Abstract
The clinical implications of the biopsy findings in cases of drug-induced liver injury (DILI) are not fully elucidated. The aim of this study was to evaluate the histopathological findings of cases diagnosed as DILI and to correlate them with clinical and biochemical findings (such as causality assessment algorithms). We searched our department database for all cases of liver biopsy with findings consistent with toxic liver disease and selected those with a clinical diagnosis of DILI. The causative relationships were established according to Roussel Uclaf Causality Assessment Method (RUCAM). A total of 53 cases of DILI were reviewed, most of them diagnosed in hospitalized patients (83%). The analytical toxicity profile was hepatocellular (R > 5) in 60% of the cases and cholestatic (R < 2) in 26.4% of cases. The group of drugs most implicated was the anti-microbials (18, 34%). The predominant histological patterns were “necroinflammation” (67.9%) and “cholestasis” (28.3%). The hepatocellular biochemical pattern was not associated with the presence of predominantly necroinflammatory findings in the biopsy (p = 0.44), and the biochemical cholestatic pattern was not associated with the presence of predominantly cholestatic findings in the biopsy (p = 0.51). This study supports that a better insight into the pathologic mechanisms associated with DILI should be based on liver biopsy due to the lack of a uniform correlation between clinical and biochemical patterns. Also, a liver biopsy may be used in those cases where clinical suspicion of DILI persists despite a low score on current causality assessment algorithms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug-induced liver injury (DILI) is an important cause of liver injury with significant morbidity and mortality [1, 2]. Hepatotoxicity is one of the most common black-box warnings placed on medications and is a major cause for failure to receive initial regulatory approval or withdrawal after initial approval [3, 4].
Accurate and early diagnosis is important, but the diagnosis of DILI is complicated and non-standardized because of the difficulty in the identification of drug(s) causing liver injuries and lack of reliable markers to facilitate and establish a diagnosis [5]. DILI is a great imitator, capable of mimicking all types of liver disease from other causes, in clinical presentation and pathologic features [6].
When clinically suspicious, liver function tests (LFTs) can be the first test to identify patients with DILI. Based on LFTs, the liver injury can be classified as hepatocellular, cholestatic, or mixed, based on the ratio between alanine aminotransferase (ALT) and alkaline phosphatase (ALP) [7, 8].
In patients with abnormal LFTs without an obvious DILI suggestive semiology, and when the initial laboratory tests and imaging studies do not reveal an apparent cause, the patient is often proposed for liver biopsy. Histologically, most DILI can be categorized as acute and chronic cholestatic, acute and chronic hepatitis, or mixed hepatitic-cholestatic pattern of injury [9]. Because drug hepatotoxicity can simulate nearly any clinical syndrome or pathologic lesion that may occur in the liver, the diagnosis cannot be made on morphologic grounds alone, but a liver biopsy can define a histological pattern of injury [10]. In addition to describing the pattern of injury, the pathology report should contain information about the severity of the injury as suggested in previous reports [9, 11, 12].
However, the relationship between biochemical and histological patterns of injury has not been clearly defined. In this retrospective study, we aimed to evaluate the histopathological findings of cases diagnosed as DILI and to correlate them with clinical and biochemical patterns of injury, as well as the standard causality assessment algorithms.
Methods
Case inclusion
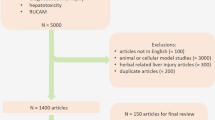
A retrospective case review was performed in all adult patients found to have a liver biopsy performed between January 2007 and December 2017 at “Centro Hospitalar e Universitário São João”. The Hospital is located in Porto, the second-largest city of Portugal, and serves as a referral center for the population of the North of the country. The Department of Gastroenterology and Hepatology is a tertiary, academic, non-transplant one. It is the largest in the region both in terms of the number of physicians and the number of patients’ referrals.
The pathology reports were reviewed, and a list of all biopsies performed in patients with clinical suspicion of DILI was generated from the Pathology Department database. We have also excluded from the analysis cases in which the biopsy was performed at another institution or when relevant clinical data were missing from the files.
