Abstract
Causes of peritoneal carcinomatosis (PC) in patients with a history of breast carcinoma include both metastatic breast carcinoma (MBC) and primary peritoneal/ovarian carcinoma (PPOC). The origin of PC is important to determine the appropriate treatment strategy. Cytological examination of the peritoneal fluid (PF), which may be the first diagnostic approach to PC, is of distinct value in confirming the presence of malignant cells and determining the origin of PC. We analyzed the clinicopathological and cytomorphological characteristics of 33 patients with a history of breast carcinoma whose PF cytology contained malignant cells. Cases showing positive immunoreactivity for PAX8 and a lack of GATA3 expression were considered as PPOC. Sixteen patients developed PC caused by PPOC. PPOC patients were characterized by early-stage primary breast carcinoma, absence of non-peritoneal MBC before PC, and normal serum levels of CEA and CA15-3. Fourteen PPOC patients had pathogenic germline BRCA mutations. Cytological examination revealed that most of the PPOC cases had a dominant papillary arrangement of the tumor cells with severe nuclear pleomorphism, occasional bizarre nuclei, and atypical mitotic figures. Patients with PPOC who underwent cytoreductive surgery had a significantly longer survival time compared to those who did not, or MBC patients. In patients with a history of breast carcinoma presenting with PC, the presence of early-stage primary breast carcinoma, no prior non-peritoneal MBC, and a dominant papillary cellular arrangement pattern in the PF cytology were independent predictors of PPOC. Cytoreductive surgery significantly improved survival for patients with PPOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast carcinoma is the most common malignancy among women in developed countries [1]. With the improvements in the diagnosis and management of breast carcinoma, there appears to be a shift in the pattern of recurrence. Better locoregional control is now achievable with combined modality therapy, and this has translated into a higher rate of observed distant metastasis [2]. Patients who develop distant metastasis have extremely poor prognoses as well as long-term survival. The most common sites of breast carcinoma metastases are, in the order of frequency, the bones, liver, lungs, and brain, but other secondary localizations have been described in the literature, including those of the peritoneal cavity [3, 4]. Peritoneal metastasis of breast carcinoma presents with ascites and seeding nodules involving the omentum, mesentery, and abdominopelvic cavity, and is also known as peritoneal carcinomatosis (PC) [3, 4]. Cases of PC from breast carcinoma are uncommon, while those of PC as the initial presenting sign of metastatic breast carcinoma (MBC) are even rarer. In fact, PC is a typical feature of tumor spread in patients with primary advanced ovarian carcinoma. The incidence of second primary peritoneal/ovarian carcinoma (PPOC) significantly increases in patients with a history of breast carcinoma [5]. It is well known that a majority of PPOC patients are diagnosed in the advanced stages of the disease with malignant ascites and PC. Previous studies demonstrated that approximately 37% of the pelvic masses and 75% of the PCs in breast carcinoma patients were caused by PPOC [6, 7].
Cytological examination of ascitic fluid is a widely used diagnostic tool for the detection of disseminated malignant cells in the peritoneal cavity. The advantages of peritoneal fluid (PF) cytology include high specificity and minimal invasiveness, as well as the fact that it may provide some morphological clues for the determination of the primary site of origin [8]. Furthermore, immunocytochemistry on cell blocks can further help in identifying the cell type and origin of metastatic tumors [9, 10]. The majority (83–90%) of breast carcinomas are positive for GATA3, and carcinomas of tubo-ovarian and peritoneal origin are positive for PAX8 [11,12,13]. The predictive value of these markers in determining the breast or peritoneal/ovarian origin of the malignant cells has been well-demonstrated [11, 13, 14].
It is important to distinguish between PPOC and peritoneal MBC (pMBC) in the diagnosis of PC for the appropriate treatment strategy: cytoreductive surgery with platinum-based adjuvant chemotherapy for PPOC versus chemoradiation therapy and/or hormonal therapy for pMBC [7]. Therefore, being aware of the diagnostic clues for PPOC is essential in preventing misdiagnosis and ensuring accurate management. The examination of PF cytology specimens is one of the most critical points to be assessed at the time of PC detection in breast carcinoma patients. The aim of this study is to analyze the clinicopathological characteristics of patients with a history of breast carcinoma who developed PC associated with either PPOC or pMBC, and also compare the cytomorphology of their malignant peritoneal effusions.
Materials and methods
Patient selection
The pathology database was queried for all cases diagnosed with malignant cells in the PF cytology specimens, between January 2008 and December 2017. Data on 45 patients with a history of breast carcinoma were initially extracted using the selection criteria: (1) having a history of breast carcinoma and (2) diffuse peritoneal thickening and/or omental seeding nodules on images at the time of PF cytology sampling. Then, 12 patients with the following criteria were excluded: (1) having a history of dual primary malignancies before cytological examination of the PF or (2) having breast carcinoma diagnosed subsequent to PPOC. Thirty of the remaining 33 patients underwent laparoscopic peritoneal biopsies for the diagnosis of PC, and the tumor tissues and cell blocks were used for pathological examination and GATA3 and PAX8 immunostaining. In the remaining three patients, cell blocks were only available for immunocytochemistry.
