Abstract
We assessed the value of cytokeratin 17 (CK17) expression for the differential diagnosis between primary ovarian mucinous tumors and metastases from the gastrointestinal tract (GIT) and the significance of CK17 expression in a broad spectrum of primary ovarian tumors with respect to their prognosis. The sample set consisted of 554 primary ovarian tumors and 255 GIT tumors. In the primary ovarian tumors, a higher CK17 expression (in > 10% of tumors cells) was present only in 0–11.4% of all tumors (including mucinous tumors, micropapillary serous borderline tumors, clear cell, endometrioid, and high-grade serous carcinomas). The only exception was low-grade serous carcinoma, where higher CK17 expression was present in 24% of cases. Concerning GIT tumors, the higher levels of CK 17 expression (in > 10% of tumor cells) were observed in the upper GIT tumors (68.5% of pancreatic ductal adenocarcinoma, 61.6% of gallbladder adenocarcinoma, and 46% of gastric adenocarcinoma), which differs substantially not only from most of the primary ovarian tumors, but also from colorectal carcinoma (3.7%; p < 0.001). The results of our study suggest that expression of CK17 can potentially be used as an adjunct marker in differential diagnosis between primary ovarian mucinous tumors and metastases from the upper GIT, but not from colorectal carcinoma. However, in GIT tumors, CK17 can be used in the differential diagnosis between adenocarcinomas of the upper and lower GIT. Statistical analysis did not reveal strong association of CK17 expression with clinicopathological variables or patient outcomes in any primary ovarian tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytokeratin 17 (CK17) is a low molecular weight cytokeratin (type 1 cytokeratin family), which is normally expressed in the ectodermal layer during embryogenesis, but is silenced in most somatic epithelial tissues [1]. In adults, CK17 can be found in the basal cells of complex glandular epithelia including myoepithelial cells of breast and salivary glands, a subset of skin adnexal epithelial cells, respiratory and prostate basal cells, and reserve cells of the endocervix [2,3,4]. Normal urothelium and squamous epithelium is usually CK17 negative, but there may be expression in regenerative, reactive, and dysplastic squamous epithelia [4,5,6,7,8,9]. Mechanistic studies showed that under the influence of mitogenic signaling, CK17 translocates into the nucleus and binds with cell cycle inhibitor p27. The CK17-p27 complex is then exported to the cytoplasm, where p27 undergoes degradation, leading to sustained cell proliferation [10]. Despite being extensively studied in several solid tumors, including gastrointestinal tract (GIT) tumors, the literature concerning CK17 expression in primary ovarian tumors, including mucinous tumors, is very limited with only one study analyzing a small series of ovarian mucinous carcinomas (n = 12), which were all negative [11]. Based on this very limited data concerning primary mucinous ovarian tumors and the fact that according to the literature expression of CK17 is common in pancreatic, biliary tract, and gastric adenocarcinomas, the possible role of CK17 in the differential diagnosis between primary ovarian mucinous tumors and metastatic adenocarcinomas, especially from the upper GIT, has been discussed [12]. However, evidence for the use of CK17 for this purpose is currently very limited. The goal of our study was to assess the expression of CK17 in a well-defined sample set of 554 primary ovarian tumors, including mucinous carcinomas (MC), mucinous borderline tumors (MBT), endometrioid carcinomas (EC), clear cell carcinomas (CCC), low-grade serous carcinomas (LGSC), micropapillary serous borderline tumors (mSBT), and high-grade serous carcinomas (HGSC) with respect to the extent of expression and its prognostic significance. A further goal was to assess the potential use of CK17 in the differential diagnosis of primary ovarian mucinous tumors and ovarian metastases (especially from the upper GIT) and in the differential diagnosis between upper and lower GIT adenocarcinomas, based on an analysis of our data (255 of GIT tumors) and the available literature.
Material and methods
Material
The archive files of participating departments from the Czech Republic and Hungary were searched for cases diagnosed as MC, MBT, EC, CCC, LGSC, mSBT, and HGSC. For all primary mucinous tumors (n = 125), the consensus diagnosis was based on the results of an international interobserver variability study (based only on HE stained slides), which included 14 participants (who are the co-authors of this study), focusing on the diagnostics of primary ovarian mucinous tumors on this sample set. The results of this study are currently undergoing preparation for publication. All EC, CCC, LGSC, mSBT, and HGSC were reviewed by two experienced pathologists (PD and KN), and only those cases fulfilling the diagnostic criteria were included in the study. The final sample set consisted of 554 primary ovarian tumors (Fig. 1) including 44 cases of MC, 81 cases of MBT, 121 cases of CCC, 52 cases of EC, 100 cases of LGSC, 43 cases of mSBT, and 113 cases of HGSC (HGSC partially represent a dataset used in a previous study) [13]. The GIT tumor sample set consists of 255 tumors including primary colorectal carcinoma (CRC; n = 104), ovarian metastases from CRC (n = 56), primary gastric adenocarcinoma (intestinal type according to Lauren classification; n = 50), primary pancreatic ductal adenocarcinoma (n = 19), and primary gallbladder adenocarcinoma (n = 26). The GIT tumor samples were selected from the archive files of the Institute of Pathology, First Faculty of Medicine, Charles University and General University Hospital in Prague (primary tumors and ovarian metastases) and the Fingerland Department of Pathology, Charles University, Faculty of Medicine in Hradec Králové and University Hospital in Hradec Králové (ovarian metastases). They represented either a dataset used for previous studies (CRC) or recently obtained cases [14].