The histopathological specimens were obtained through percutaneous/transjugular needle-liver biopsies. Liver tissue specimens were processed according to standard histological techniques and routinely stained for hematoxylin and eosin (H&E), Perls’, and trichrome. Periodic acid–Schiff reagent after diastase (D-PAS), Ziehl–Neelsen (ZN), and modified Ziehl–Neelsen stains was performed if clinically indicated. Histopathology was evaluated independently by two experienced liver pathologists (F.C., J.L.) who were initially blinded to the type and clinical characteristics of the lesions after individual analysis; the two pathologists discussed the findings and sent a common final report.
Histological findings were categorized in major patterns of injury [13]: “necroinflammatory patterns” (zonal necrosis, submassive to massive necrosis, acute/lobular hepatitis, chronic/portal hepatitis, granulomatous hepatitis)(Fig. 1) “cholestatic injury patterns” (acute or chronic cholestasis, cholestatic hepatitis)(Fig. 2), “steatotic injury patterns” (microvesicular–macrovesicular, steatohepatitis), “vascular injury patterns” (sinusoidal obstruction syndrome, hepatoportal sclerosis, peliosis), and “cytoplasmic alterations” (glycogenosis, ground-glass change).
Histological assessment of the severity of liver inflammation and the stage of fibrosis was based on the scoring system proposed by Batts and Ludwig [14].
Etiological workup and Causality Assessment Criteria
Demographic, clinical, and laboratory data were obtained from the medical records of patients. Detailed alcohol, drug, and medication intake history were recorded based on the clinical registry. Diagnostic investigations to exclude other causes of liver injury included serology for hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis E virus (HEV), and human immunodeficiency virus (HIV) in all patients. Serum immunoglobulins and a panel of autoantibodies (including anti-nuclear (ANA), anti-smooth muscle, anti-liver–kidney microsomal type 1 and anti-mitochondrial antibodies) were taken as evidence of autoimmune hepatitis (AIH) or primary biliary cholangitis (PBC) according to established criteria [15, 16]. All laboratory workup was performed at the same central laboratory at our Institution. Patients whose laboratory workup had been performed elsewhere were excluded from the study.
An attempt to assign a specific etiology was made based on clinical presentation, laboratory workup and histological features.
The biochemical injury pattern (hepatocellular, mixed, or cholestatic) was calculated as the ratio (R) of ALT to ALP normalized by their respective upper limits of normal (ULN) from laboratory data at the time of onset. R ratios > 5 define a hepatocellular, < 2 a cholestatic, and between 2 and 5 a mixed pattern of enzymes [17].
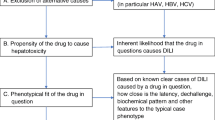
The overall diagnosis of DILI and the causal relationship between the implicated agent(s) and the liver injury event are defined by the Council for International Organizations of Medical Sciences (CIOMS) Roussel Uclaf Causality Assessment Method (RUCAM) [18]. This score was based on time to onset/“latency” of the hepatic injury, course of illness after drug discontinuation, the presence of risk factors for DILI (alcohol use, age > 55 years, pregnancy) or other drugs or potential hepatotoxins, the appropriate exclusion of other causes of liver injury, the presence of published information about the agent’s potential to cause drug-induced liver injury, and the response to re-administration. The CIOMS/RUCAM scale provides the likelihood of hepatic injury due to DILI, defining the suspicion as “definite or highly probable” (score > 8), “probable” (scores 6–8), “possible” (scores 3–5), “unlikely” (scores 1–2), and “excluded” (score ≤ 0).
Clinical severity of each case was defined with the criteria suggested by Aithal et al. [17]: “mild” (elevated ALT/ALP concentration but bilirubin concentration < 2× upper limit of normal, ULN), “moderate” (elevated ALT/ALP concentration and bilirubin concentration ≥ 2× ULN, or symptomatic hepatitis), “severe” (elevated ALT/ALP concentration bilirubin concentration ≥ 2× ULN, and one of the following: (1) international normalized ratio ≥ 1.5; (2) presence of ascites and/or encephalopathy, disease duration < 26 weeks, and absence of underlying cirrhosis; or (3) other organ failure considered to be due to DILI), and “fatal/transplantation” (when occur death or liver transplantation due to DILI).
