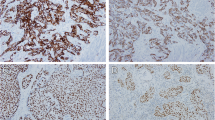
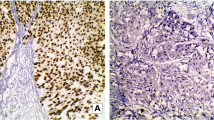
Abstract
Differential expression of cytokeratins (CK) is a characteristic feature of chemoresistant luminal (KRT20) and chemosensitive intrinsic aggressive basal (KRT5) subtypes in muscle-invasive bladder cancer (MIBC). We investigated mRNA expression of KRT5 and KRT20 and its predictive value in stage pT1 bladder cancer. In retrospective analysis of clinical data and formalin-fixed paraffin-embedded tissues (FFPE) of patients with stage pT1 NMIBC who underwent transurethral resection of the bladder, a single-step RT-qPCR was used to measure mRNA expression. Furthermore, immunohistochemical (IHC) staining of CK20, panCK, and MIB1 was performed. Valid measurements were obtained from 231 samples out of a series of 284 patients. Spearman correlation revealed significant associations between mRNA and protein expression of KRT20/CK20 (ρ 0.6096, p < 0.0001) and MKI67/MIB1 (ρ 0.5467, p < 0.0001). A positive correlation was found between MKI67 and KRT20 expression (ρ 0.3492, p < 0.0001), while MKI67 and KRT5 were negatively correlated (ρ −0.1693, p = 0.01). High KRT20 expression (≥40.26) was significantly associated with worse recurrence free survival (RFS) (p = 0.001), progression-free survival (PFS) (p = 0.0003), and cancer specific survival (CSS) (p = 0.0414). The combination of high KRT20 expression and low KRT5 expression (<36.83) was associated with unfavorable RFS (p = 0.0038) and PFS (p = 0.0003) and proved to be the only independent predictor for RFS (p = 0.0055) and PFS (p = 0.0023) in multivariate analysis. KRT20 mRNA determination was superior to CK20 protein estimation with regard to RFS and PFS prediction. KRT20 and KRT5 mRNA quantification can predict recurrence and progression of stage pT1 NMIBC reflecting basal and luminal subtypes of MIBC and is superior to CK20 protein expression determined by IHC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the ninth most common malignancy worldwide considering both genders, while 75% of all cases occur in male patients [1]. Approximately 75% of patients are initially diagnosed with non-muscle-invasive bladder cancer (NMIBC) [2]. Depending on stage and grade after transurethral resection, 50–70% of the patients develop disease recurrence and up to 20% of the tumors progress to a more invasive stage [2, 3]. Patients with NMIBC are monitored with cystoscopy over many years, which impose heavy costs to society which is why bladder cancer carries the highest cost among cancers per patient from diagnosis to death [4]. Therefore, molecular markers for stratifying patient treatment and application of novel therapeutic drugs are highly appreciated especially for stage pT1 bladder cancer.
NMIBC is stratified into three risk groups according to clinical (diameter, focality) and pathological (stage, grade, concomitant carcinoma in situ (Cis)) criteria [2]. This is the basis for treatment decision and follow-up strategy. To predict recurrence and progression of NMIBC, the European Organization for Research and Treatment of Cancer (EORTC) developed a score (EORTC score) [5]. To improve this limited clinicopathological risk calculator, various molecular markers have been investigated.
More recently, tumor heterogeneity has been investigated at molecular level, which allowed identification of distinct molecular classes beyond histopathological classification [6–8]. These resemble molecular features of the luminal and basal breast cancer subtypes with similar differences in clinical outcome [6–8]. Muscle-invasive bladder cancer (MIBC) has been shown to be of mainly basal- and luminal-cell origin [6–8].
Recently, a prospective comprehensive transcriptional study investigated gene expression in 460 NMIBC patients [10]. It was shown that NMIBC can be subclassified into three major classes with basal- and luminal-like characteristics and different clinical outcome [10].
Differential cytokeratin (CK) expression is a characteristic feature of muscle-invasive disease [11]. Cytokeratins are crucial for cell integrity and in different epithelia different combinations of the 20 CKs are found. Normal urothelium displays a combination of simple keratins including cytokeratin 20 (KRT20) and cytokeratin 5 (KRT5) [12]. KRT20 is physiologically exclusively expressed in umbrella cells [13], whereas KRT5 is expressed in basal cells [14]. In MIBC, it could be shown that KRT5 is a feature of the chemosensitive and intrinsic aggressive basal subtype, whereas KRT20 is a feature of the chemoresistant and less aggressive luminal subtype [11]. Sjödahl et al. studied bladder cancer of all stages and grades and found that KRT5 is expressed in the “Urobasal B” and “squamous cell carcinoma-like (SCC-like)” subgroups, whereas KRT20 is expressed in a subpopulation of the “Urobasal A” and of the “genomically unstable” groups [9].