CONSORT diagram. CCC clear cell carcinomas, EC endometrioid carcinomas, HGSC high-grade serous carcinomas, LGSC low-grade serous carcinomas, MC mucinous carcinomas, MBT mucinous borderline tumors, mSBT micropapillary serous borderline tumors, MUC primary mucinous tumors, AWD alive with disease, DOD died of disease, DOC died of other cause, DUC death of unknown cause, NED no evidence of disease, IHC immunohistochemical analysis of CK17, n number of cases
Immunohistochemical analysis
The immunohistochemical (IHC) analysis was performed using 4-μm-thick sections of formalin-fixed and paraffin-embedded (FFPE) tissue using tissue microarrays (TMAs). The eligible areas of each tumor were identified, and two tissue cores (each 2.0 mm in diameter) were taken from the donor block using the tissue microarray instrument TMA Master (3DHISTECH Ltd., Budapest, Hungary). If a representative tumor area was not present or the cores were lost to processing, new cores were taken for additional TMAs. Whole tissue sections were used in selected cases, in which the TMA approach would not be technically optimal due to small tumor size or low tumor cellularity. These included one ovarian MC with infiltrative invasion, 10 other cases of primary ovarian tumors, and 9 colorectal carcinomas. Moreover, whole tissue sections were used for all gastric adenocarcinomas including 32 cases from endoscopic biopsy, which were not eligible for TMA, and 18 cases from resection specimens (used as control group for endoscopic specimens which were in some cases limited in quantity).
The CK17 staining (clone E3, 1:200, Zeta Corporation, Sierra Madre, CA, USA) was performed by the Ventana BenchMark ULTRA (Roche, Basel, Switzerland) with the OptiView detection kit. The immunohistochemical results were assessed semi-quantitatively according to the overall percentage of positive cells (0–100%) and then also using the H-score method as described previously [15]. For the comparison of our results with the literature data concerning the results of immunohistochemical studies, cases were classified based on the overall percentage of positive cells as negative (totally negative or < 5% of positive tumor cells; CK17−) or positive (5–100% of positive tumor cells; CK17+).
Scoring
All cases were double-blinded and scored by two experienced pathologists (mucinous neoplasms, EC, GIT tumors: PD, MB; CCC, LGSC, mSBT, HGSC: KN, MB) and some cases also by a pathologist in training (GIT tumors: BB). The cases in which consensus between experienced pathologists was not reached (difference in scoring more than 10% or differing results leading to the case being assigned a different category in the 5–10%, 11–50%, and > 50% groups) were discussed and consensually scored. In EC, squamous morules were excluded from assessment.
Literature review
A review of the literature concerning the expression of CK17 in any of the tumor types included in our study was carried out. The data was collected from the PubMed database and included entries published up to May 2021. The search resulted in 379 articles using the term “keratin 17” and 451 articles using the term “cytokeratin 17.” Only 14 studies focused on the expression of CK17 in pancreatobiliary, GIT, and ovarian epithelial tumors [4, 11, 16,17,18,19,20,21,22,23,24,25,26,27].
Statistical analyses
All statistical tests were carried out using the program R (version 4.0.2, https://www.r-project.org/) or Statistica (TIBCO). Correlations between CK17 expression (CK17− vs. CK17+) and clinicopathological characteristics were analyzed by the Pearson chi-squared test or Fisher exact test. Comparison of CK17 expression (H-score) in different diagnoses was calculated using ANOVA approach (Mann–Whitney U test). All tests were two-sided, and a p value of less than 0.05 was considered significant.
For patients with available data (summarized in consort diagram, Fig. 1) time-to-event analysis was performed with four outcomes—overall survival (OS: the period from the date of diagnosis to the date of recorded death), disease-free survival (DFS: the period from the date of diagnosis to the date of death from diagnosis), local recurrence-free survival (LFS: the period from the primary diagnosis until the first local recurrence), and distant metastasis-free survival (MFS: the period from the primary diagnosis until the first distant metastasis diagnosis). The date of diagnosis was recorded as the date of the reception of the primary sample. We compared the probability of survival between negative (CK17−) and positive (CK17+) cases. The survival analyses were plotted using the Kaplan–Meier model, and the differences between curves were tested for significance using the log-rank test. If a patient did not have an event, the case was censored in a given analysis to the date of the last known follow-up. To determine whether CK17 expression is an independent prognostic factor, the multivariate Cox proportional hazard model involving CK17, age, and clinical stage was performed.
