Abstract
The moon jellyfish Aurelia exhibits a dramatic reorganization of tissue during its metamorphosis from planula larva to polyp. There are currently two competing hypotheses regarding the fate of embryonic germ layers during this metamorphosis. In one scenario, the original endoderm undergoes apoptosis and is replaced by a secondary endoderm derived from ectodermal cells. In the second scenario, both ectoderm and endoderm remain intact through development. In this study, we performed a pulse-chase experiment to trace the fate of larval ectodermal cells. We observed that prior to metamorphosis, ectodermal cells that proliferated early in larval development concentrate at the future oral end of the polyp. During metamorphosis, these cells migrate into the endoderm, extending all the way to the aboral portion of the gut. We therefore reject the hypothesis that larval endoderm remains intact during metamorphosis and provide additional support for the “secondary gastrulation” hypothesis. Aurelia appears to offer the first and only described case where a cnidarian derives its endoderm twice during normal development, adding to a growing body of evidence that germ layers can be dramatically reorganized in cnidarian life cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrulation is generally considered the defining moment when an animal embryo differentiates the cells that will generate the surficial tissues and organs (the ectoderm) from those in the interior (the endomesoderm), but this is not always the case. Some taxa exhibit “prolonged gastrulation”—where the precise beginning and end of embryonic gastrulation is ambiguous. For example, gastrulation in certain platyhelminth lineages involves one or more “transitory epidermises,” where the “true” epibolic movement creates the final ectoderm long after the point when nerve and muscle cells can be structurally identified (Morris et al. 2004; Martín-Durán and Egger 2012). In the embryonic head of Drosophila melanogaster, cells from the “ectoderm” continue to delaminate for several additional hours following gastrulation, contributing to mesodermal tissues like blood and endothelia (De Velasco et al. 2004). In some marine taxa, “maximal indirect development” or “catastrophic metamorphosis” involves a major reorganization of tissues, sometimes including the destruction and reformation of entire germ layers. Such reorganization is commonplace in echinoderms, “lophophorates,” and certain spiralian lineages (Davidson et al. 1995; Fuchs et al. 2011; Temereva and Malakhov 2015). Pilidiophoran nemerteans provide an extreme example, wherein the juvenile worm develops from the larval epidermis, and then proceeds to consume the rest of the larval body (Maslakova 2010). In all of these cases, similar structures across different life stages might be derived from unique cellular precursors, challenging their embryological homology.
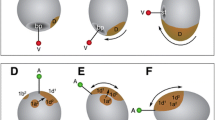
It is unclear how commonplace germ layer reorganization is in early branching animal lineages such as the Cnidaria (a phylum including sea anemones, corals, jellyfish, and hydras). Anthozoan cnidarians—such as the sea anemone Nematostella vectensis—generally develop via gradual metamorphosis, with the endoderm of the larva generating the gut of the polyp (Fritzenwanker et al. 2007). In some medusozoans—such as Hydractinia echinata and Clava multicornis—larval metamorphosis involves significant apoptosis destroying most of the ectoderm and/or endoderm, although the extent to which embryological germ layers are reorganized is unknown (Seipp et al. 2001, 2010; Pennati et al. 2013). In an immunohistochemical study of the moon jellyfish Aurelia (“sp.1” isolate sensu Dawson and Jacobs 2001), Yuan et al. (2008) hypothesized that metamorphosis of the larva (called a planula) involves the complete degradation of the endoderm, and the development of a new, secondary endoderm derived from ectodermal cells (Fig. 1a). This event occurs long after the canonical gastrulation process that generates the planula, wherein the two germ layers are formed through multipolar ingression and/or invagination (Smith 1891; Hyde 1894; Mergner 1971; Fioroni 1979). Yuan et al. (2008) subsequently named this event “secondary gastrulation.” If confirmed, it would represent the only described case in which a cnidarian’s endoderm is derived twice during normal development. However, a more recent analysis of serial semithin sections suggests that germ-layer continuity exists between the larva and polyp of Aurelia (Fig. 1b; Mayorova et al. 2012), throwing the secondary gastrulation hypothesis into doubt.
