Abstract
Purpose
There is an unmet need to develop prognostic biomarkers in post-neoadjuvant chemotherapy (NAC) muscle-invasive bladder cancer (MIBC) patients. We examine whether Ki-67 and PD-L1 expression can be used to guide adjuvant therapy.
Methods
Tissue microarrays were constructed from 130 post-NAC radical cystectomy samples. Up to 5 cores per sample were included. Expressions of Ki-67 and PD-L1 were evaluated using immunohistochemistry (IHC).
Results
Using a Cox regression model, positive Ki-67 expression in post-NAC radical cystectomy samples was associated with poorer overall survival (OS) (HR = 2.412, 95% CI, 1.076–5.408), independent of the pathological lymph node/N-stage. Positive Ki-67 expression was also associated with lack of tumor downstaging in a multivariable logistic regression model analysis (OR = 0.081, 95% CI, 0.014–0.464). PD-L1− and PD-L1+ expression was associated with a median OS of 49.8 months and 26.9 months, respectively, which did not reach statistical significance. Patients with Ki-67/PD-L1 double-negative tumors had a significantly longer median OS of 98.2 months versus 29.9 and 26.9 months in PD-L1−/Ki-67+ and PD-L1+/Ki-67+ tumors, respectively. Lack of tumor downstaging was significantly associated with positive Ki-67 and positive PD-L1 expression.
Conclusion
Positive Ki-67 and PD-L1 expression in post-NAC radical cystectomy samples was associated with inferior OS and absence of tumor downstaging. IHC on Ki-67 and PD-L1 would help to select patients for adjuvant therapy in post-NAC muscle-invasive bladder cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the ninth leading cause of cancer and ranks 13th in mortality rates worldwide [1]. In 2020, 81,400 new bladder cancer cases (62,100 men/19,300 women) and 17,980 bladder cancer-related deaths (13,050 men/4930 women) are predicted to occur. These deaths will largely be attributable to metastatic bladder cancers, which tend not to develop until the cancers in the bladder become muscle-invasive (MIBC). Despite the approval of check point inhibitors and the antibody drug conjugate enfortumab vedotin [2, 3] to treat this disease, the 5-year overall survival (OS) rate for metastatic bladder cancer remains less than 10%.
Adding cisplatin-based neoadjuvant chemotherapy (NAC) to radical cystectomy has been in shown in a randomized phase 3 trial to improve OS when compared to upfront radical cystectomy [4]. However, lack of tumor downstaging and microscopic lymph node metastases are commonly observed in post-NAC radical cystectomy tissues. Although adjuvant therapy may offer a potential cure for these patients, a retrospective review of 129 cases from the International Study of Cancers of the Urothelium database found that adjuvant chemotherapy for MIBC with lack of tumor downstaging after NAC did not improve OS [5]. This finding is confounded by the absence of effective adjuvant therapies for patients with bladder cancers which do not respond to platinum-based NAC. Furthermore, there are no validated biomarkers that can be used to risk-stratify patients with MIBC following NAC treatment beyond tumor pathological staging. Most published biomarker studies were performed using tissue samples from chemotherapy-naïve patients following radical cystectomy or precystectomy transurethral resection of bladder tumor [6,7,8].
In addition to these studies on NAC, adjuvant anti-programmed cell death 1 (anti-PD1) or anti-programmed cell death ligand 1 (anti–PD-L1) therapies for patients with bladder cancer are being tested in ongoing randomized double-blind, placebo-controlled phase 3 trials. The basis for conducting these trials is the clinical efficacy and safety data of anti-PD1 and anti–PD-L1 therapies in treating metastatic or locally advanced urothelial cancers that have progressed through frontline platinum-based chemotherapy. PD-L1 expression as detected by immunohistochemistry (IHC) in tumor-infiltrating immune cells and urothelial carcinoma cells is not a predicative biomarker for treatments with these immune check point inhibitors [9]. In terms of its potential use as a prognostic biomarker, PD-L1 IHC positivity in tumor-infiltrating mononuclear cells, but not tumor cells, has been reported to be associated with OS in patients with urothelial carcinoma [10]. However, this association has not been validated in other studies. The mouse monoclonal antibodies used in this study were different from the anti-PD-L1 antibodies developed by Ventana or DAKO for anti-PD1 or PD-L1 therapies. Compared to PD-L1, Ki-67 is a well-established tumor proliferation marker, helping to disperse individual chromosomes and organize heterochromatin during mitosis [11, 12]. Positive Ki-67 IHC in radical cystectomy tissue samples has been associated with increased disease recurrence and cancer-specific mortality in patients with non-muscle-invasive T1 bladder cancer [6, 8].