We also recorded cases that showed severity criteria according to “Hy’s Law” [19, 20]: the presence of jaundice (serum bilirubin > 2 times the upper limit of normal) in association with an elevation in serum aminotransferases (> 3 times the upper limit of normal).
Statistical analysis
Continuous variables are expressed as median (range). Categorical variables are reported as absolute (n) or relative frequencies (%). Analysis of variance was used to compare the differences in a variable between groups. Group comparisons of categorical variables were analyzed with χ2 test or Fisher’s exact test. p values < 0.05 were considered significant. Data were analyzed using SPSS 25.0 (IBM Corp, Armonk, NY, USA).
Results
Characterization of the study population
Over the 10-year study period (January 2007 to December 2017), we perform 53 liver biopsies in cases of suspected DILI. Twenty-eight (51.9%) cases were female with a median age of 54.3 years (range 25–77 years), and 25 were male patients with a median age of 49.8 years (range 18–77 years).
Most of the cases were of hospitalized patients (43 cases, 83%), with the remaining cases are from the ambulatory department (9 cases, 17%). In nearly half of the cases (54.7%), the patients did not show any clinical symptom or signal on the physical examination suggestive of liver disease.
Only in 9 cases (17%), the patients had a previous history of hepatic disease in whom 4 cases (7.5% of the total) had previous documentation of the presence of liver cirrhosis. Liver disease was caused by alcohol (4 cases), non-alcoholic steatohepatitis (4 cases), chronic hepatitis C infection, and primary biliary cholangitis (1 case each). In these cases of underlying liver disease, the cause that led to the acute decompensation of liver function was not explained by the chronic liver disease (based on the analysis of clinical and laboratory data). This sample did not include patients with a previous history of hepatitis B or autoimmune hepatitis (diseases classically related with the possibility of flare).
The median value of the basal hepatic liver function tests (based on the laboratory measurement in an ambulatory setting in the previous month to the occurrence of DILI) was in the normal range of values (Table 1).
Etiology of DILI and clinical/biochemical grading
The pharmacologic group most commonly associated with DILI was anti-infectious agents (18 cases, 34%), immunomodulator/immunosuppressor drugs (8 cases, 15.1%), and anti-inflammatory/analgesic drugs (7 cases, 13.2%). The analysis by drug showed that azithromycin was the most implicated agent (4 cases), followed by azathioprine and isoniazid (3 cases each one). Herbal and dietary supplements were responsible for 4 cases (7.5%) of DILI submitted to biopsy. Non-pharmacologic supplements were self-administered for body building (2 cases with amino acid–enriched supplements) and weight loss (2 cases with non-specified herbal supplements). In 2 cases, despite the clinical diagnosis of DILI, the responsible clinicians could not determine the causative drug for the hepatic lesion (Table 2).
Only in 4 cases, the causative drug was not administrated under medical prescription (alternative medicines or self-medication). In 22 cases (41.5%), the clinical suspicion of the presence of DILI occurred in the first 30 days after the first administration of the causative drug. In 13 cases (24.5%), the clinical diagnosis of DILI was made 90 days after the first administration of the causative drug. In 30 cases (56.6%), the putative drug was on the current medical prescription at the time of the DILI diagnosis, imposing a decision about the maintenance/suspension of the drug.
In the group of patients with underlying liver disease (n = 9), the drugs related to DILI were amlodipine, amoxicillin, azathioprine, azilsartan, idebenone, piperacillin/tazobactam, St. John’s wort (1 case each), and azithromycin (2 cases).
Regarding the cases with a physical manifestation of liver disease (30 cases, 56.7%), the most common findings were jaundice (17 cases), fever (10 cases), and abdominal discomfort (4 cases).
The biochemical injury pattern showed a hepatocellular (R > 5) pattern in 32 cases (60.4%), a cholestatic (R < 2) pattern in 14 cases (26.4%), and a mixed (2 > R > 5) pattern in 6 cases (11.3%).