Medical record review
We reviewed patients’ electronic medical records/files and pathology reports according to the time course between the initial diagnosis of primary breast carcinoma and the last follow-up. The primary endpoint was either the detection of PC by imaging studies or the cytological identification of malignant cells in the PF. The secondary endpoint was death due to malignancy. Data reviewed at the time of the initial diagnosis included patients’ age, menopausal status, histological subtype, histological grade, stage, molecular subtype, stage, and surgical treatment. The details that were reviewed at the primary endpoint included patients’ age, interval between primary breast carcinoma diagnosis and the detection of PC, prior history of bilateral breast carcinoma, prior history of nonperitoneal metastasis, distribution of metastasis, imaging findings, and serum tumor marker levels. At the secondary endpoint, data on the pathological features of PPOC, patients’ outcomes, follow-up period after PC, and germline BRCA mutation status were collected in the PPOC cases.
Cytological examination
One slide per case was created using the SurePath liquid-based preparation technique (BD Diagnostics, Franklin Lakes, NJ, USA), according to the manufacturer’s recommendations. Briefly, the PF cytology specimen was automatically dispensed into a centrifuge tube with 4 mL of BD PrepStain Density Reagent (BD Diagnostics), and the centrifuge tube was placed on the PrepMate Automated Accessory (BD Diagnostics). Next, centrifugation was performed for 2 min at 200×g, and the supernatant containing cellular debris was discarded. The sample was then centrifuged for 10 min at 800×g, the residual reagent was removed by aspiration, and the cell pellet was automatically resuspended and mixed in 1 mL of buffer. Furthermore, 200 μL of the sample was transferred to the settling chamber for cell sedimentation by gravity for 10 min, and the cell sediments were then washed to remove excess cells and air-dried for 60 s for cell attachment. An automated slide processor (PrepStain Slide Processor; BD Diagnostics) was used to prepare a thin-layer smear within a 13-mm microscopic field. Each slide was stained using a modified Papanicolaou method. All available slides were reviewed by two board-certified pathologists (K.N. and H-S.K.) specializing in gynecological pathology and cytopathology.
Immunostaining
Immunostaining was performed using an automatic instrument [Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ, USA)] according to the manufacturer’s recommendations. Antigen retrieval was performed using Cell Conditioning Solution CC1 (Ventana Medical Systems). The 4-μm-thick, formalin-fixed, paraffin-embedded slices were incubated with primary antibodies (Supplementary Table 1). After chromogenic visualization using an ultraView Universal DAB Detection Kit (Ventana Medical Systems), slices were counterstained with hematoxylin. Appropriate positive and negative controls were concurrently stained to validate the staining method. Negative control was prepared by substituting non-immune serum for primary antibody, and this resulted in no detectable staining. For GATA3, WT1, and PAX8 immunostaining, diffuse staining with a moderate-to-strong intensity in the nuclei was interpreted as a positive expression [15,16,17]. The p53 immunostaining was interpreted as mutation pattern (all or null staining) and wild-type pattern (weak-to-moderate and patchy staining) [15,16,17]. The expression statuses of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were evaluated according to the American Society of Clinical Oncology/College of American Pathologists guideline recommendations [18, 19].
Statistical analysis
The chi-square test or Fisher’s exact test was used to determine whether there was an association between the categorical variables. The Student t test was used to compare the means between two continuous variables. Multivariate logistic regression with a backward stepwise elimination method was used to identify the independent predictors of PPOC. Univariate survival analyses were performed using the Kaplan-Meier method and a log-rank test. Multivariate survival analyses were performed using the Cox proportional hazards regression model with a backward stepwise elimination method. Statistical analyses were performed using PASW Statistics for Windows (version 18.0; IBM SPSS, Armonk, NY). Statistical significance was set at a P value < 0.05.
Results
Patient subgroups
Sixteen (48.5%) of the 33 cases exhibited histological features and an immunostaining pattern characteristic of PPOC (PAX8-positive and GATA3-negative). Sixteen of the remaining 17 cases displayed an immunophenotype characteristic of pMBC (PAX8-negative and GATA3-positive). A single case showing no immunoreactivity for GATA3 and PAX8 was classified as pMBC based on its morphological resemblance to the corresponding primary breast carcinoma. Overall, 17 (51.5%) and 16 (48.5%) patients with PC were assigned to the pMBC and PPOC subgroups, respectively (Fig. 1).
