especially in the case of LFS and MFS, Fig. 3).
The 2-tier model including HER2-zero and HER2-low cases revealed better OS and RFS for HER2-zero patients (Z = 2.01, p = 0.044, and Z = 2.12, p = 0.034, respectively; Fig. 3). However, the multivariate analysis involving FIGO stage and age as possible covariates determined this relationship as not significant (χ2 = 2.75, p = 0.098), which would indicate that HER2 status is not an independent prognostic factor, and that the survival parameters are better explained by FIGO stage.
Molecular findings
DNA analysis
The DNA NGS analysis of 100 eligible cases revealed an ERBB2 pathogenic or likely pathogenic variant (class 4/5) in 3 cases. The ERBB2 mutation NM_004448.2: c.2524G > A, p.(Val842Ile) was detected in one case (HER2-zero) and the mutation c.2033G > A, p.(Arg678Gln) was detected in two cases, one of which was classified as HER2-zero, while the other was scored as IHC 2 + (the FISH analysis in this case failed).
RNA analysis and correlation between immunohistochemistry and gene expression
The HER2 gene expression analysis showed a significant correlation with HER2 IHC status when examining the entire cohort (HER2-positive vs. HER2-negative)—the expression of normalized ERBB2 mRNA was 2.97-fold higher in the HER2-positive group than the HER2-low/HER2-zero (p < 0.001) (Fig. 4). In the cohort of only HER2-negative cases, the expression of ERBB2 mRNA was 1.06-fold higher in the HER2-low group than the HER2-zero group, but this result did not reach statistical significance (p = 0.590).
Discussion
HER2 is one of the receptor kinases which plays an important role in promoting and regulating cell proliferation and differentiation. The overexpression of HER2 has been linked to chemoresistance and poor outcomes, both of which represent the main characteristics of OCCC [22]. The reported overexpression/amplification of HER2 in OCCC shows striking variability. In our study, HER2 amplification/overexpression was found in 6/118 OCCC (5%), with an additional 28/118 (24%) of cases being classified as HER2-low. This result is comparable to only one other study which reported HER2 overexpression in 6,7% of OCCCs; however, their cohort contained only 15 cases of OCCC and the authors used a different method of HER2 status evaluation (only cases which scored 3 + in IHC were considered positive) [24]. As previously mentioned, other works have reported HER2 overexpression in a very wide range including values of 12.6%; 12.5%; 14%; 20%; 42.9% and 45.6% [13, 29, 31, 33, 49, 50]. The comparison of these results is problematic, because the methodology of HER2 status evaluation lacks standardization (some authors considered HER2 score 2 + and 3 + as overexpression, some required only 3 + score to be considered as overexpression), and the confirmation of amplification by FISH was performed only in some studies [29, 49, 52]. A recent work by Koopman et al. compared three different antibodies (SP3, 4B5, and HercepTest) when evaluating ovarian clear cell carcinoma and pointed out that there is a considerable difference in HER2 overexpression by different antibodies, as well as a marked discordance with ISH [29]. Another important aspect regarding the scoring of HER2 expression by IHC is tumour heterogeneity, which seems to be more prominent in tumours of other sites apart from the breast and may result in false negative results and contribute to the observed discordance [48]. Given that HER2 may be a useful predictor for patients with OCCC, the question of a suitable method to evaluate HER2 status effectively is an important one.
In our cohort, none of the examined clinico-pathological parameters showed any significant correlation with HER2 IHC status. Considering the prognostic significance of HER2 expression in OCCC, the literary data is sparse. One review and meta-analysis has shown that increased levels of HER2 could predict worse survival, but the included tumours represent a heterogeneous group of ovarian carcinomas without further specification of their histotype [55]. Similarly, the results of the Danish “MALOVA” study (which included 9 OCCCs among the 181 investigated cases) also showed that increased HER2-expression correlated with reduced survival [16]. In contrast, others found that HER2 expression does not correlate with survival and lacks prognostic meaning [17, 50]. Our results showed that in a 3-tier model using the categories of HER2-zero, HER2-low, and HER2-positive there were no statistically significant differences in any of the outcomes. However, these results are limited by the low number of cases in the HER2-positive group. When comparing only the groups HER2-zero and HER2-low, there was significantly better survival in the HER2-zero patients in case of both overall survival and relapse-free survival. Although this relationship lost its significance when the multivariate analysis was applied (suggesting that HER2 status is not an independent prognostic factor), these results could be of potential therapeutic value, given the recent discovery that patients with HER2-low breast cancer could also benefit from anti-HER2 therapy. However, the number of cases with complete available follow up represents a limitation of our study in this respect.