A receiver operating characteristic (ROC) curve and AUC (area under the ROC curve) analysis was performed using the “pROC” library implemented in R to evaluate the biomarker potential to discriminate different diagnostic categories. The optimal cut-off values were calculated using the “cutpointr” library in R software.
Results
The detailed results of our study together with a comparison with the available literature data are summarized in Table 1, and representative images of CK17 expression in the various neoplasms are shown in Figs. 2 and 3. The ROC analyses showed that CK17 expression can potentially be used to differentiate between primary ovarian mucinous tumors and upper GIT tumors or between upper and lower GIT tumors (Fig. 4). The AUC values were 0.802 and 0.839, respectively, which represents good discrimination. The cut-off value for the purposes of discriminating between primary mucinous ovarian tumors and upper GIT tumors was 4% of overall tumor cell positivity. For differentiating between upper GIT and lower GIT adenocarcinomas, the optimal cut-off value was 1% of overall positivity, but this should be validated on an independent sample set. We did not find any significant correlation between CK17 expression and clinicopathological variables (Supplementary Table 1).
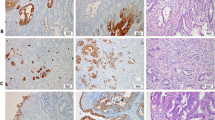
Cytokeratin 17 (CK17) expression in primary ovarian tumors. Expression of CK17 in a mucinous carcinoma with expansile and infiltrative invasion (A) (100 ×). Diffuse expression of CK17 in a mucinous carcinoma with infiltrative invasion (B) (100 ×). Expression of CK17 in squamous morules in endometrioid carcinoma. The glandular tumor cells are negative (C) (200 ×). Endometrioid carcinoma with scattered positive cells (D) (200 ×). Micropapillary serous borderline tumor with focal CK17 expression (E) (200 ×). Low-grade serous carcinoma with substantial CK17 expression (F) (200 ×)
Cytokeratin 17 (CK17) expression in gastrointestinal tumors. Focal expression of CK17 in colorectal carcinoma (A) (100 ×). Moderately differentiated pancreatic ductal carcinoma showing CK17 expression in > 50% of tumor cells (B) (100 ×). Focal expression of CK17 in gallbladder adenocarcinoma (C) (200 ×). Moderately differentiated gastric adenocarcinoma with diffuse CK17 expression (D) (100 ×)
Applicability of cytokeratin 17 immunohistochemical staining in the differential diagnosis. Graph showing receiver operating characteristic (ROC) analysis of CK17 in A 125 mucinous ovarian tumors and 95 upper GIT tumors and B 95 upper GIT and 160 lower GIT tumors. The AUC values indicate that CK17 can be used as a marker for differential diagnosis between A primary mucinous ovarian tumors and upper GIT tumors and B upper and lower GIT tumors
Ovarian mucinous tumors (MC and MBT)
Expression of CK17 was found in 18/125 (14.4%) primary ovarian mucinous tumors including 9/81 (11.1%) MBT and 3/28 (10.7%) MC with expansile invasion and 6/16 (37.5%) MC with infiltrative invasion. However, in 10/125 (8%) cases, the expression was only focal (5–10% of tumor cells). Only 7/125 (5.6%) cases showed expression in the range of 11–50% of tumor cells (3 MBT and 4 MC with infiltrative invasion), and 1/125 (0.8%) cases showed the expression in > 50% of tumor cells (MC with infiltrative invasion). In summary, only 8/125 (6.4%) of all primary ovarian mucinous tumors showed expression of CK17 in > 10% of tumor cells, and only 1/125 (0.8%) showed positivity in > 50% of tumor cells. The difference in the overall CK17 expression (H-score) between MC and MBT was not statistically significant (Mann–Whitney U test, U = 1525, Z = 1.32, p = 0.186). Similarly, there was no difference between MC with expansile invasion and MC with infiltrative invasion (U = 172, Z = -1.27, p = 0.205).
Non-mucinous primary ovarian tumors
HGSC, CCC, and EC showed generally low expression of CK17. Any extent of expression was found in 9.7% of HGSC, 3.3% of CCC, and 1.9% of EC. In 11.5% of EC, there was a focal CK17 expression present in the squamous morules, but these were excluded from the assessment. Expression in > 10% of tumor cells was found in 5.8% of HGSC, 0.8% of CCC, and 0% of EC and expression in > 50% of tumor cells in 1% of HGSC, 0.8% of CCC, and 0% of EC. On the contrary, CK17 expression in LGSC and mSBT was higher, with any extent of expression recorded in 43% and 25.6% of tumor cells, respectively. However, in mSBT, the expression was mostly focal (in < 10% of tumor cells), and only 4.6% showed expression in > 10% of tumor cells (no tumors showed expression in > 50% of tumor cells). In LGSC, expression in > 10% of tumor cells was present in 24% of cases and expression in > 50% of tumor cells in 6% of cases.