To further characterize subtypes of stage pT1 NMIBC and their biological behavior, we investigated expression of “luminal” cytokeratin KRT20 and “basal” cytokeratin KRT5 in this challenging subentity. To avoid the numerous technical limitations of IHC, we developed and used RT-qPCR assessment for objective quantification of KRT20 and KRT5 mRNA expression. Moreover, we did immunostain for CK20 to explore whether approximation of molecular subtyping by standard IHC similarly reflects biological behavior.
Patients and methods
Study population
The total study cohort consisted of 284 patients with stage pT1 NMIBC at initial diagnosis who underwent transurethral resection of the bladder (TURB) in a single center between 1989 and 2009. Histopathological parameters of all cases, including grading according to WHO 1973 and WHO 2004 classification, were reassessed by a pathologist specialized in uropathology (A.H.). All patients underwent reresection and were treated according to an organ preserving approach.
Isolation of tumor RNA
For RNA extraction from FFPE tissue, a single 10-μm section was processed according to a commercially available bead-based extraction method (XTRACT kit; STRATIFYER Molecular Pathology GmbH, Cologne, Germany). RNA was eluted with 100 μl elution buffer and RNA eluates were then stored at −80 °C until use. The section was taken from a paraffin block containing at least 30% tumor cells.
Gene expression by RT-qPCR
RT-qPCR mRNA expression levels of KRT5, KRT20, and MKI67 and of CALM2 as reference gene (REF) were determined. This involves reverse transcription of RNA and subsequent amplification of cDNA, executed using Taqman Primer/Probes. Each patient sample or control was analyzed with each assay mix in triplicate. Experiments were run on a Siemens Versant (Siemens, Germany) according to the following protocol: 5 min at 50 °C, 20 s at 95 °C followed by 40 cycles of 15 s at 95 °C, and 60 s at 60 °C. Forty amplification cycles were applied and the cycle quantification threshold (Cq) values of three markers and one reference gene for each sample (S) were estimated as the median of the triplicate measurements. Final values were generated using ΔCq from the total number of cycles, to ensure that normalized gene expression obtained by the test is proportional to the corresponding mRNA expression levels.
Immunohistochemistry: assessment and evaluation
From the total cohort, a tissue microarray (TMA) was assembled. From formalin-fixed paraffin-embedded tissue blocks, one representative 1.5-mm core was used, proven by histopathologic comparison of the TMA core with full original sections of the entire tumor. Then, 4-μm sections were cut and mounted on poly-L-lysine-coated glass slides.
IHC staining was carried out in a BenchMark IHC Full System immunostainer (Roche Diagnostics, Mannheim, Germany) using the avidin-biotin peroxidase method with diaminobenzidine as a chromogen according to the manufacturer’s instructions. The primary antibodies used were anti-CK20, anti-MIB (each mouse monoclonal, dilution 1:200, incubation time 20 min, DAKO Deutschland, Germany), and anti-panCK (mouse monoclonal, dilution 1:50, incubation time 12 min, Immunotech Laboratories, USA). IHC staining was evaluated by a specialized uro-pathologist (A.H.).
Statistical methods
Statistical analysis was performed using SPSS version 23 and JMP 9.0.0. Cut-off definitions were done by Partitioning tests and Youden Index analysis. The Spearman product-moment correlation coefficient r was used as a measure of the strength and direction of the linear relationship between variables. Statistical analyses including Kaplan-Meier survival analysis, multivariate Cox regression, and partitioning testing were performed with JMP SAS (SAS Institute, Cary, NC, USA) and Graph Pad Prism software (Version 5.04; Graph Pad Software Inc., La Jolla, CA, USA).
Results
Patient population
The total study cohort consisted of 284 NMIBC tumor samples from 1989 to 2009 from a single institution. All tumors were staged pT1 in initial histopathological assessment and confirmed by histopathological reassessment in 2015/16. As 53 samples had to be excluded, data from 231 patients (78.4% male, median age 72 years) could be used for final evaluation (Suppl. Fig. 1). Of these, 70 were graded G2 and 161 were graded G3 according to the WHO Grading system 1979 (Table 1). Median follow-up time was 43 months. Median mRNA expression levels were for KRT5 36.86, for KRT20 40.59, and for MKI67 36.35.