The frequency of HER2 overexpression/amplification indicates that anti-HER2 targeted therapy could be of value in this setting, especially in the cases of metastatic and/or recurrent tumours. Some authors reported promising results for the effect of trastuzumab on three OCCC cell lines, where trastuzumab significantly and dose-dependently reduced the growth of tumour cells in vitro and prolonged the survival of mice with a xenografted tumour [13]. However, others found that trastuzumab did not inhibit proliferation in any of the four OCCC lines tested [22]. Despite the potentially encouraging results, when trastuzumab was used in a single-agent study of 41 patients with recurrent or refractory ovarian/primary peritoneal carcinoma with an overexpression of HER2 (including 7 OCCCs) the therapeutic response was disappointing [6, 13]. The authors concluded that the value of single-agent trastuzumab therapy is not only limited by the low frequency of HER2 overexpression (in their cohort 11.4% of 837 screened tumour samples), but also by the rather poor overall response rate which reached 7.3% and included one complete and two partial responses, with no further specification provided for the subset of OCCC patients [6]. However, the inclusion criteria were set to include tumours which were HER2 2 + or HER2 3 + by IHC, without further assessment of the amplification status. The discordance between the IHC evaluation and the therapeutic responsiveness could have been caused by the different antibodies used for evaluation [29]. Based on the limited data, it seems that similarly to breast and gastric carcinoma, the efficacy of trastuzumab in OCCC patients could be improved by combining it with chemotherapy [14].
The correlation between the immunohistochemistry and gene expression analysis showed that while there was a strong correlation between HER2 gene expression and HER2 IHC status when the entire cohort was included (HER2-positive vs. HER2-negative); when looking at the HER2-negative cases only, the results were not statistically significant. In our cohort, the gene expression analysis would not be a suitable tool to confirm the subdivision of the HER2-negative group into HER2-low and HER2-zero. However, it has been suggested that evaluating HER2 expression using more precise methods such as mRNA expression may provide a higher clinical meaning, given that HER2 evaluation by immunohistochemistry can underestimate the HER2 expression in ovarian cancer [30]. Comparable data is currently missing for ovarian carcinomas, although for breast cancer Almstedt et al. reported that the IHC HER2-subdivision in their cohort was significantly confirmed by gene expression analysis (both in the entire cohort and in the HER2-negative subgroup) [1].
Our results also revealed a class 4/5 HER2 mutation in 3 cases, namely p.(Arg678Gln) in 2 cases and p.(Val842Ile) in one case. Both detected HER2 variants are known recurrent activating oncogenic mutations found in diverse types of cancers [20, 25, 39]. We did not observe any relationship between mRNA/protein expression and the presence of HER2 mutation. The frequency of HER2-mutated OCCC in our study is comparable with the results of the other authors, which varies between 0 and 4% [5, 23, 34, 43]. The frequency of HER2 mutations in our cohort (3/100, 3%) suggests that analysis of HER2 oncogenic mutations may be of clinical interest, as these patients could also benefit from anti-HER2 therapy [18, 32].
We are aware of other limitations of our study. The main limitation is the use of TMA, as the results of the immunohistochemical evaluation could be influenced by tumour heterogeneity. While that is true, the use of TMA has become a widely used routine method in research to enable the evaluation of a large number of samples while preserving the sometimes limited tissue and providing high experimental uniformity. To minimize the potential impact of heterogeneity, two cores were taken from each of the tumour samples and evaluated together, thus increasing the amount of analysed tissue.
Conclusion
We have characterized the HER2 status using immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) on a large, well-defined cohort of 118 OCCCs fulfilling strict inclusion criteria. The frequency of overexpression/amplification (HER2-positive cases) confirms that anti-HER2 targeted therapy could be of value, especially in cases of metastatic and/or recurrent tumours. This is also the first work to date which has evaluated the HER2 status of OCCC in the context of the novel paradigm of HER2-zero and HER2-low status. Although the relationship between HER2 expression (HER2-zero vs. HER2-low) and survival lost its significance in the multivariate analysis (suggesting that HER2 status is not an independent prognostic factor), these results could be of potential therapeutic value, given the recent discovery that patients with HER2-low breast cancer could also benefit from anti-HER2 therapy. Our results also emphasize the need for standardized HER2 testing in ovarian clear cell carcinoma to determine the most suitable predictor of clinical response.
