Abstract
Mammary analogue secretory carcinoma (MASC) has a specific ETV6-NTRK3 translocation and morphologically overlaps with acinic cell carcinoma (AciCC). Before the recognition of MASC, in AciCC, four histologic patterns were identified including microcystic, solid, papillary-cystic, and follicular. The aim of this study was to evaluate histologic patterns in these two neoplasms through comprehensive histologic subtyping. Using fluorescence in situ hybridization (FISH), we identified 14 cases of MASC and 21 cases of AciCC. We used comprehensive histologic subtyping to provide a semiquantitive assessment of histologic patterns in each tumor and performed immunohistochemical analyses including S100/vimentin/mammaglobin/DOG1. MASC often presented papillary-cystic patterns without a solid component, previously considered to be one of the four major patterns associated with AciCC. However, in our study, this histologic feature was rarely seen in AciCC and more characteristic of MASC. In aspiration cytology samples, MASC was associated with more cellular atypia. An immunohistochemical panel of S100/mammaglobin/DOG1 was found useful for differential diagnosis. Comprehensive subtyping of histologic patterns is a useful screening method prior to initiation of molecular testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammary analogue secretory carcinoma (MASC) of the salivary gland has recently been identified as a distinct type of salivary gland carcinoma with histologic features and gene mutations similar to breast secretory carcinomas [1]. Both tumor types contain a t(12; 15) translocation involving the ETS variant 6 (ETV6) gene on chromosome 12 and the neurotrophic tyrosine kinase receptor type 3 (NTRK3) gene on chromosome 15 [1–3]. ETV6 belongs to the family of ETS transcription factors. The carboxy-terminus of this gene harbors the DNA binding domain, and the amino-terminus contains a helix-loop-helix (HLH) domain [2]. NTRK3 encodes a membrane-anchored receptor tyrosine kinase. This enzyme regulates downstream signaling including the mitogen-activated protein kinase (MAPK) pathway [2]. Conversely, the ETV6-NTRK3 fusion gene produces a chimeric protein comprising the HLH domain of ETV6 and the tyrosine kinase domain of NTRK3. This chimeric protein is capable of spontaneous oligomerization, which in turn allows downstream signaling pathways to be activated independently of ligands [2].
Prior to the recognition of the ETV6-NTRK3 translocation in MASCs, most of these tumors were diagnosed as acinic cell carcinomas (AciCC), due to the morphological similarity between these two neoplasms. According to the World Health Organization (WHO) Classification of Head and Neck Tumours, AciCCs present four major histologic patterns: microcystic, solid, papillary-cystic, and follicular [4]. Unfortunately, this classification was based on data that included MASCs [1, 3–11]. We therefore sought to determine the validity of the notion that AciCC is characterized by four histologic patterns.
A diagnosis of MASC is based on a molecular test that confirms the presence of an ETV6-NTRK3 translocation. Common diagnostic techniques include fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR). Many studies tried to use immunohistochemistry as a screening method before applying molecular tests [1, 3–14]. We employed both comprehensive histologic subtyping and immunohistochemical methods (S100/vimentin/mammaglobin/DOG1 staining) to investigate differences between AciCCs and MASCs.
Materials and methods
Case selection and clinicopathological review
Since 2012, in our hospital, MASC diagnoses are confirmed using FISH tests. Therefore, we included cases if (1) they had been diagnosed as AciCC between 2000 and 2013 or as MASC between 2012 and 2013 and (2) associated tissue samples had been preserved as slides and paraffin blocks in the archives of the Department of Pathology, National Taiwan University Hospital. Clinical information of each patient, including age, sex, clinical symptoms, fine-needle aspiration report, tumor location, tumor size, lymph node status, pathologic stage, and local recurrence during clinical follow-up, was collected from medical records. Hematoxylin and eosin-stained slides were reviewed by two pathologists (M.S.H. and Y.H.C.), and the following histologic features were recorded: (1) histologic pattern (i.e., microcystic, solid, papillary-cystic, or follicular), (2) predominant growth pattern, (3) secretions within microcystic or tubular spaces, (4) degree of necrosis, (5) degree of hemorrhage or hemosiderin deposition, (6) presence of vacuolated cytoplasm, (7) nuclear contours, (8) nuclear size compared with small lymphocytes, (9) chromatin patterns (vesicular or condensed), (10) presence of distinct nucleoli, (11) presence of cytoplasmic zymogen granules, and (12) presence of perineural or lymphovascular invasion. This study (103-002523) was approved by the Research Ethics Committee of the National Taiwan University Hospital.
