Abstract
Main conclusion
Serine/arginine-rich (SR) proteins participate in RNA processing by interacting with precursor mRNAs or other splicing factors to maintain plant growth and stress responses.
Abstract
Alternative splicing is an important mechanism involved in mRNA processing and regulation of gene expression at the posttranscriptional level, which is the main reason for the diversity of genes and proteins. The process of alternative splicing requires the participation of many specific splicing factors. The SR protein family is a splicing factor in eukaryotes. The vast majority of SR proteins’ existence is an essential survival factor. Through its RS domain and other unique domains, SR proteins can interact with specific sequences of precursor mRNA or other splicing factors and cooperate to complete the correct selection of splicing sites or promote the formation of spliceosomes. They play essential roles in the composition and alternative splicing of precursor mRNAs, providing pivotal functions to maintain growth and stress responses in animals and plants. Although SR proteins have been identified in plants for three decades, their evolutionary trajectory, molecular function, and regulatory network remain largely unknown compared to their animal counterparts. This article reviews the current understanding of this gene family in eukaryotes and proposes potential key research priorities for future functional studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SR protein in alternative splicing
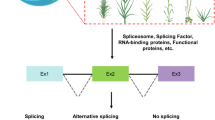
Alternative splicing (AS) is a biological process by which an mRNA precursor can be spliced in different ways to generate multiple mRNA transcripts. As a fundamental mechanism to provide transcriptome and proteome diversity, different splicing combinations of exons and introns in precursor RNA lead to different mRNA isoforms (Chen et al. 2020). Many nuclear multiexonic genes undergo AS in a broad range of eukaryotic species. For example, in animal species, humans have been shown to have alternative splicing of more than 95% of intron-containing genes (Pan et al. 2008). In model plants, more than 80% of genes in Arabidopsis thaliana can produce multiple transcripts (Zhu et al. 2017), more than 70% of endogenous genes in rice have different isoforms (Chen et al. 2021), and more than 50% of genes in maize undergo variable splicing (Chen et al. 2018). This sophisticated process is mediated by a dynamic mega RNA‒protein complex defined as the spliceosome, containing core splicing factors and regulatory splicing-related proteins (Califice et al. 2012). In particular, some RNA-binding proteins have an impact on the assembly of the spliceosome and the selection of splice sites, which in turn affects variable splicing.
The serine/arginine-rich (SR) protein, an RNA-binding protein that plays an essential role in alternative splicing, was characterized in studies nearly three decades ago as a critical regulator of constitutive splicing and AS (Fu and Maniatis 1990). In addition, subsequent studies have shown that this conserved family of proteins has multiple roles in RNA metabolism (Gu et al. 2020). Furthermore, green plants contain more SR proteins than their animal counterparts. Thus, plant-specific phylogenetic clades for this family have also been proposed (Califice et al. 2012; Richardson et al. 2011), which may play an important role during plant development and stress responses. For example, the accumulation or reduction of SRs in vivo in tomatoes in high-temperature environments affects temperature-sensitive alternative splicing (Rosenkranz et al. 2021), and the serine/arginine splicing factor RS33 in rice can regulate pre-mRNA splicing during abiotic stress responses (Butt et al. 2022). In a genome-wide analysis of the serine/arginine protein family of wheat, a total of 92 cis-type elements associated with stress response, growth, and development, and hormones were detected in the promoter region of the TaSR protein genes, indicating that they have functions in development and response to environmental stresses (Chen et al. 2019b). However, despite this, except for a few related studies, most plant SR protein genes’ functions are unclear, and most of the functional information about this protein family has been obtained from animal studies. This review article provides an update on recent progress in studying SR proteins in eukaryotic organisms, with a particular focus on their evolutionary trajectory and molecular functions. Both conserved and specific molecular mechanisms of SR proteins in eukaryotes will be discussed to provide the outlook for future investigations on the plant SR protein family.