Data availability
The bioinformatics pipeline and module settings used during the current study are available from the corresponding author on reasonable request.
References
Almstedt K, Heimes AS, Kappenberg F, Battista MJ, Lehr HA, Krajnak S, Lebrecht A, Gehrmann M, Stewen K, Brenner W, Weikel W, Rahnenfuhrer J, Hengstler JG, Hasenburg A, Schmidt M (2022) Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer 173:10–19. https://doi.org/10.1016/j.ejca.2022.06.012
Amano T, Chano T, Yoshino F, Kimura F, Murakami T (2019) Current position of the molecular therapeutic targets for ovarian clear cell carcinoma: a literature review. Healthcare (Basel) 7(3):94. https://doi.org/10.3390/healthcare7030094
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, Carrato A, Gulley ML, Jain D, Kakar S, Mackay HJ, Streutker C, Tang L, Troxell M, Ajani JA (2017) HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 35:446–464. https://doi.org/10.1200/JCO.2016.69.4836
Bolton KL, Chen D, Corona de la Fuente RI, Fu Z, Murali R, Kobel M, Tazi Y, Cunningham JM, Chan ICC, Wiley BJ, Moukarzel LA, Winham SJ, Armasu SM, Lester J, Elishaev E, Laslavic A, Kennedy CJ, Piskorz A, Sekowska M, Brand AH, Chiew YE, Pharoah P, Elias KM, Drapkin R, Churchman M, Gourley C, DeFazio A, Karlan B, Brenton JD, Weigelt B, Anglesio MS, Huntsman D, Gayther SA, Konner J, Modugno F, Lawrenson K, Goode EL, Papaemmanuil E (2022) Molecular subclasses of clear cell ovarian carcinoma and their impact on disease behavior and outcomes. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-21-3817
Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR (2003) Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol 21:283–290. https://doi.org/10.1200/JCO.2003.10.104
Dundr P, Bártů M, Bosse T, Bui QH, Cibula D, Drozenová J, Fabian P, Fadare O, Hausnerová J, Hojný J, Hájková N, Jakša R, Laco J, Lax SF, Matěj R, Méhes G, Michálková R, Šafanda A, Němejcová K, Singh N, Stolnicu S, Švajdler M, Zima T, Stružinská I, McCluggage WG (2023) Primary mucinous tumors of the ovary: an interobserver reproducibility and detailed molecular study reveals significant overlap between diagnostic categories. Mod Pathol 36:100040. https://doi.org/10.1016/j.modpat.2022.100040
Early Breast Cancer Trialists’ Collaborative g (2021) Trastuzumab for early-stage, HER2-positive breast cancer a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol 22:1139–1150. https://doi.org/10.1016/S1470-2045(21)00288-6
Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E (2021) The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) 13(5):1015. https://doi.org/10.3390/cancers13051015
Fadare O, Parkash V (2019) Pathology of endometrioid and clear cell carcinoma of the ovary. Surg Pathol Clin 12:529–564. https://doi.org/10.1016/j.path.2019.01.009
Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers S, Secord AA, Havrilesky L, O’Malley DM, Backes FJ, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi K, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi DA, Schwartz PE, Santin AD (2020) Randomized phase II trial of carboplatin-paclitaxel compared with carboplatin-paclitaxel-trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu (NCT01367002): updated overall survival analysis. Clin Cancer Res 26:3928–3935. https://doi.org/10.1158/1078-0432.CCR-20-0953
Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers SK, Secord AA, Havrilesky L, O’Malley DM, Backes F, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi KS, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi DA, Schwartz PE, Santin AD (2018) Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2neu. J Clin Oncol 36:2044–2051. https://doi.org/10.1200/JCO.2017.76.5966
Fujimura M, Katsumata N, Tsuda H, Uchi N, Miyazaki S, Hidaka T, Sakai M, Saito S (2002) HER2 is frequently over-expressed in ovarian clear cell adenocarcinoma: possible novel treatment modality using recombinant monoclonal antibody against HER2, trastuzumab. Jpn J Cancer Res 93:1250–1257. https://doi.org/10.1111/j.1349-7006.2002.tb01231.x
Gounaris I, Brenton JD (2015) Molecular pathogenesis of ovarian clear cell carcinoma. Future Oncol 11:1389–1405. https://doi.org/10.2217/fon.15.45
