Abstract
Acinic cell carcinoma (AciCC) is a common salivary gland malignancy, typically composed of neoplastic acinic cells with zymogen granules. The vast majority of cases are driven by a t(4;9)(q13;q31) leading to enhancer hijacking and upregulation of the NR4A3 gene. However, a minority of cases do not display NR4A3 overexpression on immunohistochemical examination and are negative for the rearrangement involving the NR4A3 gene when tested by FISH. Such cases overexpress NR4A2, and the protein product is detectable by immunohistochemistry. In this study, we aimed to assess the utility of NR4A2 and NR4A3 immunohistochemistry in the differential diagnosis of salivary gland tumors. Eighty-five cases of classic low-grade ACiCC, as well as 36 cases with high-grade transformation (HGT) and 7 high-grade AciCC cases were included in the analysis. NR4A3 was at least focally positive in 105/128 (82%) cases. Out of the 23 cases that were immunohistochemically negative for NR4A3, 6 displayed nuclear immunopositivity with the NR4A2 antibody. The NR4A3 rearrangement was confirmed by FISH in 38/52 (73%) cases. In addition, this is the first report of an NR4A2 rearrangement being detected by FISH in 2 AciCC cases that were negative for the NR4A3 rearrangement. Our analysis confirms that the majority of AciCC, including high-grade cases and cases with HGT, are immunopositive for NR4A3, and suggests that NR4A3 immunohistochemistry is a powerful tool in the differential diagnosis of salivary gland tumors. However, its utility is limited in sub-optimally fixed samples which often display weaker and focal positivity. Our study also indicates that in a minority of cases, AciCC might be negative for NR4A3 immunostaining, because the pathogenic genetic event in these cases is instead a rearrangement involving the NR4A2 gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinic cell carcinoma (AciCC) represents about 10% of malignant salivary gland tumors and is also the second most common pediatric salivary gland malignancy [1]. Middle-aged adults, more often female, are the most commonly affected, with the average age of about 50 years, but the age range is considerably wide. While the prognosis for patients with the classic low-grade AciCC is excellent, with 5-year and 10-year survival rates of 97% and 94%, respectively [2], some cases display a transformation into high-grade, aggressively behaving neoplasms with unfavorable outcomes [3,4,5].
In typical low-grade cases, the neoplastic cells display a serous acinic differentiation with PAS-positive cytoplasmic granules. However, some neoplastic cells can also have eosinophilic, clear-cell or vacuolated cytoplasm. The most common architectural pattern is solid-microcystic, often with abundant lymphoid stroma, including well-formed lymphoid follicles with germ centers in some cases. Cribriform and cystic architecture is often associated with intratumoral hemorrhage. Follicular, tubular, and papillary growth patterns are less common and might pose a diagnostic challenge in some instances. Areas with transformation into high-grade carcinoma usually grow as solid nests, often with central comedonecrosis. Tumor cells are positive for SOX10 and DOG1, with the latter antibody usually showing an apical/canalicular pattern of staining. Conversely, p40, p63, and S100 markers are negative in AciCC. Even though the diagnostic process in acinic cell carcinoma is usually straight-forward, the immunohistochemical markers might be of benefit in challenging cases such as zymogen granule-poor and other rare morphological variants including clear-cell changes, tumors with high-grade transformation or unusually located tumors. In fine-needle aspiration cytology specimens, immunohistochemistry might aid to differentiate among zymogen granule-rich neoplastic cells of acinic cell carcinoma and normal acinic cells.
Genetically, the vast majority of AciCC are driven by t(4;9)(q13;q31), leading to the hijacking of strong enhancer regions from the secretory calcium-binding phosphoprotein cluster of genes (SCPP) to the proximity of NR4A3 (Nor1) gene [6]. This causes the overexpression of NR4A3 which might in turn detected by immunohistochemistry [7]. Rare cases that are negative for the translocation might display upregulation of another gene from the same group of nuclear receptors called NR4A2 (Nurr1), located at chromosome band 2q24.1 [8]. Recently, immunohistochemical markers NR4A3 and, rarely, NR4A2 have been gaining importance in the differential diagnosis of salivary glands tumors, being highly specific and sensitive for AciCC [7,8,9].
