Abstract
Spindle cell squamous cell carcinoma (SpC-SCC) is rare, accounting for 0.4–4% of head and neck (HN) SCCs. Better understanding of HN SpC-SCC clinicopathologic characteristics, especially features that predict outcome, is needed. We present a clinicopathologic review of 71 HN mucosal SpC-SCC from three tertiary centers. The patient population showed a median age of 63 years (range 20–91), slight male predominance (M:F = 1.6:1), and a preponderance of smokers/ex-smokers (45/71, 64%). Most lesions involved oral cavity (42/71, 59%), especially oral tongue (n = 18), and larynx (n = 20, 28%). Polypoid/exophytic growth and surface ulceration were seen in 60% and 86% of cases, respectively. Histologically, most tumors showed sarcoma-like pattern (65/70, 93%), the remaining exhibiting granulation tissue-like or fibromatosis-like patterns, and 5 lesions showed osteosarcomatous/chondrosarcomatous elements. Most tumors (53/71, 74%) showed a conventional SCC (C-SCC) component, keratinizing (86%) or non-keratinizing/basaloid (14%). Nodal metastases, seen in 22 (31%) of resection specimens, showed SpC-SCC and/or C-SCC histomorphology. By immunohistochemistry, 76% of lesions showed immunoreactivity for keratin and 62/60% of lesions were p40/p63 positive. Ki-67 proliferation index ranged from 5 to 70%. Follow-up was available on 69 patients, median of 1.1 years from the time of SpC-SCC diagnosis. The 3-, 5-, and 10-year disease-specific survival (DSS) was 62, 37, and 12%, respectively. AJCC pN stage was an independent prognostic factor for DSS and distant metastasis-free survival (DMFS), whereas the presence of C-SCC was independently associated with improved DMFS. HN SpC-SCC is rare and might be diagnostically challenging. AJCC pN stage and co-existing C-SCC component appear to be prognostically relevant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck spindle cell squamous cell carcinoma (HN SpC-SCC) is an unusual variant of squamous cell carcinoma (SCC) that accounts for approximately 0.4 to 4% of all HN SCCs [1,2,3,4,5]. The most frequent affected anatomic sub-sites are the oral cavity [3, 5,6,7,8,9] or larynx [2, 10,11,12,13]. Although historically considered either carcinosarcoma or pseudosarcoma [4, 14], molecular and ultrastructural studies have shown that HN SpC-SCC represents a heavily dedifferentiated malignant process derived from and gnomically related to pre-existing conventional squamous cell carcinoma (C-SCC) [15,16,17], possibly through epithelial-to-mesenchymal transition. The latter is a process in which epithelial tumors lose their characteristic epithelial features and acquire a mesenchymal-like phenotype [18, 19].
The importance of recognizing HN SpC-SCC is crucial not only because it can be confused with true sarcoma or other mesenchymal lesion diagnostically, but also because this SCC variant has been reported to have worse prognosis when compared to C-SCC [3], particularly in tumors arising in the oral cavity [2, 3], oropharynx [3], and hypopharynx [1].
Given the relative paucity of clinicopathologic studies on HN SpC-SCC, we aimed to study the detailed histopathologic features and the clinical course in this retrospective multi-institutional study.
Methods
Following Institutional Review Board (IRB) approval from the participating institutions, a search was performed of the respective Anatomic Pathology Department files from Memorial Sloan Kettering Cancer Center (MSKCC, New York, USA), University of Alabama at Birmingham (UAB, Birmingham, USA), and Sunnybrook Health Sciences Centre (SHSC, Toronto, Canada). A total of 71 cases of SpC-SCC originating from HN mucosal sites diagnosed between 2000 and 2019 were selected (44 from MSKCC, 20 from UAB, and 7 from SHSC). Cases that originated from skin or metastases from unknown primary were excluded from the study. All cases were centrally reviewed by at least two HN pathologists (BX and NK) and key clinical and histopathologic parameters were recorded.