Haruta S, Furukawa N, Yoshizawa Y, Tsunemi T, Nagai A, Kawaguchi R, Tanase Y, Yoshida S, Kobayashi H (2011) Molecular genetics and epidemiology of epithelial ovarian cancer (review). Oncol Rep 26:1347–1356. https://doi.org/10.3892/or.2011.1456
Hogdall EV, Christensen L, Kjaer SK, Blaakaer J, Bock JE, Glud E, Norgaard-Pedersen B, Hogdall CK (2003) Distribution of HER-2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: from the Danish MALOVA Ovarian Cancer Study. Cancer 98:66–73. https://doi.org/10.1002/cncr.11476
Hoopmann M, Sachse K, Valter MM, Becker M, Neumann R, Ortmann M, Gohring UJ, Thomas A, Mallmann P, Schondorf T (2010) Serological and immunohistochemical HER-2/neu statuses do not correlate and lack prognostic value for ovarian cancer patients. Eur J Cancer Care (Engl) 19:809–815. https://doi.org/10.1111/j.1365-2354.2009.01112.x
Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, Juric D, Quinn DI, Moreno V, Doger B, Mayer IA, Boni V, Calvo E, Loi S, Lockhart AC, Erinjeri JP, Scaltriti M, Ulaner GA, Patel J, Tang J, Beer H, Selcuklu SD, Hanrahan AJ, Bouvier N, Melcer M, Murali R, Schram AM, Smyth LM, Jhaveri K, Li BT, Drilon A, Harding JJ, Iyer G, Taylor BS, Berger MF, Cutler RE Jr, Xu F, Butturini A, Eli LD, Mann G, Farrell C, Lalani AS, Bryce RP, Arteaga CL, Meric-Bernstam F, Baselga J, Solit DB (2018) HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189–194. https://doi.org/10.1038/nature25475
Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz G, Ejlertsen B, Chia SKL, Mansi J, Barrios CH, Gnant M, Buyse M, Gore I, Smith J 2nd, Harker G, Masuda N, Petrakova K, Zotano AG, Iannotti N, Rodriguez G, Tassone P, Wong A, Bryce R, Ye Y, Yao B, Martin M, Exte NETSG (2016) Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:367–377. https://doi.org/10.1016/S1470-2045(15)00551-3
Chang MT, Bhattarai TS, Schram AM, Bielski CM, Donoghue MTA, Jonsson P, Chakravarty D, Phillips S, Kandoth C, Penson A, Gorelick A, Shamu T, Patel S, Harris C, Gao J, Sumer SO, Kundra R, Razavi P, Li BT, Reales DN, Socci ND, Jayakumaran G, Zehir A, Benayed R, Arcila ME, Chandarlapaty S, Ladanyi M, Schultz N, Baselga J, Berger MF, Rosen N, Solit DB, Hyman DM, Taylor BS (2018) Accelerating discovery of functional mutant alleles in cancer. Cancer Discov 8:174–183. https://doi.org/10.1158/2159-8290.CD-17-0321
Chuang J, Klempner S, Waters K, Atkins K, Chao J, Cho M, Hendifar A, Gangi A, Burch M, Mehta P, Gong J (2022) Therapeutic advances and challenges in the management of HER2-positive gastroesophageal cancers. Diseases 10(2):23. https://doi.org/10.3390/diseases10020023
Itamochi H, Kigawa J, Terakawa N (2008) Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci 99:653–658. https://doi.org/10.1111/j.1349-7006.2008.00747.x
Itamochi H, Oishi T, Oumi N, Takeuchi S, Yoshihara K, Mikami M, Yaegashi N, Terao Y, Takehara K, Ushijima K, Watari H, Aoki D, Kimura T, Nakamura T, Yokoyama Y, Kigawa J, Sugiyama T (2017) Whole-genome sequencing revealed novel prognostic biomarkers and promising targets for therapy of ovarian clear cell carcinoma. Br J Cancer 117:717–724. https://doi.org/10.1038/bjc.2017.228
Iwamoto H, Fukasawa H, Honda T, Hirata S, Hoshi K (2003) HER-2/neu expression in ovarian clear cell carcinomas. Int J Gynecol Cancer 13:28–31. https://doi.org/10.1046/j.1525-1438.2003.13028.x
Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, Searleman AC, Shen W, Monsey J, Trusolino L, Jacobs SA, Bertotti A, Bose R (2015) HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov 5:832–841. https://doi.org/10.1158/2159-8290.CD-14-1211
Kobel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, Seidman JD, Gilks CB, Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency VBC (2010) Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 29:203–211. https://doi.org/10.1097/PGP.0b013e3181c042b6
Koopman T, Louwen M, Hage M, Smits MM, Imholz AL (2015) Pathologic diagnostics of HER2 positivity in gastroesophageal adenocarcinoma. Am J Clin Pathol 143:257–264. https://doi.org/10.1309/AJCPCX69HGDDGYCQ