Materials and methods
Case selection
For the current study, 128 AciCC cases were collected from institutional files of Biopticka laboratory. The diagnosis was confirmed by two pathologists (NK and AS) based on histomorphology and immunohistochemistry, in accordance with the current WHO classification of head and neck tumors [1]. Classic low-grade AciCC cases as well as cases with high-grade features or high-grade transformation (HGT) were included. Tumors with HGT were defined by the presence of a transformed area consisting of poorly differentiated carcinoma with high-grade features (with high mitotic activity and/or necrosis), within an otherwise well-defined, low-grade AciCC. The diagnosis of high-grade AciCC (without HGT) was rendered if areas with increased mitotic activity (> 5 mitotic figures/10 HPF (2.4 mm2)) and/or necrosis were present in the tumor, without the presence of a classic low-grade area, in accordance with a grading system proposed by Xu et al. [3]. Similarly to previous studies [7], these high-grade cases were included based on the concordant immunohistochemistry and clinicopathological correlation, as most of the samples represented recurrent tumors, metastases, or probatory biopsy specimens.
Histology and immunohistochemistry
For conventional microscopy, the excised tissues were fixed in formalin, processed routinely, embedded in paraffin (FFPE), cut, and stained with hematoxylin & eosin. For the DOG1, SOX10, NR4A3, and NR4A2 immunohistochemistry, the 4-μm-thick sections cut from paraffin blocks were processed in accordance with institutional standard on BenchMark ULTRA (Ventana Medical Systems, Tucson, AZ). All primary antibodies with their respective epitope retrieval method used in this study are summarized in Table 1. Visualization was performed using the ultraView Universal DAB Detection Kit (Roche, Tucson, AZ) and ultraView Universal Alkaline Phosphatase Red Detection Kit (Roche, Tucson, AZ). The slides were counterstained with Mayer’s hematoxylin. Appropriate controls were employed.
NR4A3 immunohistochemical examination was recognized as positive if moderate to strong nuclear staining was present at least focally (≥ 5% tumor cells). Cytoplasmic or membranous staining was considered non-specific and was not counted as positive. NR4A2 immunohistochemical stain displayed a strong background affinity to multiple, even non-neoplastic structures. Consequently, only strong diffuse nuclear staining was regarded as positive.
FISH
For the detection of NR4A2 and NR4A3 rearrangements, custom-design SureFISH NR4A2 break-apart probe (SureFISH/Agilent Technologies, Santa Clara, CA, USA) and ZytoLight SPEC NR4A3 Dual Color Break Apart Probe (ZytoVision Gmbh, Bremerhaven, Germany) were used. Chromosomal locations (build Human Genome version hg19) used for custom NR4A2 break-apart probe oligos were chr2:156,780,798–157,181,092 and chr2:157,198,713–157,599,007. The FISH procedure and interpretation of the results were performed as described previously [10].
Results
Demographic and clinical characteristics
The clinicopathological data for the 128 AciCC cases are summarized in Table 2. Eighty-five cases of low-grade AciCC, 7 cases of high-grade AciCC, and 36 cases with HGT were included in the study. The patients’ age ranged from 11 to 86 years (mean = 56, median = 61). Patients with HGT and high-grade tumors were more than a decade older than patients with low-grade AciCC (mean = 65, median = 68 vs. mean = 52, median = 56). Whereas 72% of patients with low-grade AciCC were female, both sexes were affected almost equally in the HGT and high-grade groups. One hundred and sixteen cases were primary tumors, most commonly occurring in the parotid gland, while 1 case each originated from the sublingual gland, submandibular gland, and small salivary gland in the parapharyngeal space. Location was unknown in 1 case. In addition, 9 cases represented recurrences, and 3 metastases in the lung, pleura, and omentum, respectively, were sampled. Tumor size ranged from 4 to 90 mm (mean 26.1, median 23).
Histopathologic and molecular-genetic features
Various architectural patterns were seen on microscopic examination, with the solid-microcystic pattern (Fig. 1A) being most prevalent (n = 115), commonly accompanied by abundant lymphoid stroma surrounding the neoplastic cell nests. Solid growth was present in 4% of low-grade AciCC cases (n = 3), while all cases with high-grade features contained at least minor parts with solid growth pattern. Occasionally, cystic or cystopapillary growth patterns were present (n = 39), often associated with intratumoral hemorrhage, with erythrocytes located in the lumina (Fig. 1B). Cribriform and follicular areas were revealed in 16 and 3 cases, respectively. Clear-cell change was frequent in both grade groups (n = 33), while oncocytoid or eosinophilic cytoplasm was observed in 2 cases with HGT. A collision with Warthin tumor was seen in 1 case of a parotid lesion of an 83-year-old female.