This sample did not include patients with a previous history of autoimmune hepatitis. The positivity for ANA (≥ 1/100) was seen in 11 patients (29.8%), one of them with a borderline increase in the immunoglobulin G (1650 mg/dL). Other autoimmunity markers (anti-smooth muscle, anti- liver-kidney microsomal type 1 and anti-mitochondrial antibodies) were negative in all patients. The drugs related to a positivity for ANA were metronidazole, terbinafine, allopurinol, amlodipine, azathioprine, azithromycin, trimethoprim/sulfamethoxazole, infliximab, methotrexate, and St. John’s wort (1 case each drug). In one patient, the causative drug was not identified. After liver biopsy, none of these patients met the criteria for the autoimmune hepatitis according to the revised original score for autoimmune hepatitis. [21]
Concerning the “Hy’s Law” [19, 20], 23 cases showed that criteria of clinical severity criteria. However, according to the classification of Aithal et al. [17], the majority of cases had a mild clinical impact (52.8%), without a significant elevation in the bilirubin value. A severe evolution was documented in 9 cases (17%) with the development of hepatic failure and the need for intensive care unit admission. Of those cases, five patients had documented hepatic encephalopathy, and three cases had a fatal outcome (the other two patients with encephalopathy gradually recovered with medical support after the suspension of the causative drug). All of the cases with a fatal outcome were in patients with a previous history of alcoholic chronic liver disease, all of them in the cirrhotic stage.
Causality assessment
Based on the clinical and laboratory data obtained previously to the performance of a liver biopsy, the causality assessment between the clinical scenario and the suspicion of a DILI was obtained by CIOMS/RUCAM scale [18]. A similar percentage of the cases (43.4%, 23 cases) was classified as “definitive DILI” (> 8 points) and “probable DILI” (6–8 points). In 7 cases, despite the modest causality assessment based on this score (3–5 points, “possible DILI”), the liver biopsy showed pathological findings compatible with DILI, supporting the clinical suspicion.
In the patients with a previous diagnosis of chronic liver disease (n = 9), only 3 were classified with “definitive DILI” (> 8 points) probably due to the underclassification of the parameter “exclusion of non-drug-related causes” in the CIOMS/RUCAM scale. However, none of the patients had a chronic liver disease compatible with a biochemical flare (as excluded in the serological workup).
After the suspension of the causative drug (performed in all the patients after the clinical suspicion/diagnosis of DILI), the majority of patients showed an improvement in LFT: 19 cases showed a decrease > 50% in the first week, and 23 cases showed the same decrease in liver tests in the first month after suspension. Despite the proper suspension of the causative drug, 1 case showed a long term (> 1 month) biochemical dysfunction. In 3 cases, a progressive biochemical worsening after suspension of the causative drug was noted (probably related to multiorgan dysfunction); all of them had a fatal outcome.
On the follow-up (> 6 months), only three patients persist with abnormalities in liver function tests presumably caused by DILI.
Histological characterization
After categorization of the liver biopsy findings according to the major histological characteristics, our sample showed a predominance of necroinflammatory patterns (36 cases, 67.2%). A predominant cholestatic pattern was shown in 15 cases (28.3%), and the remaining 2 cases showed a steatotic injury pattern (Table 3). Nearly half of patients (24 cases, 45.3%) did not show fibrosis; however, ten patients showed extensive fibrosis (stage 3/4).
Among the patients with a positivity for ANA (n = 11), the liver biopsy showed a necroinflammatory pattern in 8 cases and a cholestatic pattern in 3 cases. Regarding patients with underlying liver disease (n = 9), the biopsy showed a necroinflammatory pattern in 4 cases and a cholestatic pattern in 5 cases.
When we analyzed the specific histological features for each morphological group of pathological findings, we observed that all of the cases showed at least one finding suggestive of a necroinflammatory lesion. The most predominant were the presence of acute lobular hepatitis (n = 16, 30.2%), massive necrosis (n = 15, 28.3%), and portal hepatitis (n = 12, 22.6%). On the other hand, 24 cases (45.3%) did not have any findings suggestive of cholestasis. The most predominant cholestatic finding is an acute cholestatic pattern (n = 16, 30.2%). In 12 cases (22.6%), the liver biopsy showed the concomitant presence of cholestasis with lobular necroinflammatory findings.