Koopman T, Smits MM, Louwen M, Hage M, Boot H, Imholz AL (2015) HER2 positivity in gastric and esophageal adenocarcinoma: clinicopathological analysis and comparison. J Cancer Res Clin Oncol 141:1343–1351. https://doi.org/10.1007/s00432-014-1900-3
Koopman T, van der Vegt B, Dijkstra M, Bart J, Duiker E, Wisman GBA, de Bock GH, Hollema H (2018) HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: discordance between antibodies and with in-situ hybridisation. Histopathology 73:852–863. https://doi.org/10.1111/his.13704
Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, Coukos G, Powell DJ Jr (2012) Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS One 7:e49829. https://doi.org/10.1371/journal.pone.0049829
Lee CH, Huntsman DG, Cheang MC, Parker RL, Brown L, Hoskins P, Miller D, Gilks CB (2005) Assessment of Her-1, Her-2, And Her-3 expression and Her-2 amplification in advanced stage ovarian carcinoma. Int J Gynecol Pathol 24:147–152. https://doi.org/10.1097/01.pgp.0000152026.39268.57
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, Nagasaka M, Bazhenova L, Saltos AN, Felip E, Pacheco JM, Perol M, Paz-Ares L, Saxena K, Shiga R, Cheng Y, Acharyya S, Vitazka P, Shahidi J, Planchard D, Janne PA, Investigators DE-LT (2022) Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 386:241–251. https://doi.org/10.1056/NEJMoa2112431
Li M, Li H, Liu F, Bi R, Tu X, Chen L, Ye S, Cheng X (2017) Characterization of ovarian clear cell carcinoma using target drug-based molecular biomarkers: implications for personalized cancer therapy. J Ovarian Res 10:9. https://doi.org/10.1186/s13048-017-0304-9
Maru Y, Tanaka N, Ohira M, Itami M, Hippo Y, Nagase H (2017) Identification of novel mutations in Japanese ovarian clear cell carcinoma patients using optimized targeted NGS for clinical diagnosis. Gynecol Oncol 144:377–383. https://doi.org/10.1016/j.ygyno.2016.11.045
Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, Cacciatore M, Genovesi E, De Bartolo D, Vernaci G, Amato O, Porra F, Conte P, Guarneri V, Dieci MV (2022) HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 8:66. https://doi.org/10.1038/s41523-022-00434-w
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA, Investigators DE-BT (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38:1887–1896. https://doi.org/10.1200/JCO.19.02318
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I, Investigators DE-B (2020) Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610–621. https://doi.org/10.1056/NEJMoa1914510
Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, Chaudhuri S, Kljavin NM, Antony A, Phalke S, Kumar P, Mravic M, Stawiski EW, Vargas D, Durinck S, Gupta R, Khanna-Gupta A, Trabucco SE, Sokol ES, Hartmaier RJ, Singh A, Chougule A, Trivedi V, Dutt A, Patil V, Joshi A, Noronha V, Ziai J, Banavali SD, Ramprasad V, DeGrado WF, Bueno R, Jura N, Seshagiri S (2018) Actionable activating oncogenic ERBB2/HER2 transmembrane and juxtamembrane domain mutations. Cancer Cell 34:792-806 e795. https://doi.org/10.1016/j.ccell.2018.09.010
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant Trial Study T (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672. https://doi.org/10.1056/NEJMoa052306
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684. https://doi.org/10.1056/NEJMoa052122
Ross JS, Yang F, Kallakury BV, Sheehan CE, Ambros RA, Muraca PJ (1999) HER-2/neu oncogene amplification by fluorescence in situ hybridization in epithelial tumors of the ovary. Am J Clin Pathol 111:311–316. https://doi.org/10.1093/ajcp/111.3.311
Shibuya Y, Tokunaga H, Saito S, Shimokawa K, Katsuoka F, Bin L, Kojima K, Nagasaki M, Yamamoto M, Yaegashi N, Yasuda J (2018) Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosomes Cancer 57:51–60. https://doi.org/10.1002/gcc.22507
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. https://doi.org/10.1056/NEJM200103153441101
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ, team Hs (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369:29–36. https://doi.org/10.1016/S0140-6736(07)60028-2