We recently reported the clinical outcomes of 824 patients with MIBC who underwent radical cystectomy at Moffitt Cancer Center between January 29, 2007 and March 1, 2012 [13]. Of the 824 patients identified, 332 received platinum-based NAC, of whom 130 had available post-NAC cystectomy tissue blocks. Tissue microarrays (TMAs) were constructed from these 130 samples. To test whether Ki-67 and PD-L1 expression can be used to guide adjuvant therapy, we conducted IHC studies of Ki-67 and PD-L1 on these TMAs and correlated IHC positivity with clinical outcome data.
Methods
Data sources and study population
The 130 cases included in this retrospective study are patients who underwent platinum-based NAC followed by radical cystectomy at Moffitt Cancer Center January 29, 2007 and March 1, 2012. The clinical, administrative, pharmaceutical, and cancer registry data used in our retrospective analyses were integrated by the Health and Research Informatics system at Moffitt Cancer Center. Study compliance and regulation were overseen by the Moffitt Cancer Center Scientific Review Committee and Institutional Review Board.
Study measures and definitions
Available demographic information included age, gender, race, and education level. Chemotherapy agents for each patient were recorded with start and finish dates. These dates were compared against radical cystectomy dates to confirm neoadjuvant administration. The most common regimens were gemcitabine–cisplatin, gemcitabine–carboplatin, MVAC (methotrexate, vinblastine, adriamycin, cisplatin), and dose-dense MVAC; the remaining regimens, such as fluorouracil, etoposide, and paclitaxel-based chemotherapy, were categorized as other. Clinical staging was determined by pathological findings from transurethral resections of the bladder tumor and supplemented with results from radiological studies. Pathological staging was determined following radical cystectomy from reports measuring tumor, node, metastasis (TNM) classification, histology, lymph node counts, and surgical margins. Disease status, vital status, and follow-up duration were determined using the Health and Research Informatics cancer registry death index.
Immunohistochemistry
Radical cystectomy samples were embedded in paraffin to construct TMAs. Up to 5 cores were taken from each sample. Matched lymph node metastases were included if present. IHC on Ki-67 was performed at Moffitt Cancer Center Tissue Core with the Ventana Discovery XT automated system (Oro Valley, AZ). A heat-induced antigen retrieval method and a rabbit primary antibody reactive to Ki-67 (#790-4286 [Ventana]) was used. IHC intensity on a scale from 0 to 3 was multiplied by cellularity to obtain H-scores of Ki-67. Average H-scores of Ki-67 were calculated for samples with multiple cores. The DAKO 22C3 assay for PD-L1 IHC was performed at Moffitt Cancer Center’s clinical pathology laboratory and scored by a clinical pathologist. A combined positive score of 10 or above was considered positive for PD-L1. For samples with multiple cores, PD-L1 was deemed positive if 1 or more cores were positive for PD-L1 on the DAKO 22C3 assay. Binary expression status was determined by the presence or absence of any staining across each patient’s combined core samples.
Outcomes
Outcomes of interest included post-NAC pathological staging, cancer-specific survival and OS time. OS was measured from the date of cystectomy to the date of death from any cause.