Detection of ETV6 gene translocation by FISH
Commercial Vysis ETV6 Dual Color Break Apart FISH Probe (Abbott Molecular Inc., Des Plaines, IL) was used to assess ETV6 translocation. Briefly, 4 μm of paraffin-embedded tissue section slides were deparaffinized in xylene (three times, 10 min each), followed by two 5-min washes in 100 % ethanol. Sections were then treated with pretreatment reagent (Abbott Molecular) at 80 °C for 30∼50 min, whereupon sections were treated with protease mixed with a protease buffer. Sections were hybridized using an ETV6 dual color probe with a Spectrum Orange-labeled segment at the 5′-end (telomeric side) of the ETV6 breakpoint and a Spectrum Green-labeled segment at 3′-end (centromeric side) of the ETV6 gene breakpoint. Results were analyzed using a fluorescence microscope (Zeiss AXIO Imager.D2) and AxioVision 4.5 software. Two pathologists (M.S.H. and Y.H.C.) individually scored 50 selected, non-overlapping nuclei for each case. Cases were considered positive when ≧15 % of the tumor cells presented split signals.
Detection and sequencing of ETV6-NTRK3 fusion transcripts by RT-PCR
The fusion transcript was detected by RT-PCR according to a previously described method [1]. Briefly, RNA from the formalin-fixed, paraffin-embedded tissue was extracted using the RNeasy FFPE kit (Qiagen, Hilden, Germany), and complementary (cDNA) was synthesized using the Superscript III First-Strand Synthesis System for RT-PCR (RNA input 1 μg) (Life Technologies Corporation, USA). The cDNA was applied to PCR with primers TEL971, which is complementary to ETV6 (5′-ACCACATCATGGTCTCTGTCTCCC-3′), and TRKC1059, which is complementary to NTRK3 (5′-CAGTTCTCGCTTCAGCACGATG-3′). A 110-bp product of the ETV6-NTRK3 fusion transcript was then amplified. The quality of the extracted RNA was tested using an amplified 353-bp product of the beta-actin gene (sense primer: 5'-GCTCGTCGTCGACAACGGCTC-3', antisense primer: 5'-CAAACATGATCTGGGTCATCTTCTC-3'). Successfully amplified products of the ETV6-NTRK3 fusion transcript were purified, and both sides were sequenced by DNA sequencing services using the Big Dye Terminator kit (Applied Biosystems, Foster City, CA, USA) and ABI Prism 3700 DNA Analyzer (Applied Biosystems). All sequencing reactions were conducted in both forward and reverse directions, using tracings from at least two independent PCRs. Specimens with mutations were confirmed in two rounds; only specimens that yielded the same result in both rounds were recorded as mutation positive.
Immunohistochemistry
Immunohistochemistry was performed using an automated stainer (Ventana Benchmark; Roche Ventana, Tucson, AZ). Slides were allowed to react with S100 (clone Ab2, 1:500 dilution, Thermo Scientific), vimentin (clone V9, 1:200 dilution, Biogenex), mammaglobin (clone 304-1A5, 1:200 dilution, Dako), and DOG1 (clone SP31, prediluted, Roche Ventana), respectively. Staining results were categorized as negative (0), focally positive (<1/2 tumor cells) (1+), or diffusely positive (≧1/2 tumor cells) (2+) by two pathologists (M.S.H. and Y.H.C.).
Statistical analysis
Fisher’s exact test was used to determine differences in categorical data between cases of MASC and AciCC. The student’s t test was used to determine differences in continuous variables including age. Two-sided p values of less than 0.05 were considered statistically significant. STATA software (version 8.0; Stata, College Station, TX) was used for statistical analysis.