Histomorphologic features of AciCC. A Neoplastic acinic cells with zymogen granules organized in a solid-microcystic pattern were the most common finding (case LG9). B Cystic-hemorrhagic pattern in low-grade AciCC (case LG15). C Clear cell change and necrotic foci in a case with HGT (case HGT9). D High-grade area with foci of comedonecrosis and high mitotic activity (case HGT3)
The high-grade cases and the high-grade areas in cases with HGT displayed moderate to high-grade cytologic atypia with larger round to oval, often vesicular nuclei, and well-visible nucleoli. The mitotic activity was moderate to high, reaching up to 69 mitotic figures/10 HPF (2.4 mm2). In addition, necrotic areas were revealed in 95% of these cases (n = 41), usually in the form of comedo-type necrosis (Fig. 1C-D). In some cases, larger areas of geographic necrosis were revealed. Conversely, no necrotic foci were present and no more than 4 mitotic figures/10 HPF (2.4 mm2) were counted in the low-grade cases.
One case with HGT revealed further sarcomatoid dedifferentiation. The tumor, arising in the parotid gland of a 78-year-old male, contained three components: a small typical low-grade solid-microcystic components, larger sarcomatoid component consisting of pleomorphic spindled cells growing in short fascicular and vaguely storiform patterns on the background of hyalinized fibrous stroma, and a tubular to cribriform adenocarcinoma-like component with high-grade nuclear atypia growing in nests inside the sarcomatoid area. The mitotic activity was similar in both the tubular and sarcomatoid regions, reaching 14 and 11 mitotic figures/10 HPF (2.4 mm2), respectively, while no mitosis was revealed in the low-grade area. Small necrotic foci were present in the tubular component. On immunohistochemical examination, both the low-grade and the tubular-to-cribriform component were positive for NR4A3 but the sarcomatoid component was negative. FISH examination of an NR4A3 rearrangement was negative in all parts of the analyzed slide.
DOG1 immunomarker was positive in 126/128 (98%) cases (Fig. 2A), while SOX10 displayed nuclear positivity in 127/128 (99%) cases (Fig. 2B). NR4A3 was at least focally positive in 105/128 (82%) cases (Fig. 2C-D). A similar proportion (82% vs. 81% vs. 86%) of NR4A3-positive cases were revealed in the low-grade, HGT, and high-grade groups of AciCC.
Immunohistochemical findings Immunohistochemical staining for DOG1 (A) and SOX10 (B) was positive in 126/128 (98%) and 127/128 (99%) cases, respectively (case LG15). NR4A3 immunostaining revealed strong (C) (case LG10) to moderate (D) (case HGT9) nuclear positivity in 105/128 (82%) cases. Twenty-four cases did not stain for NR4A3 (E) (case LG13), six of which showed strong nuclear immunopositivity for NR4A2 (F) (case HGT23)
Out of the 23 cases that were immunohistochemically negative for NR4A3 (Fig. 2E), all were positive for both DOG1 and SOX10. All NR4A3-negative and 33 NR4A3-positive cases were subsequently stained for NR4A2 (n = 56). Six of the NR4A3-immunonegative cases displayed unequivocal nuclear immunopositivity with the NR4A2 antibody (Fig. 2F), while all the 33 NR4A3-immunopositive cases tested negative for NR4A2 on immunohistochemistry (Table 3).
The NR4A3 rearrangement was analyzed by FISH in 57 cases. Firstly, 47 NR4A3-immunopositive cases were tested. The rearrangement was verified in 23/28 (82%) low-grade AciCC cases, 11/14 (79%) cases with HGT, and 2/2 (100%) high-grade AciCC cases. In total, NR4A3 rearrangement was confirmed by FISH in 36/44 (82%) NR4A3-immunopositive analyzable cases, while 8/44 (18%) cases were negative for the aberration. Secondly, 10 NR4A3-immunonegative cases were assessed, with 6/8 (75%) analyzable cases being negative on the FISH examination and 2/8 (25%) cases displaying a positive NR4A3 split signal. Out of the whole cohort, 5 cases were not able to be analyzed by FISH.