Diagrams illustrating competing hypotheses regarding the metamorphosis of Aurelia from planula to polyp. a The “secondary gastrulation” hypothesis proposed by Yuan et al. (2008). In this scenario, the endoderm generated during gastrulation (the primary endoderm) degrades during metamorphosis, and is replaced by a secondary endoderm generated from ectodermal cells. b The hypothesis proposed by Mayorova et al. (2012). In this scenario, the endoderm from the planula is retained in the polyp. A small amount of ectoderm moves into the endoderm during the formation of the oral cavity (mouth), but this migration of ectodermal tissue ends at the pharynx, where ectodermal cells meet the original endodermal tissue
These competing interpretations of Aurelia metamorphosis, illustrated in Fig. 1, could be distinguished by lineage tracing of the planula ectoderm. If Yuan et al. (2008) are correct, then labeled ectodermal cells in the planula should be found throughout the endoderm of the polyp. If the model proposed by Mayorova et al. (2012) is accurate, then labeled ectodermal cells from the planula should be absent from endoderm in the polyp body column. Previous work suggests that cell division is low or absent in the planula endoderm (Yuan et al. 2008), supporting the argument that a cell proliferation assay could provide a robust ectodermal marker. In this study, we successfully labeled dividing cells in the planula ectoderm using 5-ethynyl-2′-deoxyuridine (EdU) and followed these cells through metamorphosis. We found that these cells and their descendants localize at the future oral pole of the polyp, and then spread throughout the entirety of the polyp endoderm. This study provides renewed support for the secondary gastrulation hypothesis and suggests that germ layers can be dramatically reorganized in some cnidarians.
Materials and methods
Aurelia planulae were collected from several brooding female medusae at the Cabrillo Marine Aquarium in San Pedro, CA. Actively swimming planulae were transferred into 10 mL of artificial seawater with 100 μM EdU, and incubated for two hours at room temperature. Animals were then washed three times in >250 mL of filtered seawater, and transferred into 250 mL of filtered seawater using a 10 μl pipette. Planula were kept in the dark at 18 °C and allowed to go through metamorphosis over the following week. Animals were collected through a series of time points following EdU exposure, ranging from 0 h to 7 days. Individuals were fixed in 4 % formaldehyde for 1 h, and then washed at least four times in PBSTr (0.3 % Triton X-100 in PBS buffer). EdU was conjugated to a fluorescent azide using the Click-iT EdU Alexa Fluor 488 Imaging Kit (Invitrogen, Cat # C10337) according to the manufacturer’s protocol. Following the Click-iT protocol, animals were labeled with additional fluorescent stains and/or antibodies, using the protocol described in Yuan et al. (2008). Primary antibodies used include anti-tyrosinated tubulin (mouse; 1:800) and anti-acetylated tubulin (mouse; 1:1000). Following antibody staining, animals were incubated for 15 min in TO-PRO®-3 Iodide nuclear stain (1:1000; Life Technologies, Cat # T3605). Specimens were mounted onto slides using ProLong Gold (Invitrogen) and viewed on a Zeiss Imager M2 Confocal Microscope. Digital stacks were analyzed using ImageJ.
Results
Following EdU exposure, all cell labeling was restricted to the planula ectoderm (Fig. 2a–d). This result was robust (N > 50), despite slight variation in the age of planula housed by the brooding females (Fig. 2b). There was no obvious pattern of EdU incorporation across the primary axis, suggesting that cells across the ectoderm undergo division at equivalent rates. Labeled nuclei are most common in the superficial layer of the ectoderm, although it is unclear if they are restricted to this region. By day 7, most larva had begun metamorphosis, but many remained actively swimming in the water column. In these 7-day old planulae, all EdU-positive cells had shifted towards the future oral pole of the polyp (N > 50; Fig. 2e, f). When young polyps derived from these planulae were analyzed, EdU-positive cells were discovered throughout the endoderm (N = 20; Fig. 2g, h).
EdU labeling of ectodermal cells in the planula, and their migration through development. All scale bars represent 50 μm. a Digital cross section of a planula larva following 2 h of EdU exposure. b The same image as (a), with only EdU-positive cells visible. The ectoderm (Ec) and endoderm (En) are separated by a dotted line. c Digital cross section of multiple planulae collected from a single adult female, following 2 h of EdU exposure. These individuals represent a diversity of young planula, ranging from the earlier and more spherical individual at the bottom, to the older, more elongated individual at the top left. d The same image as (c), with only EdU-positive cells visible. e A planula 4 days after EdU exposure. In this individual, all EdU-positive cells have shifted towards the posterior (future oral) pole, which can be readily distinguished by the flattened ridge and stronger tyrosinated tubulin staining of the aboral pole (Yuan et al. 2008). f Planula 7 days after EdU exposure, demonstrating the consistency of this cell migration in late stage larva. Arrows mark the aboral pole of the future polyp. g A young polyp derived from planula, 7 days after EdU exposure. The animal was dissected following fixation (partially noted with the dotted yellow line) so that endodermal EdU staining could be imaged at low magnification. h Partial digital cross section from another young polyp. This image demonstrates that the endodermal EdU signal seen in (g) is not a consequence of tissue damage
A series of digital longitudinal sections from metamorphosing planula elucidate the process of cell migration into the polyp endoderm (Fig. 3). Early in metamorphosis (Fig. 3a–c), a reduction in acetylated tubulin demarcates the boundary between the hypothesized primary and secondary endoderms (Enp and Ens in Fig. 3a; compare to Fig. 5 h in Yuan et al. 2008). At this stage of development, EdU-positive cells first labeled in the planula are now concentrated in the oral region of the ectoderm (similar to Fig. 2e–f) as well as the hypothesized secondary endoderm. Later in development—as the oral opening of the primary polyp develops and the pharynx takes shape—this field of EdU-positive cells moves aborally (Fig. 3d–f). The gap in nuclear staining caused by the acellular mesoglea allows for the clear demarcation of ectoderm and endoderm (Fig. 3f). At this stage, nearly all EdU-positive cells reside in the endoderm, where they extend from the oral cavity to the aboral base of the endoderm. The presence of EdU-positive cells throughout the endoderm is retained in the primary polyp (Fig. 3g–i).