Results
This study accessed the archive and retrieved 21 cases diagnosed as AciCC between 2000 and 2011 as well as six cases of AciCC and eight cases of MASC confirmed by FISH tests between 2012 and 2013. Based upon FISH testing, 29 % (6/21) of cases originally diagnosed as AciCC between 2000 and 2011 were reclassified as MASC.
All cases of MASC presented ETV6 split signals in more than half of tumor cells (range 58∼88 %, average 77 %), while AciCCs presented split signals in fewer than 10 % (range 0∼8 %, average 1.4 %) of tumor cells (Table 1). In 34 cases, the quality of extracted RNA was sufficient to perform RT-PCR, and all RT-PCR results were in agreement with results from the FISH test (Table 1). Finally, gene sequencing revealed that all MASC cases possessed the same break-apart point at the 5′-end of ETV6 exon (supplementary Table S1).
Patient clinical features (supplementary Table S2) are summarized in Table 1. No significant differences were observed between AciCCs and MASCs with regard to age, sex, laterality, clinical symptoms, tumor size, lymph node involvement, and local recurrence during follow-up. However, all patients with AciCC subjected to fine-needle aspiration (FNA) cytology prior to surgery were reported as being negative for malignancy. Conversely, patients with MASC that underwent FNA cytology tested positive for malignancy or were deemed suspicious of malignancy in 56 % (5/9) of cases.
Results of pattern analysis (supplementary Table S3) and microscopic features (supplementary Table S4) are summarized in Table 2. Comprehensive histologic subtyping revealed a microcystic pattern in most cases of AciCC and in all cases of MASC. A solid pattern was frequently observed in AciCC but rarely observed in MASC (71.4 vs.7.1 %, p < 0.001). A papillary-cystic pattern was common in MASC but not observed in AciCC (71.4 vs. 0 %, p < 0.001). A follicular pattern was uncommon in both groups. The predominant growth pattern of AciCCs included microcystic (52 %) and solid (43 %) types, while those in MASCs were microcystic (71 %) followed by papillary-cystic (21 %). Representative patterns of MASCs and AciCCs are illustrated in Figs. 1 and 2, respectively.
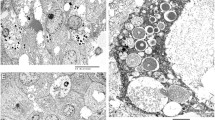
Characteristic histologic patterns of AciCC. a A predominant solid pattern mixed with a minor microcystic pattern. b Microcystic pattern: also a common pattern in AciCC. c AciCC characterized by the presence of cytoplasmic zymogen granules. d DOG1 showing cytoplasmic and luminal stains, highlighting the microcystic spaces of AciCC
Other significant microscopic differences between AciCCs and MASCs included (1) secretion within microcystic space, (2) cytoplasmic vacuolization, (3) nuclear contour, (4) nuclear size, (5) chromatin pattern, (6) distinct nucleoli, (7) necrosis, (8) hemorrhage/hemosiderin deposition, and (9) cytoplasmic zymogen granules. Perineural and lymphovascular invasion were rarely observed in either type of tumor.
Immunohistochemical results are summarized in Table 3. All cases of MASC stained positive for S100 (1+ 21 %, 2+ 79 %), vimentin (100 %), and mammaglobin (100 %). Most cases of MASC stained negative for DOG1 (93 %). In most AciCC cases, staining for DOG1 (95 %) yielded a positive result while staining for S100 (95 %) and mammaglobin (86 %) was negative. Vimentin was variably expressed in AciCC between 0 and 2+.