Clinicopathological and cytomorphological characteristics of peritoneal metastatic breast carcinoma
The median age at the time of initial breast cancer diagnosis was 45 years. In a majority (14/17; 82.4%) of the cases, the histological subtype of the primary breast carcinoma was invasive carcinoma of no special type (IC-NST). The stage distribution was as follows: I, 0% (0/17); II, 23.5% (4/17); III, 58.8% (10/17); and IV, 17.6% (3/17). The molecular subtypes of the primary breast carcinoma included the luminal A (3/17; 17.6%), luminal B (7/17; 41.2%), HER2-enriched (2/17; 11.8%), and triple-negative (5/17; 29.4%) subtypes. Fourteen of the 17 patients (except for 3 patients who had stage IV disease) underwent surgery. All patients received chemoradiation therapy with or without hormonal therapy. The clinicopathological characteristics of those with primary breast carcinoma are summarized in Supplementary Table 2.
The median interval between the diagnosis of the primary breast carcinoma and the detection of PC was 45 months. Fifteen (88.2%) patients had a prior history of non-peritoneal metastasis in 2 or more organs. The common sites of metastases before the development of PC included the pleural cavity (10/17), liver (10/17), bone (8/17), skin (6/17), and lungs (5/17). The involved lymph nodes included those of the abdominopelvic cavity (7/17), mediastinum (7/17), and supraclavicular region (7/17). The percentages of pMBC patients with elevated serum tumor marker levels were as follows: CEA (> 3 ng/mL), 70.5% (12/17); CA15–3 (> 30 U/mL), 94.1% (16/17); and CA125 (> 35 U/mL), 83.3% (5/6). PC was associated with pelvic mass in 11 (64.7%) patients. The clinicopathological characteristics at the time of PC detection are summarized in Supplementary Table 3.
Cytologically, 13 (76.5%) pMBC cases showed hypercellular smears. The histological subtype was IC-NST in 12 (92.3%) cases. Representative photomicrographs showing the cellular arrangement on the liquid-based preparations of the PF specimens are shown in Fig. 3. The most common morphological arrangement of the malignant cells was the spheroid pattern (tightly cohesive cell clusters with an almost perfectly round contour and smooth border), which was observed in 8 (47.1%) cases of IC-NST. Four (23.5%) IC-NST cases predominantly exhibited the formation of three-dimensional cellular clusters, characterized by cohesive cell clusters with an oval or elliptic contour and an irregular border. Overall, the malignant cells exhibited an irregular nuclear contour, a high nuclear-to-cytoplasmic ratio, a delicate and homogeneously stained cytoplasm, and hyperchromatic nuclei. The frequency of the presence of prominent nucleoli, chromatin patterns, and multinucleated giant cells varied between the cases. One (7.7%) case showing hypercellular smear was invasive micropapillary carcinoma. The tumor cells showed oval to angulated clusters with papillary configuration lacking a fibrovascular core. In many papillae, the nuclei were at a central location, with a rim of cytoplasm toward the periphery. They exhibited mild to moderate atypia, irregular nuclear contours, hyperchromasia, inconspicuous nucleoli, and finely dispersed chromatin.
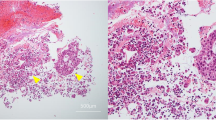
The remaining four (23.5%) pMBC cases showed low cellular smears, and their histological subtypes included IC-NST (2/4; 50.0%) and invasive lobular carcinoma (2/4, 50.0%). In three (75.0%) cases showing hypocellular smears, the malignant cells were predominantly arranged as single cells. Cytoplasmic vacuolization was prominent in two metastatic invasive lobular carcinoma cases, in which signet-ring-like cells were frequently observed (Fig. 2).
Clinicopathological and cytomorphological characteristics of primary peritoneal/ovarian carcinoma
The median age at the time of initial breast cancer diagnosis was 49 years. All patients had IC-NST. The stage distribution was as follows: I, 75.0% (12/16); II, 25.0% (4/16); III, 0% (0/16); and IV, 0% (0/16). Ten (62.5%) of the 16 patients had triple-negative breast carcinoma. All patients underwent surgery with adjuvant chemoradiation therapy with or without hormonal therapy (Supplementary Table 2).
The median interval between the diagnosis of the primary breast carcinoma and the detection of PC was 79 months. None of these patients had a prior history of non-peritoneal metastasis. Lymph node metastases were identified in 8 (50.0%) patients, and the locations were the abdominopelvic (5/16), mediastinal (2/16), supraclavicular (2/16), and inguinal (2/16) regions. Pelvic masses were identified in 10 (62.5%) patients. Elevations in the serum CEA, CA 15–3, and CA 125 levels were noted in 4 (25.0%), 7 (43.7%), and 16 (100.0%) patients, respectively. Twelve (75.0%) patients with PPOC underwent cytoreductive surgery. From these patients, detailed data on the pathological features of PPOC were collected (Supplementary Table 3). Tubo-ovarian involvement was observed in all the 12 cases. The median size of the radiologically visible pelvic tumors was 5.0 cm, and that of the radiologically invisible pelvic tumors was 0.7 cm. Histological subtypes were confirmed by immunostaining in either the debulking (12/16) or peritoneal biopsy (4/16) specimens. Fifteen cases were high-grade serous carcinoma (WT1-positive and p53-mutation pattern), and the remaining 1 case was an endometrioid carcinoma (WT1-negative, p53-wild-type pattern, ER-positive, and AT-rich interaction domain 1A-loss).