Movement of EdU-positive cells into the endoderm during larval metamorphosis. All images represent digital cross sections, and all scale bars represent 50 μM. a–c A planula at an early stage of metamorphosis, following Fig. 2c. a Acetylated tubulin staining, with the hypothesized primary endoderm (Enp) and secondary endoderm (Ens) labeled according to Yuan et al. (2008). An insert is included to show the relevant boundary between the two endoderms at higher magnification. b The same individual, illustrating the distribution of EdU-positive cells. Note the lack of EdU staining in the presumptive primary endoderm. c Acetylated tubulin and EdU staining overlaid with TO-PRO nuclear stain. The gap in staining demonstrates the boundary between ectoderm (Ec) and endoderm (En). d–f A metamorphosing larva in the process of developing the oral cavity. g–i A primary polyp, featuring a complete oral cavity (gut)
Discussion
In this study, we found that ectodermal cells in the planula of Aurelia sp.1 congregate at the posterior pole and then migrate into the endoderm during polyp metamorphosis. This observation was possible because EdU functions as a robust marker of ectodermal cells in Aurelia planula, which suggests that cell division in the endoderm is minimal or absent at this stage. This is consistent with Yuan et al. (2008), who found no significant signal for another cell division marker (phosphorylated histone H3) in planula endoderm. In contrast, this is unlike what is observed in the endoderm of mature polyps, where cellular proliferation occurs at a constant rate comparable to the ectoderm (Gold and Jacobs 2013; Takashima et al. 2013). While the data presented here is insufficient to demonstrate that this small set of non-dividing primary endodermal cells is destroyed in conjunction with the migration of the secondary endoderm, such a scenario is supported by evidence of nuclear degradation and caspase activity in the primary endoderm during metamorphosis (Yuan et al. 2008). However, it is worth noting that caspase is not a specific marker for cellular apotosis, and future work is required to definitively confirm that the primary endoderm is destroyed.
We envision two interpretations regarding the nature of Aurelia’s “secondary gastrulation” event. Secondary gastrulation could be an example of catastrophic metamorphosis, where the larval endoderm degenerates, and postembryonic endoderm is formed by cells of the definitive planula ectoderm. This would suggest that cells in the planula ectoderm transdifferentiate into endodermal cells or were never differentiated following gastrulation. Alternatively, one might consider this event an extreme form of “prolonged gastrulation.” In such a scenario, the planula ectoderm is, in reality, a mixed “ecto-endoderm” containing a population of cells fated to be endoderm. An early, incomplete gastrulation process that moves only a subset of cells with endodermal fate into the embryo, leaving behind an outer layer that still contains much of the presumptive (postembryonic) endoderm. Gene expression studies comparing germ-layer makers between Aurelia and cnidarians with more gradual metamorphosis (such as Nematostella) could help resolve these competing interpretations.
While larval metamorphosis in Aurelia sp.1 represents, to our knowledge, the first described case of secondary gastrulation in a cnidarian, this observation is consistent with a larger trend in cnidarian development where the de novo generation of structures is favored over the reorganization of structures from the prior life stage. Examples of this phenomenon include the destruction and redevelopment of cnidarian nervous systems (Nakanishi et al. 2008; Pennati et al. 2013), musculature (Helm et al. 2015), and tentacles (Gold et al. 2015; Kraus et al. 2015). The extent to which mechanisms of gut formation have been modified through cnidarian evolution should be further examined and ought to be taken into account in current debates regarding animal gut homology, such as the possibility of independent gut evolution in early branching animal lineages such as ctenophores (Martindale and Hejnol 2009).