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, Armonk, NY, USA). Follow-up data was available in 69 cases. Overall survival (OS), disease-specific survival (DSS), disease-free survival (DFS), and distant metastasis-free survival (DMFS) were calculated from either the date of squamous cell carcinoma diagnosis of any type or from the date of the first SpC-SCC. Prognostic value of each clinicopathologic feature was determined using univariate log rank test. Factors significant on univariate analysis were subsequently subjected to multivariate analysis using Cox proportional hazards model. P values less than 0.05 were considered to be statistically significant.
Results
Clinical features of the patients with HN SpC-SCC
The clinicopathologic parameters of the study cohort are shown in Table 1. The median age at diagnosis of SpC-SCC was 63 years (range 20–91). There was a male predominance with a male to female ratio of 1.6:1. Although the majority of patients were either current or former smokers (n = 45, 64%), 25 individuals were never smokers.
Lesions of SpC-SCC occurred most frequently in oral cavity (42/71, 59%), in particular oral tongue (n = 18), followed by larynx (n = 20, 28%), oropharynx (n = 4, 6%), hypopharynx (n = 3, 4%), and sinonasal tract (n = 2, 3%).
Not all patients presented with SpC-SCC. In this cohort, the tumor type at first presentation was mixed C-SCC and SpC-SCC in 31 patients (44%), pure C-SCC in 30 patients (42%), and pure SpC-SCC in the remaining 10 individuals (14%). Out of the 10 patients who presented with pure SpC-SCC, three had their diagnosis made on a biopsy specimen and not on a surgical resection. Thirty patients (42%) had a history of C-SCC with one or multiple (up to 4) recurrences prior to the development of SpC-SCC. Among them, the median time between the first onset of C-SCC and SpC-SCC was 4.7 years (range 0.3–20.5).
Pathologic characteristics of the tumors
SpC-SCC was diagnosed in primary resection/wide local excision specimen (n = 65, 92%), small biopsies (n = 4, 6%), incisional biopsies (n = 1, 1%), and neck lymph node dissections (n = 1, 1%).
The median tumor size was 2.5 cm (range 0.3–10.0), the median tumor thickness was 11 mm (range 0.3–55), and the median depth of invasion was 8 mm (range 0–55).
A minority of lesions showed pure polypoid/exophytic growth (13/68, 19%), 27 (40%) tumors showed pure endophytic growth, while the remaining 28 (41%) had both endophytic and exophytic components.
A C-SCC component was seen in 53 tumors (74%). Among them, the SpC-SCC components accounted for a median of 70% of total tumor volume (range: 3–100%) and appeared to be intermixed with the SpC-SCC in 74% (39/53). In the remaining 14 lesions, the spindle cell component was separated from the C-SCC. The C-SCC component was keratinizing in 86% and non-keratinizing/basaloid in 14%. The histologic grade of C-SCC was well-differentiated with verrucous features in 6%, moderately differentiated in 65%, and poorly differentiated in 29%.
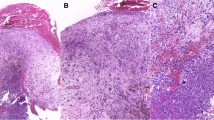
The most common histologic features of SpC-SCC were sarcoma-like (seen in 65 patients, 92%) with pleomorphic to spindle tumor cells in an intervening to storiform fascicular arrangement, and granulation tissue-like (seen in 13%) with pleomorphic malignant cells in an abundant inflammatory background with or without myxoid stroma (Fig. 1). In 4 cases, both patterns co-existed. There was also one case demonstrating a pauci-cellular fibromatosis-like pattern with scattered pleomorphic tumor cells in a fibrotic background.
Histologic features of head and neck spindle cell squamous cell carcinoma (SpC-SCC). A A laryngeal SpC-SCC with exclusive polypoid growth and ulcerated surface. B A SpC-SCC of the oral tongue with ulcerated surface and an associated conventional squamous cell carcinoma (C-SCC) component. Note that the two components are located separately (C-SCC left and SpC-SCC right) in this tumor. C Sarcoma-like SpC-SCC with tumor cells arranged as loose intervening fascicles. D Granulation tissue-like SpC-SCC with malignant pleomorphic cells scattered within a myxoid stroma enriched with inflammatory infiltrate. Note the presence of multiple mitotic figures. E A SpC-SCC and C-SCC intimately intertwined with each other (intermixed pattern). F Both components are positive for CK5/6 immunostaining
Divergent differentiation (heterologous components) was identified in five cases (7%) with four cases showing osteosarcomatous differentiation and one containing a chondrosarcomatous component (Fig. 2).