Statistical analyses
The Wilcoxon rank sum test, χ2 independent test, and Fisher’s exact test were used to compare demographic and clinical characteristics between protein marker expression groups. OS was estimated using the Kaplan–Meier method. The marker expression groups were compared with the use of the log-rank test, and the Cox proportional hazards regression model was used to estimate hazard ratios (HRs). Logistic regression analyses were performed to associate protein expression data with binary outcomes in regard to tumor downstaging and complete response. In all regression analyses, predictive variables on univariate model analyses (P < 0.05) were included in an initial multivariable regression model. Backward variable selection was then used to remove statistically insignificant variables and obtain a parsimonious model.
Results
Post-NAC T staging, bladder cancer histology, NAC regimen, adjuvant chemotherapy and OS
The median age at diagnosis of MIBC was 65 years (range 33–84) for the 130 patients included in the post-NAC TMA analyses. Overall survival and gender information were available for 116 patients. Consistent with prior publications, univariate Cox proportional model analysis showed lack of tumor downstaging (log rank P < 0.001), lack of pathological complete response (log rank P = 0.003) and positive lymph node involvements (log rank P < 0.001) after NAC are significantly associated with worse OS. Among the 116 cases, 74 had pure urothelial carcinoma and 42 had urothelial carcinoma mixed with variant histology; 90 received cisplatin-based regimen and 22 received carboplatin-based regimen. Only 17 out of the 116 cases received adjuvant chemotherapy. As shown in Fig. 1, no statistically significant association was observed between OS and cancer histology, NAC regimen, or the adjuvant chemotherapy status.
Positive Ki-67 expressions were associated with inferior OS and lack of tumor downstaging in post-NAC bladder cancer patients
The median Ki-67 IHC H-score was 60.5 (0.300). The median OS for the 92 Ki-67+ patients was 27.7 months (range 1.7–127), which was significantly shorter (P = 0.0035) than the 98.2-month (range 1–103) median OS among the 23 Ki-67− patients (Fig. 2a; Log-rank test, P = 0.0041). As shown in Fig. 2c, cancer-specific survival was also significantly inferior in Ki-67+ patients. Consistent with the inferior survivals, positive Ki-67 IHC was significantly associated with the absence of complete response (P < 0.001) and tumor downstaging (P < 0.001) (Table 1). Using a multivariable Cox regression model of OS consisted of the average Ki-67 H-score and pathological N-stage. Positive Ki-67 IHC (H-score > 0) in post-NAC radical cystectomy samples was associated with worse OS (Table 2), independent of the pathological N-stage. Ki-67 presence was also significantly associated with the lack of tumor downstaging in a multivariable logistic regression model (odds ratio = 0.081; 95% CI, 0.014–0.464; P = 0.004) while adjusting for adjuvant chemotherapy and pathological complete response.
Expression of PD-L1 and the prognostic value of combining PD-L1 and Ki-67 expressions in post-NAC bladder cancer
Overall, 38% of post-NAC bladder cancer tissue samples had PD-L1+ IHC [combined positive score (CPS) > 10] on the DAKO 22C3 assay. Although the 64 PD-L1− patients had a median OS of 49.83 months compared to the median OS of 26.97 months in the 52 PD-L1+ patients, the difference in OS based on PD-L1 positivity was not statistically significant (Fig. 2b; Log-rank test; P = 0.1266). A trend toward better cancer-specific survival was noted in PD-L1− patients, but this trend is not statistically significant (Fig. 2d). PD-L1 IHC positivity was significantly associated with lack of pathological complete response (OR = 0.16; 95% CI, 0.05–0.59; P = 0.006) and tumor downstaging (OR = 0.29; 95% CI, 0.13–0.67; P = 0.003) in the logistic regression analyses. Lymph node invasion (81% vs 53%; P = 0.052) and perineural invasion (44% vs 27%; P = 0.041) were more common in PD-L1+ than PD-L1− post-NAC bladder cancer patients (Table 1).