In the cases with a lower causality assessment (3–5 points, “possible DILI”, n = 7), the histological findings were crucial in the final diagnosis. The predominant histological pattern was a necroinflammatory pattern in 5 cases (4 cases with massive necrosis and 1 case with acute lobular hepatitis) and cholestatic in 2 cases (acute cholestasis in the liver biopsy). In such cases, the multidisciplinary review of the medical and biochemical data (by the hepatologist and the pathologist), supported with the presence of acute histological lesions in liver biopsy, gave the final diagnosis of DILI.
The presence of inflammatory cells in liver biopsy was shown in 77.4% of the cases, most of them with the participation of lymphocytes (24 cases, 45.3%). The presence of eosinophils in the liver biopsy specimen was present in only 6 cases. Also, despite steatosis was not a predominant pattern of injury, the presence of lipidic inclusions is high prevalent (n = 29, 54.7%). The majority showed macrovesicular mixed steatosis (27 cases).
The vascular involvement was shown in 3 liver biopsies: one case with sinusoidal dilation (“peliosis”) and two cases with findings suggestive of sinusoidal obstruction.
Relationship between clinical, biochemical, and histological features
Based on the statistical distribution of frequencies between groups created by clinical/laboratory/histological findings (and analyzed by χ2 test or Fisher’s exact test), we showed that the presence of signs/symptoms attributed to DILI was associated the presence of “Hy’s law” criteria (p = 0.01) and a necroinflammatory predominant pattern in liver biopsy (p = 0.01). In a similar mode, the presence of encephalopathy was associated with a fatal outcome (p = 0.02), without a statistically significant relationship with the presence of histologic necroinflammatory findings (p = 0.62) or massive necrosis (p = 0.68).
The comparison between the biochemical and histological patterns was performed with the comparison of the observed and expected frequencies of the results of the “R” classification and predominant histological pattern. It was shown that the hepatocellular biochemical pattern (R > 5) was not associated with the presence of predominantly necroinflammatory findings in the biopsy (p = 0.44), and the biochemical cholestatic pattern (R < 2) was not associated with the presence of predominantly cholestatic findings in the biopsy (p = 0.51) (Table 4).
Discussion
In this sample of patients submitted to liver biopsy following clinical suspicion of DILI, we confirmed the heterogeneity of the histological presentation of such disease. A chi-squared test was done to verify that there was not a statistically significant difference between the histological and biochemical patterns of injury, as we expected previously to the performance of liver biopsy.
Because drug hepatotoxicity can simulate nearly any clinical syndrome or pathologic lesion that may occur in the liver, the diagnosis cannot be made on morphologic grounds alone or based on any specific laboratory test or biomarker [6]. However, a liver biopsy may provide critical features that can help point to and narrow the differential of DILI or to confirm the clinical diagnosis. Previous reports showed that in addition to the pattern of injury, the biopsy provides information on the severity of the injury, which may inform prognosis [9, 11, 12]. According to these studies, specific features, including the degree of necrosis and fibrosis and presence of neutrophils, ductular reaction, and microvesicular steatosis, are associated with a higher chance of liver failure.
In accordance with recent clinical guidelines [22], liver biopsy is not a routine procedure in the presence of clinical suspicion of DILI and may be reserved in cases in which careful drug history and serologic exclusion of other causes of liver injury would have revealed the likely underlying cause. This fact explains the heterogeneity of our group, with a limitation in the prognostic extrapolation of the convenience sample data.
However, previously to the liver biopsy, all of the cases were evaluated by an experienced hepatologist. As we previously shown, most of the cases were in hospitalized patients (83%) without any symptom or signal suggestive of liver disease. These patients were hospitalized for a non-liver diagnosis, and, having started a target therapy during hospitalization (causative drug), presented abnormal liver function tests requiring a gastroenterology consultation. The fact that the most prevalent class of causative drugs were antibiotics (34%) supports that, as well as the most common drug (azithromycin), frequently administered in an inpatient environment.
Consultation cases had a major dilemma in the clinical evaluation, in which the consultant gastroenterologist had to decide about the capacity to maintain or to resume the causative drug (that may be an essential therapy). To our knowledge, there are no systematic studies published on the utility of liver biopsy in the diagnosis or management of DILI, although suspected DILI is a reasonable indication for liver biopsy [23].