Gastrointestinal tumors
Expression of CK17 differed substantially between CRC and upper GIT tumors. CRC showed any positivity in 10.6% of cases, but this was mostly focal (< 10% of tumor cells). Higher expression (11–50% of tumor cells) occurred in only 3.7% of tumors. No CRC case showed expression in > 50% of tumor cells. When comparing primary and metastatic CRC, CK17 expression was higher in primary tumors than in metastases (any extent of expression was seen in 14.4% vs. 3.6%). On the contrary, the upper GIT tumors generally showed a higher expression of CK17 with any extent of CK17 expression observed in 58% of gastric adenocarcinomas, in 73.1% of gallbladder adenocarcinomas, and in 73.7% of pancreatic ductal adenocarcinomas. However, most tumors showed higher levels of CK17 expression (in > 10% of tumor cells), which was found in 46% of gastric adenocarcinomas, in 61.6% of gallbladder adenocarcinomas, and in 68.5% of pancreatic ductal adenocarcinomas. Moreover, high levels of CK17 expression (in > 50% of tumor cells) were present in 16% of gastric adenocarcinomas, in 23.1% of gallbladder adenocarcinomas, and in 63.2% of pancreatic ductal adenocarcinomas.
In the group of gastric adenocarcinomas, we also compared the results between biopsies (n = 32) and resection specimens (n = 18) and found a high concordance. Any extent of positivity was found in 56.2% of biopsies and in 61.2% of resection samples and positivity in > 50% of tumor cells in 15.6% of biopsies and in 16.7% of resection samples. The only difference was found in the categories of 5–10% and 11–50%, where biopsies more often were categorized in the category of 5–10% (15.6% vs 5.6%) and less frequently in the category of 11–50% (25% vs 38.9%).
Summary of literature
The data obtained from the 14 included studies were analyzed with an emphasis on the number of CK17 positive cases and on the extent of the positivity [4, 11, 16,17,18,19,20,21,22,23,24,25,26,27]. Seven of these studies used the TMA approach (one of them combined both whole tissue sections for a third of their cases and TMA for the remaining cases). The cut-offs for positivity differ among the studies with 10% cut-off used in 2 studies (in one of those the criteria was specified as “10% in any of 3 TMA cores”), 5% cut-off in 4 studies, 1% cut-off in 2 studies, and any extent of positivity in 2 studies (in one of those the expression had to be of strong intensity). In 4 studies, the cut-off value based on the percentage of positive cells was not specified, and in one of those, the detailed results were not available. There was a total of 671 pancreatobiliary tumors, which were categorized as primary pancreatic carcinomas, primary gallbladder carcinomas, primary intrahepatic, and extrahepatic biliary carcinomas or grouped together into the category of pancreatobiliary carcinomas. Out of all cases, expression of CK17 was found in 415 (61.9%) cases. Studies dealing with primary colorectal adenocarcinomas included 409 cases, of which 156 (38.1%) showed CK17 expression. Studies concerning gastric adenocarcinoma included 362 cases, from which 39.8% showed CK17 expression.
Prognostic significance of cytokeratin 17 expression
A significant relationship between the expression of CK17 and age was observed in the group of 113 HGSCs. However, the ANOVA analysis with the H-score as a continuous variable did not confirm a significant correlation (p = 0.199). We did not find any other significant correlations between the CK17 expression and clinicopathological variables in other examined histological types of ovarian cancer (Supplementary Table 1).
To investigate the prognostic value of CK17 expression in ovarian tumors, we performed a time-to-event analysis with a total of four outcomes (OS, DFS, LFS, and MFS) for all histological subgroups of ovarian tumors with respect to CK17 expression (Supplementary Table 2). A sufficient number of events for survival analyses were found in the subgroups of HGSC and LGSC, where CK17 expression had no effect on survival rates.
Discussion
Expression of CK17 has been found to be an adverse prognostic factor in several tumors, including squamous cell carcinoma and adenocarcinoma of the uterine cervix, high-grade endometrial carcinoma, breast carcinoma, gastric adenocarcinoma, gallbladder adenocarcinoma, CRC, pancreatic adenocarcinoma, esophageal carcinoma, and head and neck squamous cell carcinoma [6, 17, 18, 20,21,22, 28,29,30,31,32,33]. One recent study also analyzed the significance of CK17 expression as a predictive marker of response to chemotherapy in pancreatic adenocarcinoma [34].