Nomeclature and evolution of plant SR proteins
The first SR protein (SRSF1/ASF/SF2) was identified in animals in the early 1980s in studies related to identifying RNA binding proteins with splicing activity (Krainer and Maniatis 1985). Subsequently, based on sequence homology with mammalian counterparts, the first plant SR protein SR34/SR1 was identified in Arabidopsis thaliana (Lazar et al. 1995). Prototypical SR proteins are characterized by their minimal structure, including a first category containing a single RNA recognition motif (RRM) and a second category containing dual RRMs at the N-terminus for RNA binding. Both categories have conserved arginine/serine-rich (RS) repeats enriched at the C-terminus to mediate protein–protein interactions (Fig. 1A) (Califice et al. 2012). In the third category of RRM containing ZnK-like SR proteins, one or two CCHC zinc-knuckle (ZnK) domains are present between the N-terminal RRM and C-terminal RS repeats (Fig. 1A). To further explore the evolutionary trajectory of the SR gene family in different species. Members of the SR protein family in different species were summarized based on the TimeTree web tool (http://timetree.org/). The number of the three SR proteins in different species is represented as a heatmap (Fig. 1B). The figure shows that the SR protein family is strongly conserved in plants, animals and microorganisms. The statistics of the three types of SR proteins in different species show that single RRM, dual RRMs are more common than RRM ZnK-Like types. These data suggest that SR proteins have played an important role in the evolution of each species. Given that the RRM domain is the most common RNA binding domain among eukaryotic organisms and that over 350 RRM-containing proteins have been identified in humans (Califice et al. 2012), additional criteria were needed to define the SR protein family further. Therefore, in 2010, Manley et al. proposed a simple definition and uniform nomenclature of SR proteins based on the sequence characteristics of SR proteins (Manley and Krainer 2010). In addition, plants have twice as many SR proteins as animals, and some subfamilies are plant-specific (Barta et al. 2008). As mentioned earlier, SR proteins have three classifications: (a) single RRM, (b) dual RRMs, and (c) single RRM & Znk-like (Fig. 1A). Six subfamilies of SR proteins have been identified in plants, of which three subfamilies, SC (single RRM), RSZ (single RRM Znk-like), and SR (dual RRM), correspond to homologs in humans (Califice et al. 2012). Moreover, there are three subfamilies of SCL (single RRM), RS2Z (single RRM & zkn -like), and RS (double RRM), which have the specific structural organization in plants (Barta et al. 2010; Morton et al. 2019b). Among them, RS repeats are limited to at least 20% RS or SR dipeptide (Barta et al. 2010) (Fig. 1C).
Overview of the classification and evolutionary timeline of the serine/arginine-rich (SR) gene family. A. Three classifications of SR proteins. B. The members of the SR protein family from various species are summarized. The main graph is made using the TimeTree web tool (http://timetree.org/). The approximate time points of the selected cluster are indicated, The number of three SR proteins in different species is presented as a heatmap. C. Six subfamilies of SR proteins have been identified in plants. Plant-specific gene families are highlighted in green color. RRM, RNA binding motif
The RRM domain was the first to be identified in eukaryotes and is the most abundant RNA-binding structural domain in eukaryotic genomes (Dreyfuss et al. 1988). In prokaryotes, however, RRM proteins encode few or no RRM features in the genome and are only found to be prevalent in cyanobacteria, but their role remains incompletely elucidated (Maruyama et al. 1999). In addition, some genes encoding RRM proteins have been identified in viral genomes (Kenan et al. 1991). In plants, a growing number of RNA-binding proteins have been shown to mediate specific RNA processing steps in chloroplasts (Ruwe et al. 2011).