It is a generalized practice in our group to perform a liver biopsy to support this clinical decision. The role of the pathologist in evaluating cases of DILI is to provide expert interpretation of the morphologic changes considering a patient’s medical and pharmaceutical history [24]. It is a challenging work for the liver pathologist, who needs to correlate the findings with the history, sorting out findings that can be attributed to non-drug etiologies. Another utility of liver biopsy is when the DILI was clinically suspected, but the biopsy showed either no support for the diagnosis or suggested an alternative explanation. However, we do not document this type of cases in this sample.
The distribution of causative drugs in this sample can be an illustration of the epidemiology of DILI in a hospital setting, in which there is a lack of organized data. Becker et al. [25] recently report in a systematic review of one-country published DILI cases that while DILI does seem to have an impact on hospital admission, the number of patients hospitalized for other causes who developed DILI during hospitalization was twice as high as the number of patients initially hospitalized due to DILI. We agree with the authors about the need to carefully monitor patients’ liver function and also gather more data on potentially hepatotoxic drugs used in hospitals.
In the clinical evolution of the patients submitted to liver biopsy, the causality assessment was performed with CIOMS/RUCAM scale. This algorithm has been described as the best method for detecting DILI and determining its causality [26]. However, none of the scales was used for causality assessment address all risk factors in all patients, and none is used routinely in clinical practice [27]. In this sample, 7 cases (13.2%), classified as “possible cases” (3–5 points) based on RUCAM scale, were firmly diagnosed with DILI after liver biopsy. This supports the use of liver biopsy in cases of clinician’s suspicion of DILI, despite the lack of causality assessment punctuation in the current algorithms.
After the suspension of the causative drug, we observed the worsening in liver function tests in 4 cases, which includes the 3 cases of the documented mortality in this sample. This factor may be a significant prognostic index, in which the progressive worsening in liver function tests is related to the multi-organic dysfunction despite an isolated contribution of idiosyncratic injury. However, it did not punctuate in the RUCAM algorithm [18] because it was not suggestive of DILI. This fact may underestimate the role of DILI in cases of greater clinical severity, particularly in the presence of multiorgan dysfunction (and possible referral for transplantation). In such critical care cases, when an accurate clinical history intake with the patient is not possible, and with a paradoxical evolution in liver function tests, the liver biopsy may be the only possibility to have a correct diagnosis of liver dysfunction.
In conclusion, this descriptive study supports that a better insight into the pathologic mechanisms associated with DILI should rely on liver biopsy due to the lack of a uniform correlation between clinical and biochemical patterns. Also, liver biopsy may be used in those cases where clinical suspicion of DILI persists despite a low score on current causality assessment algorithms.
References
Fontana RJ, Seeff LB, Andrade RJ, Bjornsson E, Day CP, Serrano J, Hoofnagle JH (2010) Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology 52(2):730–742. https://doi.org/10.1002/hep.23696
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137(12):947–954. https://doi.org/10.7326/0003-4819-137-12-200212170-00007
Temple RJ, Himmel MH (2002) Safety of newly approved drugs: implications for prescribing. Jama 287(17):2273–2275. https://doi.org/10.1001/jama.287.17.2273
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH (2002) Timing of new black box warnings and withdrawals for prescription medications. Jama 287(17):2215–2220. https://doi.org/10.1001/jama.287.17.2215
Chalasani N, Bjornsson E (2010) Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology 138(7):2246–2259. https://doi.org/10.1053/j.gastro.2010.04.001
Goodman ZD (2017) Phenotypes and pathology of drug-induced liver disease. Clin Liver Dis 21(1):89–101. https://doi.org/10.1016/j.cld.2016.08.006
Watkins PB, Seeff LB (2006) Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology 43(3):618–631. https://doi.org/10.1002/hep.21095