Cellularity of the smears were high in 14 (87.5%) PPOC cases. All of them were high-grade serous carcinomas. They showed numerous, large arborizing papillary fragments (papillary pattern) and singly dissociated cells in the background of abundant necrotic debris and inflammatory cells. Many papillary clusters exhibited compactly overlapped tumor cells. Some of clusters were less compact, and thin fibrovascular cores were identified in the center of the papillae. The tumor cells were large with round to oval nuclei, high nuclear-to-cytoplasmic ratio, severe nuclear pleomorphism, hyperchromasia, prominent nucleoli, and a delicate and homogeneously stained cytoplasm. Occasionally, the cells with bizarre nuclei or multinucleation were observed. Mitoses were frequent and some of which showed atypical figures. Psammomatous microcalcifications were identified in two cases. The remaining 2 (12.5%) PPOC cases with low cellularity showed malignant cells arranged in small three-dimensional clusters (endometrioid carcinoma) and as single cells (high-grade serous carcinoma), respectively.
Differences in the clinicopathological characteristics and patient outcomes according to the origin of peritoneal carcinomatosis
We evaluated the associations between the origin of PC and clinicopathological characteristics (Table 1). Four characteristics including the presence of early-stage primary breast carcinoma (P < 0.001), no prior history of non-peritoneal metastasis (P < 0.001), and normal serum CEA (P = 0.015) and CA15–3 (P = 0.002) levels at the time of PC detection were significantly associated with the presence of PPOC. In the multivariate analysis, the former two parameters remained significant (Table 1), suggesting that having early-stage primary breast carcinoma (P = 0.017) and no prior non-peritoneal metastasis (P < 0.001) are independent factors in the prediction of the development of PPOC in breast carcinoma patients.
The median survival times for patients with pMBC and PPOC were 7 months (range, 2–39 months) and 37 months (range, 8–80 months), respectively (Supplementary Table 3). A majority (88.2%; 15/17) of the pMBC patients had a carcinoma-related death, while half (8/16) of the PPOC patients were alive at the last follow-up. The median survival time for the 12 PPOC patients who underwent cytoreductive surgery was 42 months (range, 9–80 months), while that for the remaining 4 patients who did not undergo cytoreductive surgery was 22 months (range, 8–35 months). Univariate survival analyses revealed that PPOC patients who underwent cytoreductive surgery had a significantly longer survival time than those who did not undergo cytoreductive surgery (P = 0.046), or pMBC patients (P < 0.001; Fig. 3). There was no significant difference in the survival between PPOC patients who did not undergo cytoreductive surgery and those with pMBC (P = 0.229).
Differences in the cytomorphological characteristics according to the origin of peritoneal carcinomatosis
Univariate analyses revealed that the presence of dominant papillary patterns (P < 0.001), severe nuclear pleomorphisms (P = 0.002), bizarre nuclei (P < 0.001), and atypical mitotic figures (P = 0.032) were significantly associated with PPOC (Table 2). Multivariate analyses revealed that the presence of a dominant papillary pattern was an independent cytomorphological factor for the prediction of the development of PPOC in breast carcinoma patients (P = 0.002; Table 2).
Hormone receptor/HER2 expression status
We investigated whether there were any differences in the concordance rates of the ER, PR, and HER2 expression between the pMBC and PPOC cases. In the pMBC cases, the positive ER, PR, and HER2 expression rates in the primary breast carcinoma tissues (58.8%/41.1%/11.7%) were similar to those observed in the pMBC tissues (58.8/35.2/5.8%). The concordance rates of the ER, PR, and HER2 expression between the primary breast carcinomas and corresponding metastases were 64.7, 58.8, and 94.1%, respectively. The combined concordance rates were 47.0 and 41.1% for the ER/PR and ER/PR/HER2 expressions, respectively. In the PPOC cases, the positive ER/PR/HER2 expression rates in the primary breast carcinoma and PPOC tissues were 31.2/12.5/12.5% and 75.0/12.5/0.0%, respectively. The concordance rates of the ER, PR, and HER2 expression between the primary breast carcinoma and PPOC cases were 43.7, 87.5, and 87.5%, respectively. The combined results were concordant in 37.5 and 31.2% of the cases for the ER/PR and ER/PR/HER2 expressions, respectively. The differences in the concordance rates of these markers between the pMBC and PPOC cases were not statistically significant (Supplementary Table 4). The expression status of ER, PR, and HER2 in each case is summarized in Supplementary Table 5.