Correlation of cytokeratin mRNA expression and protein expression by IHC
Non-parametric Spearman rank correlation revealed a positive, statistically significant association between mRNA expression and protein expression measured by RT-qPCR and IHC between KRT20/CK20 (ρ 0.6096, p < 0.0001) and MKI67/MIB1 (ρ 0.5467, p < 0.0001) (Suppl. Fig. 2). MKI67 and KRT20 showed a positive correlation (ρ 0.3492, p < 0.0001), whereas both MKI67 and KRT5 (ρ −0.1693, p = 0.010) and KRT20 and KRT5 (ρ −0.1804, p = 0.006) were negatively correlated (Suppl. Fig. 2).
Predictive value of cytokeratin expression
Kaplan-Meier analysis revealed a statistically significant association between high KRT20 mRNA expression (≥40.26) and poor recurrence-free (RFS) (p = 0.001), progression-free (PFS) (p = 0.0003), and carcinoma-specific survival (CSS) (p = 0.0414) (Fig. 1). High protein expression of CK20 in IHC with a cut-off of ≥80% of cells positive for CK20 was only associated with PFS (p = 0.0047) (Suppl. Fig. 3).
Taken together, high expression of KRT20 (≥40.26) and low expression of KRT5 (<36.83) showed a statistically significant association with poor RFS (p = 0.0038) and PFS (p = 0.0003) without an effect on CSS (Fig. 2). Expression levels of KRT5 were associated with PFS, but not with RFS and CSS.
Multivariate Cox regression analysis adjusted for gender, associated Cis, tumor size, focality, and WHO 1973 grading revealed subtyping according to the KRT mRNA expression (KRT20 ≥ 40.26 and KRT5 < 36.83) as the only statistically significant risk factor for RFS (L-R Chi2 10.40, p = 0.0055) and PFS (L-R Chi2 12.13, p = 0.0023) (Table 2). For CSS, tumor size (L-R Chi2 5.17, p = 0.0229) and grade 3 after WHO classification of 1973 (L-R Chi2 5.29, p = 0.0215) were the only statistically significant risk factors (Table 2).
KRT20 mRNA expression is predictive for grade 3 tumors
High KRT20 mRNA expression (≥40.26) subdivided high risk pT1G3 tumors into a high and a low risk group with 65% PFS versus 95% PFS after a 5-year follow-up, the latter with the same risk as pT1G2 tumors (p < 0.0001) (Fig. 3b). This was also observed for RFS (p = 0.0121) (Fig. 3a). Low KRT20-expression in a pT1G3 tumor was associated with intermediate CSS comparable to that of a pT1G2 tumor, while a pT1G3 tumor with high KRT20 expression carried the highest risk (p = 0.038) (Fig. 3c).
Discussion
Recently, different studies reported that MIBC can be subdivided into luminal and basal subtypes with different biological behavior [6–10]. The basal subtype presents with advanced stage and metastatic disease is intrinsically aggressive but chemosensitive [11]. The luminal subtype has less aggressive potential but often emerges as chemoresistant [11]. To distinguish between these subtypes, CK expression seems to be a potentially useful marker [11]. KRT5, which marks stem or progenitor cells and is expressed in the basal compartment of urothelium, characterizes the basal subtype [6, 14–16]. KRT20, which is expressed in superficial umbrella cells, is a marker for luminal subtypes [11, 13, 16]. As these findings have been reported for MIBC, we investigated whether differential CK expression has a predictive role in stage pT1 NMIBC. Conventional clinical and pathological risk factors have limited potential for risk stratification of this subentity, even though reliable and reproducible risk stratification is needed for these patients.