Discussion
Our results suggest that a papillary-cystic pattern without a solid component is a major histologic feature of MASC but rare in AciCC. MASC is a salivary gland tumor with an ETV6-NTRK3 fusion oncogene [1]. In recent years, recurrent chromosome rearrangements have been identified in several tumors of the salivary gland, including a recurrent t(6;9) translocation resulting in a MYB-NFIB fusion gene in adenoid cystic carcinoma, a recurrent t(11;19) translocation resulting in a CRTC1-MAML2 fusion gene in mucoepidermoid carcinoma, and a t(12;22) translocation resulting in a EWSR1-ATF1 fusion gene in hyalinizing clear cell carcinomas and PLAG1 or HMGA2 translocations in pleomorphic adenomas [9, 15–19]. We employed FISH as gold standard and found that 6 of the 21 cases diagnosed as AciCC (6/21, 29 %) between 2000 and 2011 were actually MASC. In addition, FISH and RT-PCR were found to have equal power in detecting ETV6 translocations.
ETV6 is an important hematopoietic regulatory factor, and rearrangement of this gene is implicated in many hematological malignancies with fusion partners such as RUNX1, JAK2, ABL1, ABL2, NCOA2, SYK, and PAX5 [20]. All ETV6 fusion transcripts involve the oligomerization domain of ETV6 and the tyrosine kinase domain of its fusion partner. In agreement with previous reports, all cases of MASC included in our study presented NTRK3 as the fusion partner of ETV6 with the same break point at the 5′-end of ETV6 exon, as revealed by Sanger sequencing [1].
Contrary to previous assumptions, this study demonstrates that a papillary-cystic pattern is actually rare in AciCC. In fact, a papillary-cystic pattern is characteristic of MASC, while a solid pattern is frequently encountered in AciCC (but not in MASC). The microcystic pattern is shared by both AciCC and MASC. In the past, many cases of MASC have been misdiagnosed as AciCC; therefore, the belief that AciCCs are characterized by four common histologic patterns need to be re-evaluated. Cystic change is not uncommon in AciCC (43 %) but there are no typical papillary structures found in MASC. Papillary-cystic structures in MASCs have irregular inner surfaces due to epithelial papillary proliferations, while cysts in AciCCs have smooth inner surfaces (Fig. 3). A recent study conducted by Urano et al. also showed different morphologic features of these two salivary gland tumors as MASC exhibited papillary-cystic and follicular patterns with vacuolated cells, and AciCC exhibited microcystic and solid patterns with acinar cells [13]. Comprehensive subtyping of histologic patterns may provide a useful clinical method to screen for suspicious cases that warrant additional molecular tests.
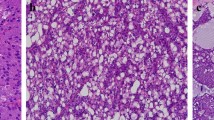
Papillary-cystic pattern is characteristic of MASC. a Tumor with cystic change, papillary structures, and an irregular inner surface. b Papillary structures with fibrovascular cores lined by tumor cells. c Another case with typical papillary-cystic pattern and tumor cells showing hobnail features. d Cystic change in AciCC associated with a smooth inner surface and no papillary epithelial proliferations
A number of interesting trends were observed in the clinical features of the two tumors. Firstly, males appear to have a predilection for MASC, while AciCC occurs predominantly in female patients. Secondly, lymph node metastasis was found only in cases of MASC (21 %) and limited to patients in stage T2 or T3. Thirdly, no local recurrence was observed in MASC, whereas a local recurrence rate of 14 % was observed among patients with AciCC. Finally, examination of FNA cytology in cases of AciCC revealed a high rate of false negatives; however, this was not observed among MASC patients. We believe that this discrepancy can be attributed to differences in microscopic findings between the two tumors, based on the fact that MASC is more likely to present cellular atypia (Table 2).