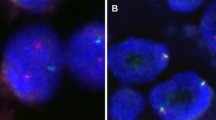
Finally, we assessed the presence of a rearrangement involving NR4A2 using a custom FISH break-apart probe (Table 3). In total, 9 cases were tested, including the 6 NR4A2-immunopositive cases and 3 cases that were negative for both NR4A2 and NR4A3 by immunohistochemistry. The NR4A2 break-apart was confirmed in 2/8 (25%) analyzable cases, while 6/8 (75%) analyzable cases were negative and 1 case was not analyzable. Both cases with the NR4A2 rearrangement confirmed by FISH were also positive for the NR4A2 immunohistochemical marker (Fig. 3B-C). The first case that harbored the break was an unusual low-grade AciCC affecting the parotid gland of a 19-year-old male. It displayed a solid-trabecular growth of uniform neoplastic cells with abundant eosinophilic and occasionally clear cytoplasm and was therefore originally diagnosed as oncocytoma (Fig. 3A). FISH analysis of an NR4A3 break-apart was negative in this case. The second case was a tumor with HGT occurring in a 61-year-old male. The low-grade areas with solid-microcystic architecture gradually devolved into high-grade solid tumor displaying a conspicuous cytoplasmic clearing, nuclear atypia, and central necrosis. The NR4A3 rearrangement was not analyzable by FISH.
Overall, 17 cases were immunohistochemically negative for both NR4A3 and NR4A2 markers. Four of these were able to be tested for the NR4A3 rearrangement by FISH, and the break was confirmed in 2/4 (50%) cases. The NR4A2 break-apart was further analyzed by FISH in 3 of these cases, with a negative result in all of them.
Discussion
In this study, we present an extensive analysis of the immunohistochemical and molecular genetic features of AciCC, a common malignant salivary gland tumor driven in most cases by t(4;9)(q13;q31) leading to upregulation of the NR4A3 gene. NR4A3 immunostain is a powerful tool in differential diagnostics in AciCC cases. As much as 82% of cases were positive for NR4A3 in our series. However, previous reports documented an even higher proportion (up to 98%) of AciCC cases to be immunopositive for NR4A3 with a homogenous diffuse nuclear pattern [7, 9]. The discordance was likely caused by the inclusion of archival, sub-optimally fixed samples, which were slightly more prevalent in the low-grade cohort. The NR4A3 antigenicity was probably limited or completely lost in such cases, and the NR4A3 immunohistochemistry was apparently highly sensitive to sub-optimal processing in our laboratory. Although FISH analysis of the NR4A3 rearrangement might be informative in some of these troublesome cases, a negative result of the molecular-genetic examination does not necessarily exclude the possibility of the t(4;9)(q13;q31) being present. As indicated by Haller et al. [7], some cases with chromosomal breakpoints of chromosome 9 located higher upstream from the NR4A3 gene might show apparently normal, non-translocated fluorescence signals. Other methods might thereafter be employed to reveal the NR4A3 overexpression (qRT-PCR) and the exact location of the chromosome breaks involved in the rearrangement, especially in rare cases with unusual morphology that might render the diagnostic process exceptionally difficult. Notably, since the mechanism of oncogenesis in acinic cell carcinoma is enhancer hijacking which upregulates NR4A3, and not a gene fusion that would code for an active fusion oncoprotein, no fusion transcript is detectable by RT-PCR or RNA-sequencing.
Importantly, both low-grade and high-grade (including cases with HGT) cases were able to be stained with the NR4A3 antibody. In cases with HGT, nuclear positivity for NR4A3 was observed in both low-grade and transformed areas. NR4A3 immunostaining might therefore be utilized in challenging cases, i.e., small samples containing poorly differentiated high-grade areas only, fine-needle aspiration cytology specimens [11, 12], tumors with unusual morphology etc.
In addition, AciCC might sporadically be driven by overexpression of NR4A3 paralog NR4A2. Both genes code for proteins belonging to the steroid-thyroid hormone retinoid receptor family that act as transcription factors when activated by their respective ligands. The carcinogenetic process in tumors with the NR4A2 rearrangement is therefore likely analogous to the enhancer hijacking described in NR4A3-rearranged acinic cell carcinomas. While NR4A3 locates to 9q31, NR4A2 is found at 2q24. In this study, 6 NR4A3-immunonegative cases displayed a strong nuclear positivity with the NR4A2 antibody. NR4A2 immunoexpression was shown to correlate with NR4A2 upregulation in AciCC in a recent study [8]. This is the first report of a rearrangement involving NR4A2 being confirmed by FISH in 2 cases of AciCC. Interestingly, both cases displayed unusual morphologic features, with the first low-grade tumor resembling oncocytoma and the second tumor containing abundant clear-cell areas. Solid growth pattern was observed in both cases, similarly to the case reported by Haller et al. [8]. Further studies might contribute further evidence to clarify whether the NR4A2 rearrangement occurs more frequently in AciCC cases with less usual morphology.