In primary ovarian carcinomas, the prognostic significance of CK17 expression has been assessed in only one study which included 87 “serous” and 17 “non-serous” carcinomas [22]. The authors found that the expression of CK17 is statistically significantly correlated with tumor stage and overall survival, but they did not perform their analyses according to the histological tumor type. According to our results, no statistically significant association between CK17 expression and clinicopathological characteristics was found. In contrast to the study mentioned above, we did not detect any correlation between CK17 expression and survival for any of the analyzed ovarian tumor types. Our results are in accordance with a recent study focusing on pan-cancer analysis and oncogenic role of CK17 based on its expression profile data from publically available databases, in which the authors found no relation between CK17 expression in ovarian serous carcinoma and OS or DFS [33].
Only a few studies have focused on the possible use of CK17 as an adjunct in helping to diagnose the primary site of the tumor [24, 26]. These studies focused mostly on tumors of the GIT, especially on the differential diagnosis between pancreatic/pancreatobiliary carcinoma, gastric carcinoma, and colorectal carcinoma. However, the value of CK17 expression in the differential diagnosis of primary ovarian mucinous tumors and ovarian metastases (especially from the GIT) has never been analyzed in detail. It is well known that in the past a significant number of metastatic tumors were diagnosed as primary ovarian MCs or even MBTs [35,36,37]. This knowledge, together with an improved understanding of the features which can be helpful in the differential diagnosis of these tumors, allows us to differentiate between primary and metastatic mucinous tumors in most cases. Achieving the correct diagnosis should be based on the combination of macroscopic, microscopic, immunohistochemical, and clinicopathological features [38,39,40,41,42,43].
Immunohistochemistry can be very helpful for the diagnostic procedure, but we should be aware of certain limitations. In general, there is no single antibody which can be used alone, and a panel of antibodies is always needed, as we have summarized in our recent review [38]. For metastases from the lower GIT (colorectal and appendiceal tumors), a combination of cytokeratin 7 (CK7), cytokeratin 20 (CK20), CDX2, SATB2, and PAX8 is commonly used. Recent study of these markers showed that the most effective combination is CK7 and SATB2, which outperformed the usual immunostaining set of CK7, CK20, and CDX2 [44]. However, we should be aware that primary ovarian mucinous tumors arising in teratoma can have the same immunohistochemical profile as lower GIT tumors. The results of our study showed that CK17 has no value in this differential diagnosis, as its expression is low in both primary ovarian mucinous tumors and CRC.
The differential diagnosis between primary ovarian mucinous tumors and metastases from the upper GIT (including pancreas, biliary tree, and stomach) is more complicated. This is partly due to the fact that especially metastases from the pancreatobiliary tree are well known for mimicking the morphology of primary ovarian mucinous tumors because of the “maturation-effect,” with areas mimicking benign and borderline mucinous neoplasia [45,46,47,48]. Moreover, the use of immunohistochemistry in this setting is rather limited and antibodies used in the differential diagnosis between primary ovarian mucinous tumors, and metastases from the lower GIT tract mentioned above are (with the exception of PAX8) useless in the distinction from upper GIT metastases [38]. Moreover, the loss of DPC4 expression has been reported in about 45–58% of pancreatic carcinomas and 43% of extrahepatic cholangiocarcinomas, but only rarely (in about 2% of cases) in primary ovarian mucinous tumors [49,50,51]. Nevertheless, despite its high specificity for metastases from pancreatic/pancreatobiliary tract, the sensitivity is rather low, and as such DPC4 is not helpful in the differentiation from gastric adenocarcinoma, as > 95% of gastric tumors retain DPC4 expression [51]. No other immunohistochemical marker proved to be useful in this setting. Based on this, other markers which can have value in the differential diagnosis of primary ovarian mucinous tumors and metastases from the upper GIT are needed. One of the markers mentioned in this setting might be CK17, but the evidence for use of CK17 for this purpose is currently very limited.
According to the published literature, expression of CK17 occurs in approximately 77% (range 60–88.2%) of pancreatic and 60.5% (range 53.2–92.2%) of pancreatobiliary carcinomas [4, 11, 16, 19, 20, 22, 24, 26, 27]. Concerning gastric carcinomas, one study showed that CK17 was negative in all of tumors studied [24]. However, other studies found expression of CK17 in a substantial number of gastric carcinomas (range 27.5–52.4%) [4, 18, 26]. Some studies also focused on the possible role of CK17 expression in the differential diagnosis between upper and lower GIT tumors. Some of these reported that CK17 is negative in colorectal carcinomas, but others did not confirm this and found CK17 positivity in a broad range (5.6–68.2%) of cases [4, 21, 24, 26, 27]. The results of our study confirm the high expression of CK17 not only in primary pancreatic and gallbladder adenocarcinoma but also in gastric adenocarcinoma (73.7%, 73.1%, and 58% positivity, respectively), with expression in greater than 10% of tumor cells in 68.5%, 51.6%, and 46% of cases, respectively, and expression in > 50% of tumor cells in 63.2%, 23.1%, and 16% of cases, respectively. On the contrary, colorectal carcinomas showed CK17 positivity in only 10.6% of cases (14.4% of primary and 3.6% of metastases), and expression in greater than 10% of tumor cells was seen in only 3.7% of cases (5.8% of primary tumors and 0% of metastases). No case showed expression in > 50% of tumor cells. Based on this data, the expression of CK17 can be used as a useful adjunct marker in the differential diagnosis between colorectal and pancreatobiliary carcinomas, especially if the extent of the positivity is taken into account.