Germline BRCA mutation status
Fourteen (87.5%) of the 16 breast carcinoma patients who developed PPOC harbored pathogenic germline BRCA1 (11/16; 68.8%) or BRCA2 (3/16; 18.8%) mutations. The germline BRCA1 mutations included frameshift mutations (8/11; 72.7%), nonsense mutations (2/11; 18.2%), and missense mutation (1/11; 9.1%). The types of germline BRCA2 mutation included nonsense mutations (2/3; 66.7%) and frameshift mutation (1/3; 33.3%). The histological subtype of the 14 BRCA-mutant PPOCs was high-grade serous carcinoma. The two PPOCs without pathogenic BRCA mutations were high-grade serous carcinoma and endometrioid carcinoma, respectively. Detailed information on the DNA sequence changes and predicted consequences appears in Supplementary Table 6.
Discussion
In patients with a history of breast carcinoma, PC development is rare, but when it occurs, it is associated with high morbidity and mortality [20]. Previous studies found that approximately 0.3 and 0.7% of breast carcinoma patients develop PPOC and pMBC, respectively [3, 21,22,23]. In our PF cytology-based cohort, the incidence of PPOC (16/33) and pMBC (17/33) as a cause of PC in patients with a history of breast carcinoma was similar. Garg et al. [7] demonstrated that 74.7% of breast carcinoma patients who underwent surgery for PC were diagnosed as having PPOC. They also reported that optimal cytoreductive surgery was associated with a significant survival advantage for patients with PPOC. Consistent with the findings of previous studies, we showed that cytoreductive surgery for PPOC led to significantly improved survival, justifying the importance of a differential diagnosis for PPOC and pMBC in the setting of PC.
Ingham et al. [21] reported that women from families with BRCA1 and BRCA2 mutations showed 50- and 17-fold increased risks of ovarian carcinoma, respectively. These data are in agreement with our results, in that the vast majority of PPOC patients harbored pathogenic germline BRCA mutations. Our observations suggest that PPOC should be considered primarily a cause of PC in patients with germline BRCA mutations. With regard to cost-effectiveness, there are various guidelines globally; some of them recommend the consideration of BRCA mutation testing for patients with a history of unilateral breast cancer diagnosed at age 35 years or less or bilateral breast cancer diagnosed at age 50 years or less, and for patients with clinical or family histories otherwise suggestive of hereditary breast and ovarian carcinoma syndrome [24]. Since BRCA mutation testing does not generally precede the diagnosis of double primary breast and ovarian carcinomas [25], the assessment of the clinicopathological and cytomorphological characteristics together can be effective and practical in predicting the development of PPOC.
Bertozzi et al. [3] reported that the prevalence of peritoneal involvement in patients with MBC was 7.6%. They also stated that the prevalence rates of peritoneal and non-peritoneal MBC at the 5-year follow-up were 60.0 and 77.1%, respectively, indicating that peritoneal metastases develop significantly later than those of any other site. That notion is supported by our observation that the median survival of patients with pMBC after the diagnosis of PC was 7 months. We further assumed that the clinicopathological characteristics at the time of primary breast carcinoma diagnosis would be more aggressive in patients with pMBC. Statistical analyses revealed that pMBC patients exhibited a higher proportion of aggressive primary tumors that were characterized by a more advanced stage (III-IV) at the initial diagnosis, and prior non-peritoneal metastases. In contrast, PPOC patients had stage I-II breast carcinoma and no prior history of non-peritoneal metastasis. Consistent with our results, Garg et al. [7] documented that the presence of stage I disease and no history of recurrence were the most relevant factors in the prediction of PPOC in breast carcinoma patients who developed PC. Tserkezoqlou et al. [26] also observed that initial stage IV breast carcinoma patients with multiple metastases are likely to develop pMBC.
Lobular histology, higher histological grade, and non-luminal A subtypes are known to be significant pathological factors in the prediction of the development of metastasis in breast carcinoma cases [3]. In our series, however, those factors were not significant in distinguishing pMBC from PPOC. A possible explanation may be the unequal distribution of the cases, particularly the presence of a small number of lobular carcinoma (2/33), grade 1 tumor (2/33), and luminal A carcinoma (4/33) cases. It is well-known that triple-negative breast carcinoma has a highly aggressive nature and is likelier to cause early visceral metastasis [27, 28]. Unexpectedly, in our series, more than half (62.5%; 10/16) of the PPOC patients had triple-negative breast carcinomas, as did 5 (29.4%) of the 17 pMBC patients. A possible explanation may be the higher prevalence of BRCA mutations in PPOC patients. Eleven (68.8%) and 3 (18.8%) of the 16 PPOC patients harbored pathogenic germline BRCA1 and BRCA2 mutations, respectively. It has been reported that germline BRCA mutations are found in a high proportion of patients with triple-negative breast carcinoma [29], and the prevalence of pathogenic germline BRCA mutations is approximately twice as high as that in breast cancer, overall [30].