FISH is regarded as the gold standard for detecting ETV6 gene translocations and for diagnosing MASC. However, this method is very expensive and therefore not available in many laboratories. Many researchers have attempted to identify other immunohistochemical markers which may be used as screening tools or proxy markers in place of ETV6 FISH. For example, Skálová et al. reported strong vimentin and S100 positivity in all 15 examined cases of MASC [1]. Chiosea et al. reported similar findings and used expression of S100 to divide patients into two groups with either diffuse (53.3 % of cases) or focal staining (46.7 % of cases) [8]. Connor et al. also reported S100 immunoreactivity in five out of seven MASC cases [3]. Urano et al. reported MASC to be diffusely positive for vimentin, high molecular-weight cytokeratin, S100, cytokeratin 19, mammaglobin, MUC1, and adipophilin. In contrast, less than one-third of AciCC cases showed positive staining for these antigens [13]. In the present study, staining for S100, vimentin, and mammaglobin was diffusely positive in 79 % (11/14), 100 % (14/14), and 100 % (14/14) of MASC cases, respectively. Conversely, no case of AciCC was diffusely positive for S100. Despite the fact that the difference in vimentin staining reached statistical significance, we still consider vimentin a less discriminative marker due to its variable expression in AciCC. Specifically, 24 % (5/21) of cases presented a diffuse staining pattern. Previous studies have identified mammaglobin as a potential proxy marker for MASC [11, 13, 21, 22]. However, we observed positive mammaglobin staining in a number of AciCC cases (3/21, 14 %). Positive DOG1 staining was observed in most AciCC cases (20/21) but in only one case of MASC (1/14). DOG1 typically presents mixed cytoplasmic and luminal staining patterns, and luminal staining is particularly conspicuous in the microcystic spaces of AciCC [23]. In summary, none of the immunohistochemical markers used in this study reached 100 % specificity. Thus, though these staining techniques can serve as preliminary screening tools, molecular tests are still necessary for diagnostic confirmation.
We encountered one atypical case of MASC that presented a predominant solid pattern and a sclerotic background (case 11) (Fig. 4). However, this case displayed other features commonly associated with MASC, including microcystic spaces with secretions, eosinophilic cytoplasm, vesicular chromatin, distinct nucleoli, and an immunoprofile with staining for S100,vimentin, and mammaglobin while DOG1 negative. No other cases of MASC presented this type of sclerotic stroma or solid component in this study. We consider this aberrant morphology to reflect high-grade transformation [24]. Skálová A et al. first reported three MASC cases with high-grade transformation characterized by an accelerated clinical course and poor outcome [14, 24]. Cases with high-grade transformation had both low-grade and high-grade components; ETV6 gene rearrangement was present in both components. High-grade transformation is defined by histomorphologic criteria such as nuclear polymorphism, distinctive nucleoli, increased mitotic activity, increased MIB1 index, and areas of necrosis [24].
An unusual histologic variant of MASC (MASC case 11) with a predominant solid pattern (a) and a central scar (b). c Tumor cells with large nuclei, vesicular chromatin, nucleoli, occasional microcystic spaces filled with mucin, and S100 immunoreactivity (inset). d ETV6 rearrangement confirmed by RT-PCR (MASC case 8 served as positive control) and sequencing results
In conclusion, comprehensive subtyping of histologic patterns revealed that the papillary-cystic pattern is a major growth pattern of MASC, but not of AciCC. MASC is associated with more pronounced cellular atypia. Thus, comprehensive subtyping of histologic patterns and a screening immunohistochemical panel that includes S100/mammaglobin/DOG1 are useful, prior to confirmatory molecular testing.
References
Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RH, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599–608. doi:10.1097/PAS.0b013e3181d9efcc
Lannon CL, Sorensen PH (2005) ETV6-NTRK3: a chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin Cancer Biol 15:215–223
Connor A, Perez-Ordoñez B, Shago M, Skálová A, Weinreb I (2012) Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol 36:27–34. doi:10.1097/PAS.0b013e318231542a
Ellis G, Simpson RHW (2005) Acinic cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) World health organization classification of tumours. Pathology & Genetics of Head and Neck Tumours. IARC press, Lyon, pp 216–218