Surface ulceration was extremely common, being present in 86% (57/66) of cases. The rate of lymphovascular invasion, perineural invasion, and positive surgical margins was 17, 31, and 30% respectively. The pT stage defined by the 8th Edition AJCC staging system was pT1/T2 in 46% and pT3/pT4 in 54%.
Twenty-two (31%) patients had lymph node metastasis at the time of resection for SpC-SCC. The morphology of the lymph node metastases was mixed SpC-SCC and C-SCC in seven (33%), pure SpC-SCC in six (29%), and pure C-SCC in eight (38%).
Immunoprofile of SpC-SCC
Immunohistochemical evaluation was performed in a subset, and the results are shown in Table 1. Overall, 76% (31/43) of SpC-SCC showed immunopositivity of one or more cytokeratins, including AE1/AE3 in 61%, CAM5.2 in 44%, CK18 in 33%, 34BE12 in 64%, and CK5/6 in 64%. The extent of immunopositivity was highly variable, ranging from rare cells to focal to diffuse. The frequency of immunopositivity for p40 and p63 was 62 and 60%, respectively. Myogenic markers were immunoreactive in a subset of tumors, including smooth muscle actin (SMA) in 47% (9/19) and desmin in 28% (5/18). Myogenin and S100 were consistently negative in all cases tested (n = 7 and 31, respectively). Ki-67 proliferation index ranged from 5 to 70% with a median of 40%. Four cases originated from the oropharynx, all of which were negative for p16 by immunostaining and negative for high-risk HPV by in situ hybridization (one using DNA probe, and the others using RNA probe).
Follow-up and outcome
Follow-up data was available in 69 patients with a median follow-up time of 2.5 years (range 0.1–21.7 years) from first SCC diagnosis and 1.1 years from the first SpC-SCC diagnosis (range 0.1–14.7 years). Among them, 50 patients had recurrence, including 14 (20%) with distant metastasis, 11 (16%) with regional metastasis, and 37 (54%) with local recurrence. The majority of patients received adjuvant radiation (48/69, 70%) and/or chemotherapy (26/69, 38%).
The 3-, 5-, and 10-year OS was 54, 33, and 11%, respectively, from the diagnosis of SpC-SCC, and 74, 63 and 44%, respectively, from first diagnosis of SCC of any type. Similarly, the 3-, 5-, and 10-year DSS was 62, 37, and 12% from the diagnosis of SpC-SCC, and 77, 68, and 51% from first diagnosis of SCC (any type). The 3-year DFS and DMFS were 18 and 65% from first SpC-SCC diagnosis, and 43 and 77% from first SCC diagnosis.
The results of univariate survival analysis using log rank test are shown in Table 2 and the Kaplan–Meier curves are shown in Fig. 3. From the first SpC-SCC diagnosis, significant adverse prognostic factors included advanced AJCC pT and pN stage in predicting DSS, advanced AJCC pT stage in predicting DFS, and the absence of C-SCC component and large tumor size in predicting DMFS. From the first SCC diagnosis of any type, oral tongue location was associated with improved DSS compared with other sites. Other clinical and pathologic parameters did not impact survival.
We additionally compared the survival between laryngeal tumors and other anatomic sub-sites, as well as between oropharyngeal tumors and other anatomic sub-sites. The prognosis did not differ (data not shown, p > 0.05). When the analysis was restricted to oral cavity, DOI predict DFS (p = 0.011) but not DMFS or DSS (p > 0.05), whereas tumor thickness did not predict clinical outcome, including DSS, DFS, and DMFS (p > 0.05).
On multivariate analysis using Cox proportional hazards model, independent adverse prognostic factors identified were high AJCC pN stage for DSS (hazard ratio HR = 1.551, 95% confidence interval CI 1.068–2.252, p = 0.021); high AJCC pN stage for DMFS (HR = 1.463, 95% CI 1.046–2.047, p = 0.026), and absence of co-existing C-SCC component (HR = 4.857, 95% CI 1.690–13.959, p = 0.003) for DMFS. Other factors that were significant on univariate survival analysis failed to reach significance on multivariate analysis.