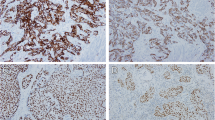
Positive Ki-67 IHC expression in post-NAC bladder cancer was significantly associated with positive PD-L1 IHC expression (P = 0.001). When PD-L1 positivity was combined with Ki-67 status, Ki-67−/PD-L1− tumors had a significantly longer median OS rate of 98.23 months (49.83–not reached) versus 23.71 (15.94–48.68) and 28.57 (16.27–55.58) months in PD-L1−/Ki-67+ and PD-L1+/Ki-67+ populations, respectively (Log-rank test, P = 0.0361) (Fig. 3a). Similar pattern was seen in cancer-specific survival (Fig. 3b). Representative positive and negative IHC on Ki-67 and PD-L1 is shown in Fig. 3c. Of note, most tumor cells coexpress Ki-67 and PD-L1 in PD-L1+/Ki-67+ post-NAC bladder cancer samples.
Kaplan–Meier curves for overall survival (a) and cancer-specific survival (b) in post-NAC bladder cancer patients stratified by combined Ki-67 and PD-L1 status. c Representative TMA cores from 2 male patients with post-NAC pT2bN0 bladder cancer; upper panels show the patient with positive IHCs on Ki-67 (brown nuclear staining) and PD-L1 (brown membrane staining); lower panel shows the negative IHCs on Ki-67 and PD-L1. Hematoxylin and eosin stain on these 2 cases are also shown
Discussion
Current clinical guidelines do not recommend adjuvant systemic therapy for patients with lack of tumor downstaging after NAC administration in part because of the lack of effective adjuvant systemic therapy beyond platinum-based chemotherapy. Recently the phase 3 clinical trial IMvigor010, in which adjuvant anti-PD-L1 with atezolizumab is compared with placebo, did not reach its primary endpoint of improving disease-free recurrence. In addition to finding more effective treatments, identifying prognostic biomarkers beyond TNM staging is equally important to identify patients who would benefit from adjuvant therapy.
Ki-67 overexpression and alterations in the expression of cell cycle regulators have been associated with poor urothelial bladder cancer outcomes in terms of both disease recurrence and OS [6, 14,15,16]. However, these studies were conducted among patients with non-muscle-invasive bladder cancer or among those who were chemotherapy naïve. In the present study, we report that positive Ki-67 IHC was associated with inferior OS and lack of tumor downstaging in post-NAC bladder cancer, and its association with OS is independent of pathological staging of the resected tumor. Unlike prior studies with H-score cutoffs on Ki-67, our study determined binary expression status as the presence or absence of any staining across each patient’s TMA cores. This binary determination on Ki-67 IHC is easier to interpret and is more repeatable compared to using H-score cutoffs. If validated by future studies, Ki-67 IHC can serve as a prognostic biomarker for post-NAC bladder cancer.
Prior studies reported increased PD-L1 expression in muscle invasive bladder cancer, but these results are mixed regarding the correlation between PD-L1 expression and OS [10, 17, 18]. Neoadjuvant trials with anti-PD1 or anti-PD-L1 therapy have reported promising clinical activity with pathological CR rate above 30% [19, 20]. The role of PD-L1 IHC as a predicative biomarker for NAC remains to be determined. In our studies on post-NAC bladder cancer, samples with PD-L1 CPS scores above 10 had a lack of tumor downstaging and inferior OS, but only the association with tumor downstaging reached statistical significance. Intriguingly, PD-L1 expression was strongly associated with Ki-67 positivity, which indicates active immune suppression in proliferating bladder cancers after NAC administration. Moreover, PD-L1−/Ki-67− tumors demonstrated a significantly longer OS than that of other subsets (Log-rank test; P = 0.0361). The prognostic value of Ki-67 will likely be further improved by adding PD-L1 IHC particularly in newly diagnosed MIBC prior to NAC.
Limitations of this study include selection bias inherent to retrospective studies. Many post-NAC cases do not have available pre-NAC tumor biopsy samples to determine the PD-L1 and Ki-67 status before NAC administration. The relative small sample size and unbalanced sample sizes among some subgroups also preclude robust subgroup and multivariate analysis. Nonetheless, our findings, if validated by future studies, may help to risk-stratify post-NAC bladder cancer patients. The inferior OS observed in the PD-L1 and Ki-67 double-positive bladder cancer cases and the strong association between PD-L1 and Ki-67 expression support combining cytotoxic chemotherapy with anti-PD1 or anti-PD-L1 immunotherapy as an adjuvant approach for this subset of patients with post-NAC bladder cancers.