All currently known RRM proteins are nuclear encoded and are imported into the chloroplast post-translationally. with characteristic structural domain organisation: an N-terminal transit peptide essential for import into the chloroplast, an acidic structural domain at the N terminus of the mature protein and two consecutive RRMs at the C terminus (Ruwe et al. 2011). Chloroplast ribonucleoproteins (cpRNP) genome-wide identification of cpRNP genes in rice and mimosa was performed based on the predicted presence of two RRM structural domains and an N-terminal chloroplast-targeting signal peptide in the proteins, identifying 10 the associated cpRNP proteins were shown to be involved in the control of splicing, editing and stability of chloroplast RNAs, and they can regulate plant development and tolerance to stress through their effects on plant photosynthesis and respiration(Wu et al. 2021). The fact that SR proteins are twice as abundant in plants as in animals, and that RRM proteins localised in chloroplasts are unique to plants, may also account for the fact that there are more RRM proteins in plants than in animals.Although the classification of SR proteins in plants has been solved, the evolutionary trajectory of plant SR proteins is still unclear. Several research groups carried out independent phylogenetic analysis using publicly available genomic and proteomic datasets in the early 2000s (Califice et al. 2012; Richardson et al. 2011; Barta et al. 2010b). The results of these studies suggested that plants or more complicated photosynthetic organisms generally possess relatively more diverse SR members among all eukaryotic organisms, and the number of identified SR proteins is particularly enriched in angiosperms (Fig. 1). Furthermore, the expansion of the plant SR protein family has been proposed to be related to genome duplication events across the plant lineage. However, several research questions remain to be elucidated in this field. First, the evolutionary relationships between each subfamily member are unclear due to the lack of synteny or transposon analysis. The combinatorial usage of these analytical methods will help us accurately estimate the effect of whole genome duplication events on the SR family in planta, further confirming whether convergence contributes to the evolution of this gene family (Califice et al. 2012). In addition to detecting any large-scale duplication at the genomic level, local transpositions and duplication events also need to be evaluated. With the development of high-throughput sequencing technology, the need to define a clear evolutionary trajectory and duplication events along the plant lineage becomes even more apparent when considering how SR proteins have evolved from the ancient common ancestor. Second, the complexity of the AS network of a particular organism seems to be positively correlated with the number of SR proteins and the number of SR splice variants (Aravind et al. 2000; Busch and Hertel 2012), suggesting a potential link between SR family size and splicing regulation across the green lineage. Thus, the selection mechanism of these confirmed AS events is worth investigating at the evolutionary level.
Structural and functional diversification
SR proteins form a conserved family of RNA-binding proteins with a unique domain structure, which is composed of one or two RNA recognition motifs (RRMs) and a characteristic C-terminal arginine/serine (RS)-rich domain (Chen et al. 2019b). SR is highly conserved in animals and plants [reviewed in (Giannakouros et al. 2011)]. RRM domain-containing proteins are generally related to RNA processing in their molecular function. Similarly, SR proteins were originally isolated for their role in constitutive and alternative splicing (Krainer and Maniatis 1985). However, subsequent studies have demonstrated that the molecular function of this protein family extends to multiple aspects of RNA metabolism, suggesting that SR proteins are versatile regulators of RNA biology in eukaryotic cells (Jeong 2017). Among these SR proteins, animal SRSF2/SC35 and SRSF1/ASF/SF2 are the most studied members, with experimental data revealing their molecular functions.
For example, biochemical and genetic evidence has demonstrated that the depletion of SRSF2/SC35 results in a dramatic decrease in nascent RNA transcribed by RNA polymerase II (Pol II), implying its role in promoting transcriptional elongation (Fig. 2) (Ji et al. 2013; Lin et al. 2008; Fededa and Kornblihtt 2008). This finding further strengthens the coupling theory between Pol II activity and RNA processing. In addition, the SR protein Npl3 in yeast has been shown to play an important role in transcriptional elongation, splicing, etc. (Holmes et al. 2015). The second function of SR family members described here is their role in regulating mRNA stability (Aznarez et al. 2018; Zhang and Krainer 2004). For example, the stability of PKCI-1-associated mRNA is controlled by the splicing factor