Discussion
SpC-SCC in the HN region is rare. The frequency of SpC-SCC among all HN SCC diagnosed was 0.4%to 0.6% in studies based on Surveillance, Epidemiology, and End Results (SEER) data [2, 3], and 0.6 to 4.2% in center-based retrospective cohort [1, 4, 5]. Given its rarity, large-scale comprehensive clinicopathologic studies on HN SpC-SCC are scant in the literature.
HN SpC-SCC commonly occurs in elderly patients but may impact a wide age range including young adults. The reported median age was 51 to 73.5 years and the age of diagnosis ranges from 18- to 90-year-old [1, 4, 6,7,8, 13, 15, 20,21,22,23]. All studies [1, 4, 5, 8, 10, 13, 15, 16, 20, 21] showed a male predilection for HN SpC-SCC, with a wide male to female ratio ranging from exclusively male (i.e., 62:0) [1] to 2:1 [6, 16]. Recognized risk factors for HN SCC were also commonly found in SpC-SCC, including smoking and other forms of tobacco consumptions, being reported in 66 to 96% of SpC-SCC patients [1, 4, 9, 13, 15], and alcohol usage in 24 to 96% [1, 4, 9, 13]. Such demographic findings were confirmed by the current study. In our cohort, the median age of diagnosis was 63 (range 18–91) and 64% of patients had a history of smoking. Although we also demonstrated a male predominance, the male to female ratio was the lowest in the literature, being 1.6:1.
The most common anatomic site of HN SpC-SCC in our cohort was the oral cavity (62%) followed by larynx (28%), confirming what have been previously reported with either oral cavity [3, 5,6,7,8,9] or larynx [2, 10,11,12,13] as the most frequent origin of HN SpC-SCC. A small percentage of HN mucosal SpC-SCC might also occur in the sinonasal tract, hypopharynx, oropharynx, and nasopharynx [2, 5, 6, 8,9,10,11,12,13].
An intriguing yet common histologic finding of HN SpC-SCC is that it is often polypoid with surface ulceration. In our cohort, 60% of the SpC-SCC had a polypoid/exophytic component, and 86% had ulceration. This histologic feature of HN SpC-SCC has already been noted in the early literature on SpC-SCC by Lane in 1957 [14] and Hyams et al. in 1975 [24], and was subsequently confirmed by multiple studies including ours. The reported frequency of polypoid configuration in HN SpC-SCC ranges from 33 to 99% [4, 8,9,10,11, 15, 16] and ulceration from 67 to 95% [8, 9, 15, 16].
In our cohort, 75% of tumors were associated with a C-SCC component and 42% had carcinoma in situ (CIS) or pre-cancerous surface alterations (e.g., dysplasia or verrucous hyperplasia). Similarly, an associated invasive C-SCC or CIS/dysplasia was reported in 31 to 84% [4, 8, 13, 15, 17] and 15 to 25% [8, 15] of HN SpC-SCC in the literature. Interestingly, the association with C-SCC and or CIS/dysplasia seems to be common and, in our opinion, it is the most helpful histologic feature in distinguishing a SpC-SCC from a true sarcoma. The SpC-SCC and C-SCC component might be seen individually or in combination in primary tumor, recurrence, and nodal metastasis as demonstrated in the current study and the literature [5, 8, 23].
When an associated epithelial component is absent, immunohistochemistry studies to demonstrate tumor immunopositivity for epithelial markers (e.g., low and high molecular weight cytokeratins) and squamous markers (e.g., p63 and p40) are useful diagnostic tools. As demonstrated in this study and in the literature [4, 8, 15, 17], 48 to 76% of HN SpC-SCC were positive for at least one epithelial marker or keratin. The reported positivity rate of individual keratins and squamous markers in the current study and in the literature was 26 to 61% for cytokeratin AE1/AE3 [4, 10, 15], 9 to 64% for 34bE12 [4, 10, 15], 0 to 15% for CAM5.2 [4, 15], and 36 to 60% for p63 [10].