We show that mRNA expression of KRT20 is significantly associated with IHC expression of CK20. As IHC analysis is limited by high inter-observer variability which is also a problem in histopathological staging and grading [2], we used KRT20 mRNA expression as an objective and reproducible method for CK20 expression. Survival analysis revealed a statistically significant worse RFS, PFS, and CSS for NMIBC with high KRT20 expression. Furthermore, we show that KRT20 and MKI67 expression are positively correlated. This allows us to conclude that stage pT1 tumors with luminal characteristics, as reflected in high KRT20 expression, have a higher proliferative activity and are more aggressive. This is in contrast to previous reports in which luminal phenotype of MIBC was associated with less aggressive behavior after chemotherapy [11]. However, a recent prospective comprehensive transcriptional study of a large series of NMIBC showed that a subgroup of BC with luminal differentiation has a higher progression rate than basal-like cancers [10]. Our findings are also in line with previous IHC studies indicating that IHC expression of CK20 in NMIBC increases with stage and grade [17]. Alsheik et al. showed that CK20 is expressed in low grade NMIBC but without any correlation between CK20 IHC expression and recurrence [18]. In stage pT1 NMIBC, high CK20 expression by IHC is associated with unfavorable RFS and CSS [19, 20]. Various studies investigated KRT20 mRNA expression in different stages and grades of urothelial carcinoma of the bladder and in other tissues. In one study comparing normal urothelium with bladder cancer tissue, increased KRT20 mRNA expression was found to be associated with higher grade tumor tissue [21]. Two other studies reported an association between KRT20 mRNA expression in peripheral blood and higher stage and worse prognosis [22, 23]. Ribal et al. found KRT20 mRNA expression in lymph nodes of patients after radical cystectomy (RC) to be associated with higher stage and suggested that this might be used as a biomarker [24]. Subsequently, Gazquez et al. showed that KRT20 mRNA expression in lymph nodes of patients with MIBC is a more sensitive marker for the presence of micrometastasis than histologic evaluation, with predictive value for survival [25]. Furthermore, patients with high KRT20 expression in bone marrow prior to RC were found to have worse outcome [26]. Sjödahl et al. found that KRT20 is expressed in genomically unstable high grade tumors [9]. This subgroup shows intermediate disease-specific survival when all stages and grades are taken together [9].
We found in contrast that KRT5 mRNA expression is negatively correlated with MKI67 and KRT20 mRNA expression. The combination of high KRT20 and low KRT5 expression proved to be a statistically significant predictor for worse RFS and PFS. This also contrasts with previous findings in MIBC, of an association of high expression of KRT5 as basal marker with worse outcome after chemotherapy. Sjöhdahl et al. identified a subgroup of patients, with a “Urobasal B” or “SCC-like” tumor with high expression of KRT5 in IHC, that showed the worst disease-specific survival when all stages and grades were taken together [9]. A consensus conference defined the “basal-squamous-like” (BASQ) subtype, which is consistently associated with worse outcome and characterized by the expression of KRT5 [27]. Volkmer et al. postulated three different subtypes that stratify overall survival into a worse (KRT14+, KRT5+, KRT20−), intermediate (KRT14−, KRT5+, KRT20−), and differentiated (KRT14−, KRT5−, KRT20+) risk group [28]. Most studies focus on MIBC and there is the need for more detailed analysis of NMIBC as was stipulated in a recent consensus conference [27].
In our cohort, high expression of KRT20 divided stage pT1G3 tumors into two risk groups in defining a group of patients with high risk for progression and worse CSS who might benefit from early cystectomy. Earlier studies showed that early cystectomy is associated with improved long-term CSS in the subgroup of high risk T1G3 tumors [29]. As radical cystectomy has a 90-day mortality rate of up to 8%, a bladder preserving approach with BCG maintenance instillation therapy should be kept in mind [2]. To date, several IHC parameters have been evaluated to support risk stratification of this challenging entity, with limited potential [30]. We show that the single assessment of KRT20 mRNA expression is a valuable and simple approach towards reliable stratification of these patients.
The major weakness of the present study is its retrospective nature with data from a single center. These results need to be validated in a prospective multi-center study. We conclude that expression of KRT20 and KRT5 is predictive for recurrence and progression of stage pT1 NMIBC, reflecting basal and luminal subtypes of MIBC. This information might be used in risk stratification of this difficult entity, i.e., in identifying patients who might benefit from early cystectomy.