ASF/SF2, which affects gene expression in vertebrate cells by regulating mRNA stability and splicing(Lemaire et al. 2002). Disease-related studies have reported that SR proteins can mediate tumourigenesis by affecting genomic stability (Wan et al. 2022). Furthermore. SRSF1/ASF/SF2 can enhance the operation of the nonsense-mediated mRNA decay (NMD) pathway, a quality control mechanism responsible for the degradation of premature termination codon (PTC)-containing mRNAs, by directly recruiting the NMD factor UPF1 (Fig. 2) (Aznarez et al. 2018; Zhang and Krainer 2004). This observation demonstrated an untraditional NMD pathway in the eukaryotic system. Animal SR proteins have been found to maintain genome stability by preventing the formation of an RNA‒DNA hybrid in an R loop structure during transcription (Li and Manley 2005), and this ability seems to be functional in a cell-specific manner (Xiao et al. 2007). Third, plant SR proteins have been observed to maintain microRNA levels by selecting the correct strand for microRNA biogenesis in nuclear speckles (Chen et al. 2015). For example, SR proteins can enhance microprocessor activity by recruiting DROSHA, and cleave pri-miRNA to promote miRNA maturation (Fig. 2) (Kim et al. 2018). Human tumor-related studies have also found that SRp20 is involved in the cleavage of primary microRNAs and is involved in the integrity and diversity of the genome (Wang and Jiang 2021). Fourth, SR proteins have been found to mediate both cap-dependent and cap-independent translation. In particular, SRSF1/ASF/SF2 can promote translation of its target mRNA by interacting with cytoplasmic cap-binding protein eIF4E (Fig. 2) (Michlewski et al. 2008), whereas SRSF3/SRp20, orthologous to the plant RSZ subfamily, can utilize cap-independent translation at an internal ribosome entry site (IRES) to facilitate the translation of a poliovirus RNA (Bedard et al. 2007). Fifth, SR proteins play an important role in mRNA transport, and post-translational modifications of SR proteins are the basis for regulating their mRNA export activity (Botti et al. 2017). And related studies have demonstrated that dephosphorylation of SR proteins has a conserved role in regulating the interaction between SR proteins and mRNA export receptors(Reed and Cheng 2005). In addition, some SR family members can function as adaptors to facilitate mRNA transport by interacting with nuclear export factor 1 (NXF1) in animals (Fig. 2) (Mullermcnicoll et al. 2016). The nucleocytoplasmic shuttling ability of SR proteins has also been observed in plants, where it is mediated by the CRM1/XPO1/exportin-1 receptor pathway and nuclear transporter MOS14 (Fig. 2) (Xu et al. 2011).
Molecular mechanisms of SR proteins in RNA processing. Existing mechanisms of SR proteins in the nucleus and cytosol are illustrated here. Components shared by animals and plants are present in orange color. Components reported only in animal studies are shown in grey color. Plant-specific components or pathways are labeled as green color. Pol II, polymerase II; U1, U2, U4, U5, U6, small nuclear RNA and binding protein complex (snRNP); U2AF, U2 snRNP auxiliary factors; Drosha, a ribonuclease (RNase) III double-stranded RNA-specific ribonuclease that is involved in microRNA biogenesis; NFX1, nuclear export protein; CRM1, Chromosomal Maintenance 1 / Exportin 1; MOS14, nuclear import receptor; UPF1, Up-frameshift suppressor 1; elF4E, translation elongation factor
In addition to the five mechanisms mentioned above, most reports have focused on the role of the SR protein family in splicing regulation. Specifically, the detection of the SR protein in the cytoplasmic S100 extract in earlier studies did not find its presence, and it is precisely due to the lack of SR protein that the extract also lacks splicing ability (Krainer and Maniatis 1985). By utilizing this system, plant SR proteins have been similarly subsequently characterized (Lopato et al. 1996), suggesting a conserved splicing function of SR proteins between animals and plants. However, some plant SR members, such as SR34 of the SR subfamily, failed to complement the S100 extract, implying potential functional diversification or alternative incompatibility of the plant SR family. Additionally, SR proteins have been observed to affect splice site choice during early spliceosome assembly in both animals and plants (Isshiki et al. 2006; Choi et al. 2021). The established mechanism of this regulation is that SR proteins bind to their binding sequences, such as exonic splicing enhancers (ESEs), recruiting and bridging U1 and U2 auxiliary factors (U2AFs) to the corresponding 5′ and 3′ splice sites, respectively (Cho et al. 2011; Graveley et al. 2001). In plants, several SR proteins have been reported to interact with U1-70 K and U2AF proteins (Yan et al. 2017; Lorkovic et al. 2008; Golovkin and Reddy 1999), suggesting that the function of SR proteins in splice site selection is conserved between animals and plants.