It is worthwhile to note that an expanding list of HN sarcomas may show keratin positivity, sometimes diffuse, in particular adamantinoma-like Ewing sarcoma [25] and rhabdomyosarcoma [26, 27]. Conversely, SpC-SCC may show expression of mesenchymal markers, e.g., desmin (2 to 28%) [4, 8], SMA (16 to 47%) [4, 8], and S100 (0 to 7%) [4, 8] as shown in the present study and in the literature. Therefore, in challenging cases, immunohistochemistry may be helpful but may not be definitive to distinguish SpC-SCC from sarcoma. Indeed, one tumor that was initially diagnosed as SpC-SCC without associated C-SCC showing immunopositivity for 34BE12, CAM5.2, CK7, desmin, and myoD1 was subsequently found to have EWSR1-TFCP2 fusion. The diagnosis was revised as rhabdomyosarcoma with TFCP2 fusion [26] and the patient was excluded from the present study. In such cases, molecular diagnosis may be necessary to better classify the tumor.
Most of the HN SpC-SCC in this study showed histologic features akin to sarcoma, in particular undifferentiated pleomorphic/spindle sarcoma. However, in 13% of cases, the tumor resembled granulation tissue with abundant inflammation, myxoid stroma, and scattered pleomorphic tumor cells. The cellularity of the neoplastic cells is relatively low and may be masked by the background inflammation and surface ulceration. Such “atypical granulation tissue-like” (or “nodular fasciitis-like”) SpC-SCC was also noted by Lewis et al. in 3 of 26 (11.5%) laryngeal SpC-SCC [15] and Watson et al. [13] in a small percentage of HN SpC-SCC. In such cases, the diagnosis of SpC-SCC is often challenging especially in biopsy material, and it may be mistaken as benign lesions, in particular pyogenic granuloma which also frequently presents as rapid-growing polypoid mass with surface ulceration. Careful examination of the tumor to identify marked nuclear pleomorphism, frequent mitoses, and atypical mitoses, as well as immunopositivity for p40 and high molecular weight cytokeratin, are the key to the correct diagnosis.
Heterologous differentiation may rarely been seen in HN SpC-SCC with a reported frequency of 7% in our cohort and 7 to 8% in the literature [4, 8, 15]. All reported cases showed either osteoid or chondroid differentiation/element.
An interesting observation in our study was that Ki-67 proliferation index in SpC-SCC had a wide range from as low as 5 to 70%. Similarly, Lewis et al. reported a Ki-67 proliferation index from 18 to 57%, and Thompson et al. showed a mitotic index from 0 to 103 per 10 high-power fields in laryngeal SpC-SCC [4, 15]. Together, these data suggest that proliferation rate and mitotic activity may vary drastically in HN SpC-SCC. It is important to keep in mind that a low mitotic count and/or proliferation index does not exclude the diagnosis of SpC-SCC.
Depth of invasion, defined as the measurement from the horizon of the basement membrane of the adjacent uninvolved mucosa perpendicularly to the deepest point of invasion, is a crucial prognostic factor for oral cavity SCC and has been included in the AJCC 8th edition pT stage [28]. Tumor thickness, another important related parameter, is measured from the surface of the invasive SCC for an endophytic or exophytic tumor and from the base of an ulcerated tumor to the deepest area of invasion. Although most SpC-SCCs were ulcerated and polypoid, neither depth of invasion nor tumor thickness were found to be prognostically significant in our cohort of HN SpC-SCC. This could be due to the fact that multiple HN sub-sites were analyzed all together rather than restricting the analysis to the oral cavity. When the analysis was performed on the oral cavity cases only, DOI was predictive of DFS but not of DMFS and DSS, whereas tumor thickness did not predict outcome. Further studies with larger number of cases are needed to assess the predictive value of DOI and thickness in oral cavity SpC-SCC.