Conclusion
Ki-67 IHC positivity is associated with worse OS and lack of tumor downstaging in MIBC patients who have been treated with platinum-based NAC. Positive Ki-67 expression is strongly associated with positive PD-L1 expression. IHC on Ki-67 and PD-L1 would help to select patients for adjuvant therapy in post-NAC muscle-invasive bladder cancer.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MIBC:
-
Muscle-invasive bladder cancer
- MVAC:
-
Methotrexate, Vinblastine, Doxorubicin/Adriamycin, Cisplatin
- NAC:
-
Neoadjuvant chemotherapy
- OS:
-
Overall survival
- TMA:
-
Tissue microarray
References
Urinary Bladder (Invasive & In Situ) Cancer Recent Trends in U.S. Mortality Rates, 2000–2016 By Sex (2000–2016) National Cancer Institute.
Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, Flaig TW, Baranda J, Lang J, Plimack ER, Sangha R, Heath EI, Merchan J, Quinn DI, Srinivas S, Milowsky M, Wu C, Gartner EM, Zuo P, Melhem-Bertrandt A, Petrylak DP (2020) EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 38(10):1041–1049. https://doi.org/10.1200/JCO.19.02044
Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, Galsky MD, Hahn NM, Gartner EM, Pinelli JM, Liang SY, Melhem-Bertrandt A, Petrylak DP (2019) Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 37(29):2592–2600. https://doi.org/10.1200/JCO.19.01140
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Raghavan D, Crawford ED (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349(9):859–866. https://doi.org/10.1056/NEJMoa022148
Martinez Chanza N, Werner L, Plimack E, Yu EY, Alva AS, Crabb SJ, Powles T, Rosenberg JE, Baniel J, Vaishampayan UN, Berthold DR, Ladoire S, Hussain SA, Milowsky MI, Agarwal N, Necchi A, Pal SK, Sternberg CN, Bellmunt J, Galsky MD, Harshman LC, Investigators R (2019) Incidence, patterns, and outcomes with adjuvant chemotherapy for residual disease after neoadjuvant chemotherapy in muscle-invasive urinary tract cancers. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2018.12.013
Shariat SF, Bolenz C, Godoy G, Fradet Y, Ashfaq R, Karakiewicz PI, Isbarn H, Jeldres C, Rigaud J, Sagalowsky AI, Lotan Y (2009) Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol 182(1):78–84. https://doi.org/10.1016/j.juro.2009.02.125(discussion 84)
Shariat SF, Tokunaga H, Zhou J, Kim J, Ayala GE, Benedict WF, Lerner SP (2004) p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol 22(6):1014–1024. https://doi.org/10.1200/JCO.2004.03.118
Vetterlein MW, Roschinski J, Gild P, Marks P, Soave A, Doh O, Isbarn H, Höppner W, Wagner W, Shariat SF, Brausi M, Büscheck F, Sauter G, Fisch M, Rink M (2017) Impact of the Ki-67 labeling index and p53 expression status on disease-free survival in pT1 urothelial carcinoma of the bladder. Transl Androl Urol 6(6):1018–1026. https://doi.org/10.21037/tau.2017.11.10
Sweis RF, Galsky MD (2016) Emerging role of immunotherapy in urothelial carcinoma-Immunobiology/biomarkers. Urol Oncol 34(12):556–565. https://doi.org/10.1016/j.urolonc.2016.10.006
Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, Signoretti S (2015) Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 26(4):812–817. https://doi.org/10.1093/annonc/mdv009
Sobecki M, Mrouj K, Camasses A, Parisis N, Nicolas E, Llères D, Gerbe F, Prieto S, Krasinska L, David A, Eguren M, Birling MC, Urbach S, Hem S, Déjardin J, Malumbres M, Jay P, Dulic V, Lafontaine DLJ, Feil R, Fisher D (2016) The cell proliferation antigen Ki-67 organises heterochromatin. Elife 5:e13722. https://doi.org/10.7554/eLife.13722
Sun X, Bizhanova A, Matheson TD, Yu J, Zhu LJ, Kaufman PD (2017) Ki-67 contributes to normal cell cycle progression and inactive X heterochromatin in p21 checkpoint-proficient human cells. Mol Cell Biol. https://doi.org/10.1128/MCB.00569-16
Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, Manley B, Spiess PE, Poch MA, Sexton WJ, Fishman M, Zhang J, Gilbert SM (2018) Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol 4(11):1535–1542. https://doi.org/10.1001/jamaoncol.2018.3542
Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, Montorsi F, Bastian PJ, Nielsen ME, Müller SC, Rigaud J, Heukamp LC, Netto G, Lerner SP, Sagalowsky AI, Shariat SF (2009) Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst 101(2):114–119. https://doi.org/10.1093/jnci/djn451
Wang L, Zhou M, Feng C, Gao P, Ding G, Zhou Z, Jiang H, Wu Z, Ding Q (2016) Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy. Int Urol Nephrol 48(4):495–501. https://doi.org/10.1007/s11255-015-1197-4
Shariat SF, Passoni N, Bagrodia A, Rachakonda V, Xylinas E, Robinson B, Kapur P, Sagalowsky AI, Lotan Y (2014) Prospective evaluation of a preoperative biomarker panel for prediction of upstaging at radical cystectomy. BJU Int 113(1):70–76. https://doi.org/10.1111/bju.12343
Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Küfer R, Zerbib M, Kwon E, Thompson RH, Boorjian SA, Shariat SF (2014) Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol 40(1):121–127. https://doi.org/10.1016/j.ejso.2013.08.023
Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED, Frank I (2008) T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 14(15):4800–4808. https://doi.org/10.1158/1078-0432.CCR-08-0731
Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Szabados B, Pous AF, Gravis G, Herranz UA, Protheroe A, Ravaud A, Maillet D, Mendez MJ, Suarez C, Linch M, Prendergast A, van Dam PJ, Stanoeva D, Daelemans S, Mariathasan S, Tea JS, Mousa K, Banchereau R, Castellano D (2019) Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 25(11):1706–1714. https://doi.org/10.1038/s41591-019-0628-7
Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, Montironi R, Giannatempo P, Farè E, Pederzoli F, Bandini M, Bianchi M, Colombo R, Gandaglia G, Fossati N, Marandino L, Capitanio U, Dehò F, Ali SM, Chung JH, Ross JS, Salonia A, Briganti A, Montorsi F (2020) Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 77(4):439–446. https://doi.org/10.1016/j.eururo.2019.10.026
Acknowledgements
Dr. Alex Lopez performed pathological scoring of immunohistochemistry on AR and Ki-67. This work is supported in part by the Moffitt’s Tissue Core performed fixing and IHC staining, and Biostatistics and Bioinformatics Shared Resources at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292). We thank them for their help. Editorial assistance was provided by the Moffitt Cancer Center’s Scientific Editing Department by Dr. Paul Fletcher & Daley Drucker. No compensation was given beyond their regular salaries.
Funding
This study is funded by Moffitt’s internal research support to Jingsong Zhang.
Author information
Authors and Affiliations
Contributions
SR: data collection and manuscript writing; YK and JZ: data analysis and manuscript writing; JD: data collection; RL, PS, MP, BJM, JP, SG, and WS: data collection and manuscript editing; JZ: project development, data management, data analysis and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Zhang has received honoraria for advisory board or speaker program from AstraZeneca, Merck and Seattle Genetics.
Ethics approval and consent to participate
This study qualified for expedited approval under the federal regulations at 45CFR46.116(d) per the USF Institutional Review Board, IRB #Pro00015860.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rubino, S., Kim, Y., Zhou, J. et al. Positive Ki-67 and PD-L1 expression in post-neoadjuvant chemotherapy muscle-invasive bladder cancer is associated with shorter overall survival: a retrospective study. World J Urol 39, 1539–1547 (2021). https://doi.org/10.1007/s00345-020-03342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03342-5