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Sugiyama T, Okamoto A, Enomoto T, Hamano T, Aotani E, Terao Y, Suzuki N, Mikami M, Yaegashi N, Kato K, Yoshikawa H, Yokoyama Y, Tanabe H, Nishino K, Nomura H, Kim JW, Kim BG, Pignata S, Alexandre J, Green J, Isonishi S, Terauchi F, Fujiwara K, Aoki D (2016) Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial. J Clin Oncol 34:2881–2887. https://doi.org/10.1200/JCO.2016.66.9010
Talia KL, Banet N, Buza N (2023) The role of HER2 as a therapeutic biomarker in gynaecological malignancy: potential for use beyond uterine serous carcinoma. Pathology 55:8–18. https://doi.org/10.1016/j.pathol.2022.11.004
Tan DS, Iravani M, McCluggage WG, Lambros MB, Milanezi F, Mackay A, Gourley C, Geyer FC, Vatcheva R, Millar J, Thomas K, Natrajan R, Savage K, Fenwick K, Williams A, Jameson C, El-Bahrawy M, Gore ME, Gabra H, Kaye SB, Ashworth A, Reis-Filho JS (2011) Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin Cancer Res 17:1521–1534. https://doi.org/10.1158/1078-0432.CCR-10-1688
Tanabe H, Nishii H, Sakata A, Suzuki K, Mori Y, Shinozaki H, Watanabe A, Ochiai K, Yasuda M, Tanaka T (2004) Overexpression of HER-2/neu is not a risk factor in ovarian clear cell adenocarcinoma. Gynecol Oncol 94:735–739. https://doi.org/10.1016/j.ygyno.2004.05.055
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142:1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Wu Y, Soslow RA, Marshall DS, Leitao M, Chen B (2004) Her-2/neu expression and amplification in early stage ovarian surface epithelial neoplasms. Gynecol Oncol 95:570–575. https://doi.org/10.1016/j.ygyno.2004.08.043
Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, Katabuchi H (2017) Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol 28:e32. https://doi.org/10.3802/jgo.2017.28.e32
Yoshida K, Yoshikawa N, Shirakawa A, Niimi K, Suzuki S, Kajiyama H, Kikkawa F (2019) Prognostic value of neutrophil-to-lymphocyte ratio in early-stage ovarian clear-cell carcinoma. J Gynecol Oncol 30:e85. https://doi.org/10.3802/jgo.2019.30.e85
Zhao D, Zhang F, Zhang W, He J, Zhao Y, Sun J (2013) Prognostic role of hormone receptors in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer 23:25–33. https://doi.org/10.1097/IGC.0b013e3182788466
Acknowledgements
The authors thank Mr Zachary Harold Kane Kendall, BA (Institute for History of Medicine and Foreign Languages, First Faculty of Medicine, Charles University), for the English proofreading.
Funding
This work was supported by the Ministry of Health, Czech Republic (RVO VFN 64165 and AZV NV19-03–00007), by Charles University (Project UNCE204065, SVV260516), and by the European Regional Development Fund (EF16_013/0001674) and BBMRI_CZ LM2023033.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Michaela Kendall Bártů and Pavel Dundr conceived the study and carried out experiments, Kristýna Němejcová has participated in the assessment of the immunohistochemistry results and the FISH analyses. Ivana Stružinská, Jan Hojný, Nikola Hájková, and Pavel Dundr conceived experiments and analysed data. Romana Michálková performed the statistical analyses. Eva Krkavcová carried out the molecular genetics part of the experiments. Jan Laco, Radoslav Matěj, Jana Drozenová, Gábor Méhes, Pavel Fabian, Jitka Hausnerová, Marián Švajdler, Petr Škapa, David Cibula, and Tomáš Zima participated in the selection and evaluation of cases, provided the clinical data and participated in the data analysis. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of the General University Hospital in Prague in compliance with the Helsinki Declaration. The Ethics Committee did not require the procurement of informed consent, given that according to the Czech Law (Act. no. 373/11, and its amendment Act no. 202/17) it is not necessary to provide informed consent in fully anonymized studies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kendall Bártů, M., Němejcová, K., Michálková, R. et al. HER2 status as a potential predictive biomarker for ovarian clear cell carcinoma. Virchows Arch 483, 497–507 (2023). https://doi.org/10.1007/s00428-023-03640-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03640-4