The presence of pelvic masses on the imaging studies was not a significant factor for the differentiation between PPOC and pMBC, indicating that the absence of pelvic mass does not exclude the possibility of PPOC development. In contrast, there were significant differences in the anatomical distributions of non-peritoneal metastasis at the time of PC detection, particularly in the context of the involvement of the bone, skin, and inguinal lymph node. PPOC metastases of the bone and skin are late complications that occur very rarely [31, 32]. Li et al. [33] reported that, in patients with breast carcinoma, the incidence of inguinal lymph node metastasis is 0.1%, and that most of the patients with inguinal lymph node metastases had multiple metastatic diseases [33]. In line with these findings, we noted that none of the PPOC patients developed skeletal or cutaneous metastasis, and that inguinal lymph node metastasis was not detected in any of the pMBC patients.
There were significant differences in the frequencies of the elevation of the serum CEA and CA15-3 levels between the pMBC and PPOC cases. Normal serum CEA and CA 15-3 levels were associated with PPOC. However, our data are not sufficient to convince clinicians that serum tumor marker levels are significant factors in the differentiation between PPOC and MBC, as four and seven PPOC patients showed elevated serum levels of CEA and CA 15-3, respectively. The cut-off levels of serum CEA and CA 15-3 for the determination of the origin of PC were not apparent this study; however, we suggest that elevated levels of serum CEA and CA15-3 favor the presence of pMBC. The serum CA 125 levels were not significantly different between the MBC and PPOC cases. Elevated serum CA 125 levels were observed in most (5/6; 83.3%) of the MBC cases examined. This finding is consistent with those of previous studies which demonstrated that serum CA 125 levels are elevated in a majority of patients with pMBC [7, 26]. Although our observation is of limited value, due to the small sample size, the serum CA 125 level may not be a useful factor in differentiating between PPOC and MBC in the setting of PC.
In our study, the most significant cytomorphological feature for the determination of the origin of PC was cellular arrangement. A papillary pattern was found to independently predict the development of PPOC. In contrast, the dominant patterns of arrangement in the MBC cases varied between the histological subtypes of the primary breast carcinoma: spheroid and three-dimensional clustering patterns for IC-NST and singly scattered cells for invasive lobular carcinoma. Additional cytomorphological features suggestive of high-grade serous carcinoma are psammoma bodies, high nuclear-to-cytoplasmic ratio, prominent nucleoli, severe pleomorphism, and hyperchromasia [34]. In our series, severe nuclear pleomorphism, bizarre nuclei, and atypical mitotic figures were more frequently observed in high-grade serous carcinoma. A single case of MBC of the micropapillary type displayed a dominant papillary pattern, which may lead to misinterpretation unless pathologists are aware of the histological subtype of the corresponding primary tumor. Cytologically, invasive micropapillary carcinoma of the breast can be distinguished from high-grade serous carcinoma by the lack of fibrovascular cores, centrally located nuclei (inside-out pattern), inconspicuous nucleoli, and low-to-intermediate-grade atypia [35, 36]. In some cases, MBC may be hardly distinguished from high-grade serous carcinoma during the assessment of PF cytology and even with the peritoneal biopsy. Causes of difficulty in differential diagnosis include low cellularity in cytology and/or biopsy specimens, overlapping high-grade nuclear features in some cases of MBC, and infrequent psammomatous calcification in high-grade serous carcinoma. In these situations, immunostaining improves diagnostic accuracy. It has been suggested that the expressions of GATA3, gross cystic disease fluid protein-15, and mammaglobin in tumor cells favors MBC [37], whereas the expression of PAX8 expression favors serous carcinoma [38].
Interestingly, we found that the concordance rates of the ER/PR/HER2 expression status were not significantly different between the MBC and PPOC cases. It is common for MBC cases to exhibit ER/PR/HER2 expression patterns that differ from those of the corresponding primary breast carcinoma. Our observations support the notion that the hormone receptors and HER2 profile of the lymph node and distant metastatic sites do not always match that of the primary tumor [39, 40]. Furthermore, the expression patterns in the primary breast carcinoma and PPOC tissues were similar in approximately one third (5/16) of the cases and included triple negative (2/5), ER-positive/PR-negative/HER2-negative (2/5), and ER-positive/PR-positive/HER2-negative (1/5) expressions. Although ER/PR/HER2 immunoprofiles are therapeutically important, they are not helpful for the differential diagnosis between PPOC and MBC.
In conclusion, among patients with a history of breast carcinoma presenting with PC, the presence of early-stage primary breast carcinoma and no prior history of non-peritoneal MBC were independent predictors of PPOC development. We also demonstrated that a dominant papillary cellular arrangement pattern in the PF cytology independently predicted PPOC. Given the significantly improved survival associated with cytoreductive surgery for patients with PPOC, further studies must focus on distinguishing PPOC from pMBC in patients with this clinical presentation.