The literature concerning the expression of CK17 in primary ovarian carcinomas is very limited, and the results are equivocal. We found only 5 studies in which CK17 expression was assessed in ovarian carcinoma [4, 11, 22, 24, 25]. Only one of these studies analyzed CK17 expression in mucinous ovarian carcinoma (n = 12), and all cases were negative [11]. All cases of serous carcinoma in the same study (n = 41) were also negative. In our study, we used a cut-off of at least 5% of tumor cells for positivity. Using this cut-off, any extent of CK17 expression was present in 14.4% of mucinous tumors, but in 8%, the expression was only focal (≤ 10% of tumor cells). Expression in > 10% of tumor cells was present in only 6.4% of cases, and expression in > 50% of tumor cells in 0.8% of cases. However, if we stratified CK17 expression according to the subgroups of mucinous tumors (MBT, MC with expansile growth pattern, and MC with infiltrative growth pattern), the expression was highest in MC with infiltrative growth pattern (37.5% of any positivity, 25% in the range > 10–50% of cases, and 6.3% with expression in > 50% of tumor cells). This may reflect generally worse prognosis of MC with infiltrative invasion comparing to MC with expansile invasion. However, these results are limited as the total number of MC cases with infiltrative invasion was low (n = 16).
Concerning the expression of CK17 in other primary ovarian tumors, one study analyzed 104 cases (87 serous carcinomas and 17 “non-serous” carcinomas without further specification) and stratified CK17 expression based on the extent of expression and staining intensity into low and high categories [22]. High CK17 expression was found in 55.1% of serous carcinomas and 47.0% of the non-serous tumors. Other studies found CK17 expression in 11/15 (73.3%) ovarian serous carcinomas, in 9/10 cases of MBT, and in 14.3% of 24 cases of ovarian “non-mucinous carcinoma” (without further specification) [4, 24, 25]. Our results show that CK17 expression in the group of “non-mucinous” carcinomas is variable, with highest expression observed in LGSC (43% of cases) and low expression in HGSC (9.7% of cases), CCC (3.3% of cases), and EC (1.9%). However, with the exception of LGSC, higher levels of expression (> 10% of tumor cells) are generally rare and found in 5.8% of HGSC, 0.8% of CCC, and 0% of EC. Interestingly, CK17-positive cells have recently been described as one of the five major secretory cell subtypes found in normal Fallopian tube [52].
Finally, the limitations of our study need to be addressed. One limitation is related to the TMA approach, which, despite its wide use, bears the risk of underestimating or overestimating the scoring. However, due to the size of the cores we used and their duplication, this risk is relatively low. Another limitation is based on the fact that in the upper GIT tumors, the CK17 expression was assessed (with one exception) in primary tumors only, and as such, we cannot exclude the possibility that CK17 expression levels change in metastatic lesions. We compared the expression between primary and metastatic tumors in CRC, and the expression levels of CK17 were lower in metastases. Another limitation is related to the absence of an independent sample set. The results of our study, including the assessment of optimal cut-off, require further confirmation by subsequent independent validation studies.
Conclusion
In conclusion, the results of our study suggest that CK17 can be a useful adjunct marker in the differential diagnosis between primary ovarian mucinous tumors and metastases from the upper GIT. Based on the results of our study, we suggest adding of CK17 to the immunohistochemical panel used in this differential diagnosis, together with DPC4 and PAX8. However, the extent of CK17 expression should always be considered. The best discriminatory threshold based on the results of our study seems to be 4% (5% from a practical point of view) for the distinction between primary ovarian mucinous tumor and upper GIT metastases. However, this cut-off may be problematic for routine use and should be validated on an independent set for confirmation. Nevertheless, CK17 expression in > 50% of tumor cells was present only in 0.8% of primary ovarian mucinous tumors and in 27.4% of upper GIT tumors. If a tumor with diffuse CK17 expression is encountered in practice, the probability of primary ovarian mucinous tumor diagnosis is very low. CK17 expression, however, is not useful in the differential diagnosis between a primary ovarian mucinous neoplasm and a metastasis from CRC, as these tumors show a similar extent of positivity. Nevertheless, it can be used as an adjunct marker when differentiating between upper and lower GIT tumors.
Data availability
All data generated or analyzed during this study is included in this published article (and its Supplementary information files).