Teschke R, Schwarzenboeck A, Hennermann KH (2008) Kava hepatotoxicity: a clinical survey and critical analysis of 26 suspected cases. Eur J Gastroenterol Hepatol 20(12):1182–1193. https://doi.org/10.1097/MEG.0b013e3283036768
Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R, Talwalkar JA, Stolz A, Gu J, Barnhart H, Hoofnagle JH (2014) Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology 59(2):661–670. https://doi.org/10.1002/hep.26709
Kleiner DE (2014) Liver histology in the diagnosis and prognosis of drug-induced liver injury. Clin Liver Dis 4(1):12–16. https://doi.org/10.1002/cld.371
Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T (2006) Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int 26(10):1225–1233. https://doi.org/10.1111/j.1478-3231.2006.01377.x
Bjornsson E, Kalaitzakis E, Olsson R (2007) The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther 25(12):1411–1421. https://doi.org/10.1111/j.1365-2036.2007.03330.x
Kleiner DE (2011) Liver injury due to drugs and herbal agents. In: Saxena R (ed) Practical hepatic pathology. 1st edition edn. Sanders, Philadelphia, pp 311–352
Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19(12):1409–1417
EASL Clinical Practice Guidelines: Autoimmune hepatitis (2015). J Hepatol 63 (4):971–1004. doi:https://doi.org/10.1016/j.jhep.2015.06.030
EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis (2017). J Hepatol 67 (1):145–172. doi:https://doi.org/10.1016/j.jhep.2017.03.022
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK (2011) Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 89(6):806–815. https://doi.org/10.1038/clpt.2011.58
Benichou C (1990) Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 11(2):272–276
Bjornsson E (2006) Drug-induced liver injury: Hy’s rule revisited. Clin Pharmacol Ther 79(6):521–528. https://doi.org/10.1016/j.clpt.2006.02.012
Reuben A (2004) Hy’s law. Hepatology 39(2):574–578. https://doi.org/10.1002/hep.20081
Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WGE, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston ALWF, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RNM, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Mieli-Vergani G, Nakanuma Y, Nishioka M, Penner E, Porta G, Portmann BC, Reed WD, Rodes J, Schalm SW, Scheuer PJ, Schrumpf E, Seki T, Toda G, Tsuji T, Tygstrup N, Vergani D, Zeniya M (1999) International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 31(5):929–938. https://doi.org/10.1016/S0168-8278(99)80297-9
EASL Clinical Practice Guidelines: Drug-induced liver injury (2019). J Hepatol 70 (6):1222–1261. doi:https://doi.org/10.1016/j.jhep.2019.02.014
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy. Hepatology 49(3):1017–1044. https://doi.org/10.1002/hep.22742
Gasmi B, Kleiner DE (2020) Liver histology: diagnostic and prognostic features. Clin Liver Dis 24(1):61–74. https://doi.org/10.1016/j.cld.2019.09.004
Becker MW, Lunardelli MJM, Tovo CV, Blatt CR (2019) Drug and herb-induced liver injury: a critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann Hepatol 18(5):742–750. https://doi.org/10.1016/j.aohep.2019.03.010
Teschke R, Frenzel C (2013) Drug induced liver injury: do we still need a routine liver biopsy for diagnosis today? Ann Hepatol 13(1):121–126
Hayashi PH (2009) Causality assessment in drug-induced liver injury. Semin Liver Dis 29(4):348–356. https://doi.org/10.1055/s-0029-1240003
Author information
Authors and Affiliations
Contributions
Pedro Costa-Moreira was responsible for the study design, acquisition, and interpretation of data, drafting the manuscript, and statistical analysis. Rui Gaspar, Pedro Pereira, Susana Lopes, Pedro Canão, and Joanne Lopes were responsible for the acquisition and interpretation of data. Fátima Carneiro and Guilherme Macedo were responsible for critical revision of the manuscript for relevant intellectual content.
Corresponding author
Ethics declarations
This study was conducted according to the Declaration of Helsinki. The ethical approval for this study was obtained from the Ethics Committee of “Centro Hospitalar e Universitário São João”/Faculty of Medicine of the Porto University. Informed consent for research use of the histopathological data was obtained from each patient before the liver biopsy was performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa-Moreira, P., Gaspar, R., Pereira, P. et al. Role of liver biopsy in the era of clinical prediction scores for “drug-induced liver injury” (DILI): experience of a tertiary referral hospital. Virchows Arch 477, 517–525 (2020). https://doi.org/10.1007/s00428-020-02824-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02824-6