References
Kim YA, Oh IH, Yoon SJ, Kim HJ, Seo HY, Kim EJ, Lee YH, Jung JH (2015) The economic burden of breast cancer in Korea from 2007-2010. Cancer Res Treat 47:583–590. https://doi.org/10.4143/crt.2014.143
Bouganim N, Tsvetkova E, Clemons M, Amir E (2013) Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat 139:603–606. https://doi.org/10.1007/s10549-013-2561-7
Bertozzi S, Londero AP, Cedolini C, Uzzau A, Seriau L, Bernardi S, Bacchetti S, Pasqual EM, Risaliti A (2015) Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. Springerplus 4:688. https://doi.org/10.1186/s40064-015-1449-x
Sheen-Chen SM, Liu YW, Sun CK, Lin SE, Eng HL, Huang WT, Ko SF (2008) Abdominal carcinomatosis attributed to metastatic breast carcinoma. Dig Dis Sci 53:3043–3045. https://doi.org/10.1007/s10620-008-0529-y
Jung HK, Park S, Kim NW, Lee JE, Kim Z, Han SW, Hur SM, Kim SY, Lim CW, Lee MH, Lee J (2017) Development of second primary cancer in Korean breast cancer survivors. Ann Surg Treat Res 93:287–292. https://doi.org/10.4174/astr.2017.93.6.287
Curtin JP, Barakat RR, Hoskins WJ (1994) Ovarian disease in women with breast cancer. Obstet Gynecol 84:449–452
Garg R, Zahurak ML, Trimble EL, Armstrong DK, Bristow RE (2005) Abdominal carcinomatosis in women with a history of breast cancer. Gynecol Oncol 99:65–70. https://doi.org/10.1016/j.ygyno.2005.05.013
Pereira TC, Saad RS, Liu Y, Silverman JF (2006) The diagnosis of malignancy in effusion cytology: a pattern recognition approach. Adv Anat Pathol 13:174–184
Bedrossian CW (1998) Special stains, the old and the new: the impact of immunocytochemistry in effusion cytology. Diagn Cytopathol 18:141–149
Nance KV, Silverman JF (1992) The utility of ancillary techniques in effusion cytology. Diagn Cytopathol 8:185–189
Shield PW, Papadimos DJ, Walsh MD (2014) GATA3: a promising marker for metastatic breast carcinoma in serous effusion specimens. Cancer Cytopathol 122:307–312. https://doi.org/10.1002/cncy.21393
Wang Y, Wang Y, Li J, Yuan Z, Yuan B, Zhang T, Cragun JM, Kong B, Zheng W (2013) PAX8: a sensitive and specific marker to identify cancer cells of ovarian origin for patients prior to neoadjuvant chemotherapy. J Hematol Oncol 6:60. https://doi.org/10.1186/1756-8722-6-60
Lee BH, Hecht JL, Pinkus JL, Pinkus GS (2002) WT1, estrogen receptor, and progesterone receptor as markers for breast or ovarian primary sites in metastatic adenocarcinoma to body fluids. Am J Clin Pathol 117:745–750. https://doi.org/10.1309/QLV6-HH0H-UCTF-WEF6
Waters L, Crumley S, Truong L, Mody D, Coffey D (2014) PAX2 and PAX8: useful markers for metastatic effusions. Acta Cytol 58:60–66. https://doi.org/10.1159/000356426
Na K, Kim HS (2017) Clinicopathological characteristics of fallopian tube metastases from primary endometrial, cervical, and nongynecological malignancies: a single institutional experience. Virchows Arch 471:363–373. https://doi.org/10.1007/s00428-017-2186-z
Na K, Kim HS (2017) Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am J Surg Pathol:1. https://doi.org/10.1097/PAS.0000000000000991
Na K, Sung JY, Kim HS (2017) TP53 mutation status of tubo-ovarian and peritoneal high-grade serous carcinoma with a wild-type p53 immunostaining pattern. Anticancer Res 37:6697–6703. https://doi.org/10.21873/anticanres.12128
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Tuthill M, Pell R, Guiliani R, Lim A, Gudi M, Contractor KB, Lewis JS, Coombes RC, Stebbing J (2009) Peritoneal disease in breast cancer: a specific entity with an extremely poor prognosis. Eur J Cancer 45:2146–2149. https://doi.org/10.1016/j.ejca.2009.04.027
Ingham SL, Warwick J, Buchan I, Sahin S, O'Hara C, Moran A, Howell A, Evans DG (2013) Ovarian cancer among 8,005 women from a breast cancer family history clinic: no increased risk of invasive ovarian cancer in families testing negative for BRCA1 and BRCA2. J Med Genet 50:368–372. https://doi.org/10.1136/jmedgenet-2013-101607
Gulhan I, Eser S, Yakut C, Bige O, Ilhan E, Yildirim Y, Saygili U (2009) Second primary gynecologic cancers after breast cancer in Turkish women. Int J Gynecol Cancer 19:648–650. https://doi.org/10.1111/IGC.0b013e3181a12e8b
Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM (2007) Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat 105:195–207. https://doi.org/10.1007/s10549-006-9446-y
Kehl KL, Shen C, Litton JK, Arun B, Giordano SH (2016) Rates of BRCA1/2 mutation testing among young survivors of breast cancer. Breast Cancer Res Treat 155:165–173. https://doi.org/10.1007/s10549-015-3658-y