Three previous studies compared the clinical features and outcome of HN SpC-SCC with HN C-SCC [1,2,3]. Dai et al. [2] studied 341 HN SpC-SCC and 67,882 HN C-SCC from 2000 to 2008 SEER database, and reported that SpC-SCC was associated with laryngeal location, lower pT stage, lower rate of nodal metastasis (in SpC-SCC, 24%; in C-SCC, 39%), and older age. The DSS and risk of distant metastasis were similar between the two groups. Bice et al. [3] used SEER database from 2004 to 2009 including 118 HN SpC-SCC and 18,298 HN C-SCC, and reported that SpC-SCC was associated with oral cavity location, larger size, higher T stage, lower risk of nodal metastasis (in SpC-SCC 41%, in C-SCC 52%), as well as significantly worse 3- (in SpC-SCC 50%, in C-SCC 66%) and 5-year (in SpC-SCC 40%, in C-SCC 59%) DSS. Age, sex, ethnicity, and rate of distant metastasis did not differ between the two groups. Lastly, Dai et al. compared 62 SpC-SCC and 1632 C-SCC from hypopharynx and reported that SpC-SCC was associated with older age, male gender, higher frequency of perineural invasion and lymphovascular invasion, and decreased 5-year DSS (20% in SpC-SCC, 34% in C-SCC). Other factors, e.g., T stage, nodal metastasis, smoking, alcohol, and extranodal extension, did not differ between the two groups. Compared to C-SCC, HN SpC-SCC was reported to have a lower rate of nodal metastasis and decreased DSS in the first two abovementioned studies but not the third one. As these three studies yielded conflicting results, it remains to be determined if there is any difference between C-SCC and SpC-SCC in terms of clinical features and outcomes.
In addition to the abovementioned studies, several other publications also calculated OS and DSS in HN SpC-SCC. The 3- and 5-year OS was 20 to 76% [6, 15, 21, 23] and 6.7 to 63% [6, 7, 20], respectively, whereas the 5-year DSS was 20 to 52.7% [1, 3, 7]. Such variability of survival data may be in part due to the small sample size given the rarity of the tumor. In the current study, the 3-year OS, 5-year OS, and 5-year DSS are 54, 33, and 37% respectively from the time of SpC-SCC diagnosis.
The reported rate of nodal metastasis in HN SpC-SCC at initial resection/presentation ranged from 13 to 42% [2, 3, 6, 8, 10, 13] and was 31% in our study. Laryngeal SpC-SCC appeared to have lower rate of nodal metastasis (0 to 5%) [4, 8, 15, 20] compared with hypopharyngeal (33 to 52%) [1, 8], and oral cavity SpC-SCC (21.5 to 39%) [8, 23]. Compared with sarcoma which preferably spreads hematogenously and rarely metastasizes to regional lymph nodes, HN SpC-SCC has the potential to spread to regional lymph nodes albeit possibly at a lower rate when compared to C-SCC.
The frequency of distant metastasis in HN SpC-SCC varied drastically from 2.4 to 75% in the literature [4,5,6, 8, 13, 15, 21, 29]. In the current study, 14 patients (20%) developed distant metastasis. Once again, the small sample size might contribute to the variability of results across studies. The 2 SEER-based studies did not find a difference between C-SCC and SpC-SCC in terms of the risk of DM [2, 3].
As most of the published studies contained only a small number of patients, less than a handful of studies investigated the prognostic factors in HN SpC-SCC. In a large study of 559 HN sarcomatoid carcinomas, Ding et al. reported that patient’s age, tumor size, nodal, and distant metastasis correlated significantly with DSS and OS [30]. Similarly, in a SEER-based study of 118 HN SpC-SCC, age, tumor size, and distant metastasis (pM stage) were independent prognostic factors for DSS [3]. In a cohort of 62 hypopharyngeal SpC-SCC, Dai et al. identified stage and nodal metastasis as the only independent prognostic factor for DSS on multivariate analysis [1]. Chang et al. reported that SpC-SCC occurring in a recurrence setting is an independent adverse prognostic factor for shortened OS in 78 HN SpC-SCC [5]. In the current study, we reported that AJCC pN stage was independent prognostic factor for DSS and DMFS, and co-existing C-SCC independently incurred an improved DMFS. Other adverse prognostic factors identified on univariate analysis reported in the literature included extranodal extension [1], older age [5], lymphovascular invasion [6, 21], advanced stage (clinical, pT, pN, and pM) [10, 20, 21], and invasion into muscle/salivary gland/bone [9]. Together, these data suggest that pN stage, pM stage, tumor size, age, concurrent C-SCC, and SpC-SCC occurring in a recurrence setting may affect the outcome of HN SpC-SCC.