References
Moll R, Divo M, Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129(6):705–733
Troyanovsky SM et al (1989) Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci 93(Pt 3):419–426
Guelstein VI et al (1988) Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer 42(2):147–153
Miettinen M et al (1997) Keratin 17: immunohistochemical mapping of its distribution in human epithelial tumors and its potential applications. Applied Immunohistochemistry 5(3):152–159
Guelstein VI et al (1993) Immunohistochemical localization of cytokeratin 17 in transitional cell carcinomas of the human urinary tract. Virchows Arch B Cell Pathol Incl Mol Pathol 64(1):1–5
Escobar-Hoyos LF et al (2014) Keratin 17 in premalignant and malignant squamous lesions of the cervix: proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod Pathol 27(4):621–630
Maddox P et al (1999) Differential expression of keratins 10, 17, and 19 in normal cervical epithelium, cervical intraepithelial neoplasia, and cervical carcinoma. J Clin Pathol 52(1):41–46
Regauer S, Reich O (2007) CK17 and p16 expression patterns distinguish (atypical) immature squamous metaplasia from high-grade cervical intraepithelial neoplasia (CIN III). Histopathology 50(5):629–635
Podoll MB et al (2017) Assessment of CK17 as a marker for the diagnosis of differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol 36(3):273–280
Escobar-Hoyos LF et al (2015) Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res 75(17):3650–3662
Goldstein NS, Bassi D, Uzieblo A (2001) WT1 is an integral component of an antibody panel to distinguish pancreaticobiliary and some ovarian epithelial neoplasms. Am J Clin Pathol 116(2):246–252
Ackroyd SA et al (2019) Pancreaticobiliary metastasis presenting as primary mucinous ovarian neoplasm: a systematic literature review. Gynecol Oncol Rep 28:109–115
Nemejcova K et al (2021) A comprehensive analysis of the expression, epigenetic and genetic changes of HNF1B and ECI2 in 122 cases of high-grade serous ovarian carcinoma. Oncol Lett 21(3):185
Bartu M et al (2020) Expression, epigenetic, and genetic changes of HNF1B in colorectal lesions: an analysis of 145 cases. Pathol Oncol Res 26(4):2337–2350
Dundr P et al (2021) Uterine cellular leiomyomas are characterized by common HMGA2 aberrations, followed by chromosome 1p deletion and MED12 mutation: morphological, molecular, and immunohistochemical study of 52 cases. Virchows Arch 480(2):281–289
Yang HS et al (2012) Clinical significance of MUC1, MUC2 and CK17 expression patterns for diagnosis of pancreatobiliary arcinoma. Biotech Histochem 87(2):126–132
Roa-Pena L et al (2019) Keratin 17 identifies the most lethal molecular subtype of pancreatic cancer. Sci Rep 9(1):11239
Ide M et al (2012) Keratin 17 expression correlates with tumor progression and poor prognosis in gastric adenocarcinoma. Ann Surg Oncol 19(11):3506–3514
Lok T et al (2014) Immunohistochemical distinction between intrahepatic cholangiocarcinoma and pancreatic ductal adenocarcinoma. Hum Pathol 45(2):394–400
Kim K et al (2017) Cytokeratin 17 expression is associated with poor prognosis in gallbladder adenocarcinoma. Appl Immunohistochem Mol Morphol 25(5):346–350
Ujiie D et al (2020) KRT17 as a prognostic biomarker for stage II colorectal cancer. Carcinogenesis 41(5):591–599
Wang YF et al (2013) Overexpression of keratin 17 is associated with poor prognosis in epithelial ovarian cancer. Tumour Biol 34(3):1685–1689
Carrasco C et al (2021) The evaluation of 17 gastrointestinal tumor markers reveals prognosis value for MUC6, CK17, and CD10 in gallbladder-cancer patients. Diagnostics (Basel) 11(2):153
Chu PG et al (2005) Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 29(3):359–367
Hamada T et al (2008) Immunohistochemical analysis of reserve cell-like cells of ovarian mullerian mucinous/mixed epithelial borderline tumor. Int J Gynecol Pathol 27(2):199–206
Sarbia M et al (2007) Differentiation between pancreaticobiliary and upper gastrointestinal adenocarcinomas: is analysis of cytokeratin 17 expression helpful? Am J Clin Pathol 128(2):255–259
Kim CY et al (2012) Proteomic analysis reveals overexpression of moesin and cytokeratin 17 proteins in colorectal carcinoma. Oncol Rep 27(3):608–620
Mockler D et al (2017) Keratin 17 is a prognostic biomarker in endocervical glandular neoplasia. Am J Clin Pathol 148(3):264–273
Merkin RD et al (2017) Keratin 17 is overexpressed and predicts poor survival in estrogen receptor-negative/human epidermal growth factor receptor-2-negative breast cancer. Hum Pathol 62:23–32
Regenbogen E et al (2018) Elevated expression of keratin 17 in oropharyngeal squamous cell carcinoma is associated with decreased survival. Head Neck 40(8):1788–1798
Zeng Y et al (2020) Keratin 17 suppresses cell proliferation and epithelial-mesenchymal transition in pancreatic cancer. Front Med (Lausanne) 7:572494