Manchanda R, Legood R, Burnell M, McGuire A, Raikou M, Loggenberg K, Wardle J, Sanderson S, Gessler S, Side L, Balogun N, Desai R, Kumar A, Dorkins H, Wallis Y, Chapman C, Taylor R, Jacobs C, Tomlinson I, Beller U, Menon U, Jacobs I (2015) Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst 107:380. https://doi.org/10.1093/jnci/dju380
Tserkezoglou A, Kontou S, Hadjieleftheriou G, Apostolikas N, Vassilomanolakis M, Sikiotis K, Salamalekis E, Tseke P, Magiakos G (2006) Primary and metastatic ovarian cancer in patients with prior breast carcinoma. Pre-operative markers and treatment results. Anticancer Res 26:2339–2344
Kumar P, Aggarwal R (2016) An overview of triple-negative breast cancer. Arch Gynecol Obstet 293:247–269. https://doi.org/10.1007/s00404-015-3859-y
Hudis CA, Gianni L (2011) Triple-negative breast cancer: an unmet medical need. Oncologist 16(Suppl 1):1–11. https://doi.org/10.1634/theoncologist.2011-S1-01
Meyer P, Landgraf K, Hogel B, Eiermann W, Ataseven B (2012) BRCA2 mutations and triple-negative breast cancer. PLoS One 7:e38361. https://doi.org/10.1371/journal.pone.0038361
Hahnen E, Hauke J, Engel C, Neidhardt G, Rhiem K, Schmutzler RK (2017) Germline mutations in triple-negative breast cancer. Breast Care (Basel) 12:15–19. https://doi.org/10.1159/000455999
Zhang M, Sun J (2013) Bone metastasis from ovarian cancer. Clinical analysis of 26 cases. Saudi Med J 34:1270–1273
Cormio G, Capotorto M, Di Vagno G, Cazzolla A, Carriero C, Selvaggi L (2003) Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol 90:682–685
Li Q, Xu BH, Zhang P, Li Q, Yuan P, Wang JY, Luo Y, Ma F, Fan Y, Li Q (2013) Clinicopathological features and prognostic factors of breast cancer patients with inguinal lymph node metastases: a report of 17 cases. Zhonghua Zhong Liu Za Zhi 35:207–211. https://doi.org/10.3760/cma.j.issn.0253-3766.2013.03.010
Raptis S, Kanbour AI, Dusenbery D, Kanbour-Shakir A (1996) Fine-needle aspiration cytology of metastatic ovarian carcinoma to the breast. Diagn Cytopathol 15:1–6. https://doi.org/10.1002/(SICI)1097-0339(199607)15:1<1::AID-DC2>3.0.CO;2-N
Bayramoglu H, Zekioglu O, Erhan Y, Ciris M, Ozdemir N (2002) Fine-needle aspiration biopsy of invasive micropapillary carcinoma of the breast: a report of five cases. Diagn Cytopathol 27:214–217. https://doi.org/10.1002/dc.10176
Jaffer S, Reid-Nicholson M, Bleiweiss IJ (2002) Infiltrating micropapillary carcinoma of the breast. Cytologic Findings Acta Cytol 46:1081–1087. https://doi.org/10.1159/000327111
Gown AM, Fulton RS, Kandalaft PL (2016) Markers of metastatic carcinoma of breast origin. Histopathology 68:86–95. https://doi.org/10.1111/his.12877
Sheikh UN, Cohen C, Siddiqui MT (2016) Utility of folate receptor alpha immunohistochemistry in cytology specimens of metastatic breast carcinoma, metastatic serous carcinoma of Mullerian origin, and primary lung adenocarcinoma. Diagn Cytopathol 44:369–376. https://doi.org/10.1002/dc.23448
Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, D'Argento E, Cassano A, Schinzari G, Barone C (2015) Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer 15:307–312. https://doi.org/10.1016/j.clbc.2015.03.010
Nakayama Y, Nakagomi H, Omori M, Inoue M, Takahashi K, Maruyama M, Takano A, Furuya K, Amemiya K, Ishii E, Oyama T (2016) Benefits of using the cell block method to determine the discordance of the HR/HER2 expression in patients with metastatic breast cancer. Breast Cancer 23:633–639. https://doi.org/10.1007/s12282-015-0615-x
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) by the Ministry of Education (2016R1D1A1B03935584) and the Ministry of Science, ICT and Future Planning (2017R1A2B4007704).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was reviewed and approved by the Institutional Review Board at Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea (4-2017-1247).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 160 kb)
Rights and permissions
About this article
Cite this article
Na, K., Lee, JY., Sung, JY. et al. Comparative clinicopathological and cytomorphological analyses of peritoneal carcinomatosis associated with metastatic breast carcinoma and primary peritoneal/ovarian carcinoma in patients with a history of breast carcinoma. Virchows Arch 473, 165–175 (2018). https://doi.org/10.1007/s00428-018-2390-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2390-5