In conclusion, we herein report 71 cases of HN SpC-SCC, a rare form of SCC that is associated with variable morphology and adverse outcome. The tumor often shows polypoid growth and surface ulceration, while a small percentage shows histologic features resembling granulation tissue, posing a diagnostic challenge. Majority of tumors are associated C-SCC which can be very helpful in distinguishing a SpC-SCC from a true sarcoma. Additionally, tumor immunopositivity for epithelial and squamous markers are useful diagnostic tools. In challenging cases, molecular testing may be necessary to classify the tumor. Low proliferative index should not exclude the diagnosis of SpC-SCC, as this tumor exhibits a wide range of proliferative index. Lymphatic and hematogenous spread is observed in 31 and 20% of the patients. AJCC pN stage and co-existing C-SCC component appear to be prognostically relevant.
References
Dai L, Fang Q, Li P et al (2019) Oncologic outcomes of patients with sarcomatoid carcinoma of the hypopharynx. Front Oncol 9:950
Gerry D, Fritsch VA, Lentsch EJ (2014) Spindle cell carcinoma of the upper aerodigestive tract: an analysis of 341 cases with comparison to conventional squamous cell carcinoma. Ann Otol Rhinol Laryngol 123:576–583
Bice TC, Tran V, Merkley MA et al (2015) Disease-specific survival with spindle cell carcinoma of the head and neck. Otolaryngol Head Neck Surg 153:973–980
Thompson LD, Wieneke JA, Miettinen M et al (2002) Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol 26:153–170
Chang NJ, Kao DS, Lee LY et al (2013) Sarcomatoid carcinoma in head and neck: a review of 30 years of experience–clinical outcomes and reconstructive results. Ann Plast Surg 71(Suppl 1):S1-7
Mingo KM, Derakhshan A, Abdullah N, et al. Characteristics and outcomes in head and neck sarcomatoid squamous cell carcinoma: the Cleveland clinic experience. Ann Otol Rhinol Laryngol 2020:3489420977778.
Rosko AJ, Birkeland AC, Wilson KF et al (2016) Tumor biomarkers in spindle cell variant squamous cell carcinoma of the head and neck. Otolaryngology-head and neck surgery official journal of American Academy of Otolaryngology-Head and Neck Surgery. 155:106–112
Viswanathan S, Rahman K, Pallavi S et al (2010) Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal region: a clinicopathologic review of 103 cases from a tertiary referral cancer centre. Head Neck Pathol 4:265–275
Leventon GS, Evans HL (1981) Sarcomatoid squamous cell carcinoma of the mucous membranes of the head and neck: a clinicopathologic study of 20 cases. Cancer 48:994–1003
de Araújo AB, Serrano TLI, Pedroso MCM et al (2020) Clinicopathologic diagnostic and prognostic factors of spindle cell carcinoma of upper airway. Pathol Oncol Res 26:1097–1104
Choi HR, Sturgis EM, Rosenthal DI et al (2003) Sarcomatoid carcinoma of the head and neck: molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am J Surg Pathol 27:1216–1220
Feng L, Cai D, Muhetaer A et al (2017) Spindle cell carcinoma: the general demographics, basic clinico-pathologic characteristics, treatment, outcome and prognostic factors. Oncotarget 8:43228–43236
Watson RF, Chernock RD, Wang X et al (2013) Spindle cell carcinomas of the head and neck rarely harbor transcriptionally-active human papillomavirus. Head Neck Pathol 7:250–257
Lane N (1957) Pseudosarcoma (polypoid sarcoma-like masses) associated with squamous-cell carcinoma of the mouth, fauces, and larynx; report of ten cases. Cancer 10:19–41
Lewis JE, Olsen KD, Sebo TJ (1997) Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol 28:664–673
Takata T, Ito H, Ogawa I et al (1991) Spindle cell squamous carcinoma of the oral region An immunohistochemical and ultrastructural study on the histogenesis and differential diagnosis with a clinicopathological analysis of six cases. Virchows Arch Pathol Anat Histopathol 419:177–182