Bai JDK et al (2019) Keratin 17 is a negative prognostic biomarker in high-grade endometrial carcinomas. Hum Pathol 94:40–50
Li C et al (2021) A pan-cancer analysis of the oncogenic role of keratin 17 (KRT17) in human tumors. Transl Cancer Res 10(10):4489–4501
Pan CH et al (2020) An unbiased high-throughput drug screen reveals a potential therapeutic vulnerability in the most lethal molecular subtype of pancreatic cancer. Mol Oncol 14(8):1800–1816
Seidman JD, Kurman RJ, Ronnett BM (2003) Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 27(7):985–993
Platz CE, Benda JA (1995) Female genital tract cancer. Cancer 75(1 Suppl):270–294
Mink PJ, Sherman ME, Devesa SS (2002) Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978-1998. Cancer 95(11):2380–9
Dundr P et al (2021) Primary mucinous ovarian tumors vs ovarian metastases from gastrointestinal tract, pancreas and biliary tree: a review of current problematics. Diagn Pathol 16(1):20
McCluggage WG, Wilkinson N (2005) Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology 47(3):231–247
McCluggage WG, Young RH (2005) Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol 22(1):3–32
McCluggage WG (2012) Immunohistochemistry in the distinction between primary and metastatic ovarian mucinous neoplasms. J Clin Pathol 65(7):596–600
Hu J et al (2018) The pathologic distinction of primary and metastatic mucinous tumors involving the ovary: a re-evaluation of algorithms based on gross features. Ann Diagn Pathol 37:1–6
Talia KL, Parra-Herran C, McCluggage WG (2022) Ovarian mucinous and seromucinous neoplasms: problematic aspects and modern diagnostic approach. Histopathology 80(2):255–278
Meagher NS et al (2019) A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ovarian mucinous tumors from colorectal and appendiceal metastases. Mod Pathol 32(12):1834–1846
Park CK, Kim HS (2018) Clinicopathological characteristics of ovarian metastasis from colorectal and pancreatobiliary carcinomas mimicking primary ovarian mucinous tumor. Anticancer Res 38(9):5465–5473
Meriden Z et al (2011) Ovarian metastases of pancreaticobiliary tract adenocarcinomas: analysis of 35 cases, with emphasis on the ability of metastases to simulate primary ovarian mucinous tumors. Am J Surg Pathol 35(2):276–288
Yoshida H et al (2021) Gross mucinous multinodular appearance aids in the identification of ovarian metastases in low-grade appendiceal mucinous neoplasms during intraoperative consultation. Ann Diagn Pathol 50:151641
Young RH, Hart WR (1989) Metastases from carcinomas of the pancreas simulating primary mucinous tumors of the ovary. A report of seven cases. Am J Surg Pathol 13(9):748–56
Alghamdi S, Alghaashamy K, Pinto A (2020) Expression of SMAD4 is retained in most gynecologic tumors with mucinous differentiation. Int J Gynecol Pathol 39(5):493–497
Zapata M, Cohen C, Siddiqui MT (2007) Immunohistochemical expression of SMAD4, CK19, and CA19-9 in fine needle aspiration samples of pancreatic adenocarcinoma: utility and potential role. Cytojournal 4:13
Ritterhouse LL et al (2019) Loss of SMAD4 protein expression in gastrointestinal and extra-gastrointestinal carcinomas. Histopathology 75(4):546–551
Hu Z et al (2020) The repertoire of serous ovarian cancer non-genetic heterogeneity revealed by single-cell sequencing of normal fallopian tube epithelial cells. Cancer Cell 37(2):226-242 e7
Acknowledgements
The authors wish to thank Mgr. Zachary Harold Kane Kendall, B.A. (Institute for History of Medicine and Foreign Languages, First Faculty of Medicine, Charles University) for the English proofreading.
Funding
This work was supported by the Ministry of Health, Czech Republic (MH CZ DRO-VFN 64165 and AZV NV19-03–00007); by Charles University (Project UNCE204065); and by the European Regional Development Fund (EF16_013/0001674 and BBMRI_CZ LM2018125).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors participated on material preparation, data collection, or analyses. The first draft of the manuscript was written by Pavel Dundr, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study has been approved by the Ethics Committee of General University Hospital in Prague in compliance with the Helsinki Declaration (No. 2140/19 S-IV). The Ethics Committee waived the requirement for informed consent; as according to the Czech Law (Act. no. 373/11, and its amendment Act no. 202/17), it is not necessary to obtain informed consent in fully anonymized studies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dundr, P., Bazalová, B., Bártů, M. et al. The cytokeratin 17 expression in primary ovarian tumors has diagnostic but not prognostic significance. Virchows Arch 481, 201–212 (2022). https://doi.org/10.1007/s00428-022-03338-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03338-z