Lastly, a small cohort of our cases was negative for both NR4A2 and NR4A3 immunomarkers. FISH analysis of the NR4A3 rearrangement proved more sensitive in two of these double-immunonegative cases, and they were finally classified as acinic cell carcinoma with an NR4A3 rearrangement. However, rearrangements of NR4A2 nor NR4A3 were not detected by FISH in some of these double-immunonegative cases. Members of this group may represent cases where both detection methods used in this study failed, and the tumors in fact harbor NR4A2 or NR4A3 rearrangements. Some cases may nonetheless belong to a very minor group of AciCC cases whose pathogenesis is based on a different genetic aberration, whether it is, for example, the rearrangement of other genes from the nuclear receptor subfamily 4A, or another alteration of a completely unrelated gene.
In summary, we present a first report of an NR4A2 rearrangement in a subset of acinic cell carcinoma cases. This genetic aberration was present in cases that were negative for the NR4A3 marker and positive for the NR4A2 marker on immunohistochemical examination. NR4A2 and NR4A3 code for highly similar, paralogous proteins; we therefore propose a similar pathogenetic process being involved in acinic cell carcinomas with the NR4A2 rearrangement that has been proposed previously in tumors with the NR4A3 break-apart. NR4A2 and NR4A3 are reliable immunohistochemical markers for acinic cell carcinoma whose importance is highlighted especially in challenging, less typical cases.
Data Availability
Data supporting the findings of this study are available within the article. The complete datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
WHO classification of tumours editorial board (2022) Head and neck tumours, 5th edn. IARC Press, Lyon, France
Patel NR, Sanghvi S, Khan MN, Husain Q, Baredes S, Eloy JA (2014) Demographic trends and disease-specific survival in salivary acinic cell carcinoma: an analysis of 1129 cases. Laryngoscope 124(1):172–178
Xu B, Saliba M, Ho A, Viswanathan K, Alzumaili B, Dogan S, Ghossein R, Katabi N (2022) Head and Neck Acinic Cell Carcinoma: A New Grading System Proposal and Diagnostic Utility of NR4A3 Immunohistochemistry. Am J Surg Pathol 46(7):933–941
Thompson LD, Aslam MN, Stall JN, Udager AM, Chiosea S, McHugh JB (2016) Clinicopathologic and immunophenotypic characterization of 25 cases of acinic cell carcinoma with high-grade transformation. Head Neck Pathol 10(2):152–160
Skálová A, Sima R, Vanecek T et al (2009) Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/neu genes. Am J Surg Pathol 33(8):1137–1145
Haller F, Bieg M, Will R et al (2019) Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun 10(1):368
Haller F, Skálová A, Ihrler S et al (2019) Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol 43(9):1264–1272
Haller F, Moskalev EA, Kuck S et al (2020) Nuclear NR4A2 (Nurr1) immunostaining is a novel marker for acinic cell carcinoma of the salivary glands lacking the classic NR4A3 (NOR-1) upregulation. Am J Surg Pathol 44(9):1290–1292
Wong KS, Mariño-Enriquez A, Hornick JL, Jo VY (2021) NR4A3 Immunohistochemistry reliably discriminates acinic cell carcinoma from mimics. Head Neck Pathol 15(2):425–432
Šteiner P, Andreasen S, Grossmann P et al (2018) Prognostic significance of 1p36 locus deletion in adenoid cystic carcinoma of the salivary glands. Virchows Arch 473(4):471–480
Millan N, Tjendra Y, Zuo Y, Jorda M, Garcia-Buitrago M, Velez-Torres JM, Gomez-Fernandez C (2022) Utility of NR4A3 on FNA cytology smears and liquid-based preparations of salivary gland. Cancer Cytopathol. https://doi.org/10.1002/cncy.22632. Online ahead of print.
Skaugen JM, Seethala RR, Chiosea SI, Landau MS (2021) Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol 129(2):104–113
Funding
This study was supported by study grant SVV 260539 from the Ministry of Education, Czech Republic (NK). This work was supported by the Cooperatio Program, research area SURG (NK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NK and AS. The first draft of the manuscript was written by NK, and all authors commented on previous versions of the manuscript. There are two senior authors of this manuscript (IL and AS) with equal contribution.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent was not required for the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klubíčková, N., Grossmann, P., Šteiner, P. et al. A minority of cases of acinic cell carcinoma of the salivary glands are driven by an NR4A2 rearrangement: the diagnostic utility of the assessment of NR4A2 and NR4A3 alterations in salivary gland tumors. Virchows Arch 482, 339–345 (2023). https://doi.org/10.1007/s00428-022-03464-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03464-8