Zarbo RJ, Crissman JD, Venkat H et al (1986) Spindle-cell carcinoma of the upper aerodigestive tract mucosa An immunohistologic and ultrastructural study of 18 biphasic tumors and comparison with seven monophasic spindle-cell tumors. Am J Surg Pathol 10:741–753
Pang A, Carbini M, Moreira AL et al (2018) Carcinosarcomas and related cancers: tumors caught in the act of epithelial-mesenchymal transition. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36:210–216
Thierauf J, Veit JA, Hess J (2017) Epithelial-to-mesenchymal transition in the pathogenesis and therapy of head and neck cancer. Cancers 9
Gamez ME, Jeans E, Hinni ML et al (2018) Outcomes and patterns of failure of sarcomatoid carcinoma of the larynx: The Mayo Clinic experience. Laryngoscope 128:373–377
Su HH, Chu ST, Hou YY et al (2006) Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc: JCMA 69:478–483
Ellis GL, Corio RL (1980) Spindle cell carcinoma of the oral cavity A clinicopathologic assessment of fifty-nine cases. Oral Surg Oral Med Oral Pathol 50:523–533
Singh A, Sharin F, Singhavi H et al (2020) Sarcomatoid variant of squamous carcinoma in recurrent and second primary tumors of the oral cavity. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 49:914–919
Hyams VJ (1975) Spindle cell carcinoma of the larynx. Can J Otolaryngol 4:307–313
Bishop JA, Alaggio R, Zhang L et al (2015) Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol 39:1267–1274
Xu B, Suurmeijer AJ, Agaram NP et al (2020) Head and neck rhabdomyosarcoma harboring TFCP2 fusions and ALK overexpression: a clinicopathologic and molecular analysis of 11 cases. Histopathology [Online ahead of print]
Thompson LDR, Jo VY, Agaimy A et al (2018) Sinonasal tract alveolar rhabdomyosarcoma in adults: a clinicopathologic and immunophenotypic study of fifty-two cases with emphasis on epithelial immunoreactivity. Head Neck Pathol 12:181–192
Amin MB, Edge SB, L. GF, et al. AJCC Cancer Staging Manual. Eighth edition. New York: Springer Nature; 2017.
Leonardi E, Dalri P, Pusiol T et al (1986) Spindle-cell squamous carcinoma of head and neck region: a clinicopathologic and immunohistochemical study of eight cases. ORL J Otorhinolaryngol Relat Spec 48:275–281
Ding L, Bi ZF, Yuan H et al (2021) Sarcomatoid Carcinoma in the Head and Neck: A Population-Based Analysis of Outcome and Survival. Laryngoscope 131:E489–E499
Funding
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Carlos N Prieto-Granada: reviewing cases and drafting manuscript. Bin Xu: reviewing data, managing database. Bayan Alzumaili: editing manuscript. Mohamed Rizwan Haroon Al Rasheed: editing manuscript. Antoine Eskander: editing manuscript. Danny Enepekides: editing manuscript. Snehal G. Patel: editing manuscript. Todd M Stevens: editing manuscript. Snjezana Dogan: editing manuscript. Ronald Ghossein: reviewing cases, editing manuscript. Nora Katabi: designing study, reviewing cases, and editing manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this retrospective study involving human participants were approved by the Institutional Review Board (IRB) in our institution. The research did not involve animals.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carlos N Prieto-Granada and Bin Xu are Co-first author
Rights and permissions
About this article
Cite this article
Prieto-Granada, C.N., Xu, B., Alzumaili, B. et al. Clinicopathologic features and outcome of head and neck mucosal spindle cell squamous cell carcinoma. Virchows Arch 479, 729–739 (2021). https://doi.org/10.1007/s00428-021-03117-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03117-2