References
Davidson EH, Peterson KJ, Cameron RA (1995) Origin of bilaterian body plans: evolution of developmental regulatory mechanisms. Science 270:1319–1325
Dawson MN, Jacobs DK (2001) Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biol Bull 200:92–96
De Velasco B, Shen J, Go S, Hartenstein V (2004) Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass. Dev Biol 274:280–294
Fioroni V (1979) Abändarungen des Gastrulationsverlaufs und ihre phylogenetische Bedeutung. In: Suewing R (ed) Erlanger Symp. Ontogenie Evolutionsforsch: Ontogenie unid Phylogenie. Parey, Hamburg, pp 100–119
Fritzenwanker JH, Genikhovich G, Kraus Y, Technau U (2007) Early development and axis specification in the sea anemone Nematostella vectensis. Dev Biol 310:264–279
Fuchs J, Martindale MQ, Hejnol A (2011) Gene expression in bryozoan larvae suggest a fundamental importance of pre-patterned blastemic cells in the bryozoan life-cycle. EvoDevo 2:1
Gold DA, Nakanishi N, Hensley NM et al (2015) Structural and developmental disparity in the tentacles of the moon jellyfish Aurelia sp.1. PLoS ONE 10:e0134741
Gold DA, Jacobs DK (2013) Stem cell dynamics in Cnidaria: are there unifying principles? Dev Genes Evol 223(1-2):53–66
Helm RR, Tiozzo S, Lilley MKS et al (2015) Comparative muscle development of scyphozoan jellyfish with simple and complex life cycles. EvoDevo 6:11
Hyde JH (1894) Entwicklungsgeschichte einiger Scyphomedusen. Z Wiss Zool 58:531–565
Kraus JEM, Fredman D, Wang W et al (2015) Adoption of conserved developmental genes in development and origin of the medusa body plan. EvoDevo 56:753–777
Maslakova SA (2010) Development to metamorphosis of the nemertean pilidium larva. Front Zool 7:30
Mayorova TD, Kosevich IA, Melekhova OP (2012) On some features of embryonic development and metamorphosis of Aurelia aurita (Cnidaria, Scyphozoa). Russ J Dev Biol 43:271–285
Martín-Durán JM, Egger B (2012) Developmental diversity in free-living flatworms. EvoDevo 3:1
Martindale M, Hejnol A (2009) A developmental perspective: changes in the position of the blastopore during bilaterian evolution. Dev Cell 17:162–174
Mergner H (1971) Chapter 1: cnidaria. In: Reverberi G (ed) Experimental embryology of marine and fresh-water invertebrates. North Holland, Amsterdam, pp 1–84
Morris J, Nallur R, Ladurner P, Egger B, Rieger R, Hartenstein V (2004) The embryonic development of the flatworm Macrostomum sp. Dev Genes Evol 214:220–239
Nakanishi N, Yuan D, Jacobs DK, Hartenstein V (2008) Early development, pattern, and reorganization of the planula nervous system in Aurelia (Cnidaria, Scyphozoa). Dev Genes Evol 218:511–524
Pennati R, Dell’Anna A, Pagliara P et al (2013) Neural system reorganization during metamorphosis in the planula larva of Clava multicornis (Hydrozoa, Cnidaria). Zoomorphology 132:227–237
Seipp S, Schmich J, Leitz T (2001) Apoptosis—a death-inducing mechanism tightly linked with morphogenesis in Hydractina echinata (Cnidaria, Hydrozoa). Development 128:4891–4898
Seipp S, Schmich J, Will B et al (2010) Neuronal cell death during metamorphosis of Hydractina echinata (Cnidaria, Hydrozoa). Invert Neurosci 10:77–91
Smith F (1891) The gastrulation of Aurelia flavidula, Pér. & Les. Bull Museum Comparat Zool Harvard College 22:115–125
Takashima S, Gold D, Hartenstein V (2013) Stem cells and lineages of the intestine: a developmental and evolutionary perspective. Dev Genes Evol 223:85–102
Temereva EN, Malakhov VV (2015) Metamorphic remodeling of morphology and the body cavity in Phoronopsis harmeri (Lophotrochozoa, Phoronida): the evolution of the phoronid body plan and life cycle. BMC Evol Biol 15:1
Yuan D, Nakanishi N, Jacobs DK, Hartenstein V (2008) Embryonic development and metamorphosis of the scyphozoan Aurelia. Dev Genes Evol 218:525–539
Acknowledgments
This work was supported by funding from the NASA Astrobiology Institute (NNA13AA90A) Foundations of Complex Life, Evolution, Preservation, and Detection on Earth and Beyond.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Angelika Stollewerk
Rights and permissions
About this article
Cite this article
Gold, D.A., Nakanishi, N., Hensley, N.M. et al. Cell tracking supports secondary gastrulation in the moon jellyfish Aurelia . Dev Genes Evol 226, 383–387 (2016). https://doi.org/10.1007/s00427-016-0559-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-016-0559-y