Abbreviations
- CSS:
-
Cancer-specific survival
- FFPE:
-
Formaline fixed paraffin embedded
- GOI:
-
Gene of interest
- HR:
-
Hazard ratio
- mRNA:
-
Messenger ribonucleic acid
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- REF:
-
Reference gene
- RFS:
-
Recurrence-free survival
- RT-qPCR:
-
Reverse transcription quantitative real time polymerase chain reaction
References
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F (2016) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. doi:10.1016/j.eururo.2016.06.010
Babjuk M, Böhle A, Burger M et al (2016) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. doi:10.1016/j.eururo.2016.05.041
Prout GR Jr, Barton BA, Griffin PP, Friedell GH (1992) Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J Urol 148:1413–1419
Hong YM, Loughlin KR (2008) Economic impact of tumor markers in bladder cancer surveillance. Urology 71:131–135
Sylvester RJ, van der Meijden A, Oosterlinck W et al (2006) Predicting recurrence and progression in individual patients with stage Ta, T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466–475
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507:315–222
Choi W, Porter S, Kim S et al (2014) Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25:152–165
Damrauer JS, Hoadley KA, Chism DD et al (2014) Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 111:3110–3115
Sjodahl G, Lauss M, Lovgren K et al (2012) A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 18:3377–3386
Hedegaard J, Lamy P, Nordentoft I et al (2016) Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30(1):27–42
Choi W, Czerniak B, Ochoa A et al (2014) Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol. 11:400–410
Schaafsma HE, Ramaekers FCS, van Muijen GNP et al (1989) Distribution of cytokeratin polypeptides in epithelia of the adult human urinary tract. Histochemistry 91:151–159
Moll R, Löwe A, Laufer J et al (1992) Cytokeratin 20 in human carcinomas: a new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 140:427–447
Reis-Filho JS, Simpson PT, Martins A, Preto A, Gärtner F, Schmitt FC (2003) Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch 443(2):122–132
Ho PL, Kurtova A, Chan KS (2012) Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol 9:583–594
Dadhania V, Zhang M, Zhang L et al (2016) Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine. doi:10.1016/j.ebiom.2016.08.036
Desai S, Lim SD, Jimenez RE et al (2000) Relationship of cytokeratin 20 and CD44 protein expression with WHO/ISUP grade in pTa and pT1 papillary urothelial neoplasia. Mod Pathol 13(12):1315–1323
Alsheikh A, Mohamedali Z, Jones E, Masterson J, Gilks CB (2001) Comparison of the WHO/ISUP classification and cytokeratin 20 expression in predicting the behavior of low-grade papillary urothelial tumors. World/Health Organization/Internattional Society of Urologic Pathology. Mod Pathol 14(4):267–272
Bertz S, Otto W, Denzinger S et al (2014) Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur Urol 65:218–226
Harnden P, Mahmood N, Southgate J (1999) Expression of cytokeratin 20 redefines urothelial papillomas of the bladder. Lancet 353(9157):974–977
Christoph F, Müller M, Schostak M, Soong R, Tabiti K, Miller K (2004) Quantitative detection of cytokeratin 20 mRNA expression in bladder carcinoma by real-time reverse transcriptase-polymerase chain reaction. Urology 64(1):157–161
Güdemann CJ, Weitz J, Kienle P et al (2000) Detection of hematogenous micrometastasis in patients with transitional cell carcinoma. J Urol 164(2):532–536
Okegawa T, Kinjo M, Nutahara K, Higashihara E (2004) Value of reverse transcription polymerase chain assay in peripheral blood of patients with urothelial cancer. J Urol 171(4):1461–1466
Ribal MJ, Mengual L, Marín M et al (2006) Molecular staging of bladder cancer with RT-PCR assay for CK20 in peripheral blood, bone marrow and lymph nodes: comparison with standard histological staging. Anticancer Res 26:411–419
Gazquez C, Ribal MJ, Marín-Aguilera M et al (2012) Biomarkers vs conventional histological analysis to detect lymph node micrometastases in bladder cancer: a real improvement? BJU Int 110(9):1310–1316
Retz M, Rotering J, Nawroth R et al (2011) Long-term follow-up of bladder cancer patients with disseminated tumour cells in bone marrow. Eur Urol 60(2):231–238
Lerner SP, McConkey DJ, Hoadley KA et al (2016) Bladder cancer molecular taxonomy: summary from a consensus meeting. Bladder Cancer 2(1):37–47
Volkmer JP, Sahoo D, Chin RK et al (2012) Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A 109(6):2078–2083
Denzinger S, Fritsche HM, Otto W, Blana A, Wieland WF, Burger M (2008) Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol 53:146–152
Park J, Song C, Shin E, Hong JH, Kim CS, Ahn H (2013) Do molecular biomarkers have prognostic value in primary T1G3 bladder cancer treated with bacillus Calmette-Guerin intravesical therapy? Urol Oncol 31:849–856
Acknowledgements
We would like to thank Stefanie Herlein, Elke Veltrup, and Silke Claas for excellent technical support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Informed consent was obtained from all the individual participants included in the study. The study was conducted after approval of the local ethics committee.
Funding
This study was funded by the German Cancer Aid (Deutsche Krebshilfe (DKH)), grant number 110541.
Competing interests
RMW and SE are founders of STRATIFYER Molecular Pathology GmbH. RMW is an employee of STRATIFYER Molecular Pathology GmbH.
Additional information
Johannes Breyer, Ralph M. Wirtz, Wolfgang Otto, Philipp Erben, Maximilian C. Kriegmair, Robert Stoehr, Markus Eckstein, Sebastian Eidt, Maximilian Burger, and Arndt Hartmann are members of the BRIDGE Consortium.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Breyer, J., Wirtz, R.M., Otto, W. et al. In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch 470, 267–274 (2017). https://doi.org/10.1007/s00428-017-2064-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2064-8