Chiosea SI, Griffith C, Assaad A, Seethala RR (2012) The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol 36:343–350. doi:10.1097/PAS.0b013e318242a5b0
Bishop JA (2013) Unmasking MASC: bringing to light the unique morphologic, immunohistochemical and genetic features of the newly recognized mammary analogue secretory carcinoma of salivary glands. Head Neck Pathol 7:35–39. doi:10.1007/s12105-013-0429-0
Jung MJ, Song JS, Kim SY, Nam SY, Roh JL, Choi SH, Kim SB, Cho KJ (2013) Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol 47:36–43. doi:10.4132/KoreanJPathol.2013.47.1.36
Chiosea SI, Griffith C, Assaad A, Seethala RR (2012) Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 61:387–394. doi:10.1111/j.1365-2559.2012.04232.x
Stenman G (2013) Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol 7(Suppl 1):12–19. doi:10.1007/s12105-013-0462-z
Skálová A (2013) Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol (Suppl 1): 30-36. doi: 10.1007/s12105-013-0455-y
Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH (2013) Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol 44:1982–1988. doi:10.1016/j.humpath.2013.03.017
Chênevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR (2012) DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 25:919–929. doi:10.1038/modpathol.2012.57
Urano M, Nagao T, Miyabe S, Ishibashi K, Higuchi K, Kuroda M (2015) Characterization of mammary analogue secretory carcinoma of the salivary gland: discrimination from its mimics by the presence of the ETV6-NTRK3 translocation and novel surrogate markers. Hum Pathol 46:94–103. doi:10.1016/j.humpath.2014.09.012
Simpson RH, Skálová A, Di Palma S, Leivo I (2014) Recent advances in the diagnostic pathology of salivary carcinomas. Virchows Arch 465:371–384. doi:10.1007/s00428-014-1639-x
Nordkvist A, Mark J, Gustafsson H, Bang G, Stenman G (1994) Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes, Chromosomes Cancer 10:115–121
Nordkvist A, Gustafsson H, Juberg-Ode M, Stenman G (1994) Recurrent rearrangements of 11q14-22 in mucoepidermoid carcinoma. Cancer Genet Cytogenet 74:77–83
Brill LB 2nd, Kanner WA, Fehr A, Andrén Y, Moskaluk CA, Löning T, Stenman G, Frierson HF Jr (2011) Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol 24:1169–1176. doi:10.1038/modpathol.2011.86
Persson M, Andrén Y, Moskaluk CA, Frierson HF Jr, Cooke SL, Futreal PA, Kling T, Nelander S, Nordkvist A, Persson F, Stenman G (2012) Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes, Chromosomes Cancer 51:805–817. doi:10.1002/gcc.21965
Fehr A, Stenman G, Bullerdiek J, Löning T (2009) Molecular markers in salivary gland tumors: their use in diagnostic and prognostic workup. Pathologe 30:466–471. doi:10.1007/s00292-009-1206-4
Zhou MH, Gao L, Jing Y, Xu YY, Ding Y, Wang N, Wang W, Li MY, Han XP, Sun JZ, Wang LL, Yu L (2012) Detection of ETV6 gene rearrangements in adult acute lymphoblastic leukemia. Ann Hematol 91:1235–1243. doi:10.1007/s00277-012-1431-4
Patel KR, Solomon IH, El-Mofty SK, Lewis JS Jr, Chernock RD (2013) Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum Pathol 44:2501–2508. doi:10.1016/j.humpath.2013.06.010
Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH (2013) Most nonparotid "acinic cell carcinomas" represent mammary analog secretory carcinomas. Am J Surg Pathol 37:1053–1057. doi:10.1097/PAS.0b013e3182841554
Chan JK (2013) Newly available antibodies with practical applications in surgical pathology. Int J Surg Pathol 21:553–572. doi:10.1177/1066896913507601
Skálová A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, Hauer L, Andrle P, Hosticka L, Branžovský J, Michal M (2014) Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and CCND1 genes. Am J Surg Pathol 38:23–33. doi:10.1097/PAS.0000000000000088
Acknowledgments
The authors would like to thank Ms. Syue-Fong Hua for her technical support and the Department of Pathology at the National Taiwan University Hospital for providing laboratory facilities. This study was supported by grants from the National Taiwan University Hospital (103-002523) and the Far East Memorial Hospital (FEMH 2014-C-052).
Conflict of interests
All authors have no potential conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsieh, MS., Chou, YH., Yeh, SJ. et al. Papillary-cystic pattern is characteristic in mammary analogue secretory carcinomas but is rarely observed in acinic cell carcinomas of the salivary gland. Virchows Arch 467, 145–153 (2015). https://doi.org/10.1007/s00428-015-1786-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1786-8