Furthermore, different SR proteins have been demonstrated to affect distinct splice sites, suggesting a specific role of each SR member in the plant SR family (Isshiki et al. 2006). In particular, the characteristics of 12 animal SR protein-binding sequences have been summarized, suggesting a set of conserved degenerate purine-rich (AG) sequences (Anko 2014). Using cross-linking immunoprecipitation (CLIP) assays combined with high-throughput sequencing technologies, multiple studies have demonstrated that SR proteins can bind a variety of RNA transcripts, including noncoding RNAs and intronless and intron-containing mRNAs. Consistent with previous findings, each SR protein has a nonoverlapping set of RNA targets, suggesting their unique role in regulating a subset of genes in animals (Anko et al. 2012; Sanford et al. 2008a, b; Brugiolo et al. 2017). Meanwhile, a few plant studies in some SR-like proteins have also revealed this finding using a similar RNA immunoprecipitation (RIP) sequencing approach (Xing et al. 2015; Zhang et al. 2017).
Given that most functional studies have been carried out in animal systems, whether plant orthologues and plant-specific members have a similar molecular function remains to be investigated experimentally. In particular, a thorough analysis of subcellular localization, shuttling ability, binding activity, and molecular regulatory pathways is required to elucidate further the function of this ancient RNA-binding protein family in planta. Furthermore, the theoretical explanation and biological function of why different SR proteins bind to distinct groups of RNA targets remain to be further studied in eukaryotic systems.
Cellular compartmentation and regulatory mechanisms
As versatile adaptors in RNA processing, SR proteins have a shuttling ability between the nucleus and cytoplasm and, therefore, need to be present in multiple cellular compartments to execute their function (Caceres et al. 1997). Specifically, the C-terminal RS repeats are essential for SR proteins’ localization and shuttling ability (Caceres et al. 1997; Ali and Reddy 2006). Furthermore, even for speckle accumulation in the nucleus, different SR category proteins may have different colocalization patterns in plants (Lorkovic et al. 2008). Given that SR proteins have a variety of molecular functions, endogenous SR proteins are tightly regulated by multiple mechanisms at different levels in both animals and plants (Sun et al. 2010; Morton et al. 2019a). Intriguingly, at the transcript level, SR genes are regulated by unproductive splicing (Fig. 3A), a regulatory mechanism controlled by so-called ultraconserved DNA elements (Lareau et al. 2007; Ni et al. 2007). In particular, these DNA elements are alternatively spliced to form an in-frame PTC or an alternative 3′ untranslated region (3′-UTR), facilitating the RNA surveillance mechanism by NMD degradation.
Regulatory mechanisms to control subcellular localization and function of SR proteins. Regulation and its corresponding components are summarized at four levels, such as transcription and splicing (A), spliceosome assembly (B), translation (C), and post-translation (D). Components that have been reported in plant studies are labeled as green color. PTC, premature termination codon; NMD, nonsense-mediated mRNA decay; U1, U2, U4, U5, U6, small nuclear RNA and binding protein complex (snRNP); U2AF, U2 snRNP auxiliary factors; hnRNP, heterogeneous nuclear ribonucleoprotein; Drosha, a ribonuclease (RNase) III double-stranded RNA-specific ribonuclease that is involved in microRNA biogenesis; Dicer, Double-stranded RNA (dsRNA) endoribonuclease; AGO2, Argonaute2; CRM1, Chromosomal Maintenance 1 / Exportin 1; NFX1, nuclear export protein; PP1, protein phosphatase 1; SRPK, SR protein kinase; Pol II, polymerase II; Clk/Sty, CDC-like kinase 1; PK12, protein kinase 12; LAMMER, LAMMER domain-containing kinase; MPK, mitogen-activated protein kinase
Further investigations involving NMD inhibition have demonstrated that these NMD-regulated isoforms account for 40–70% of total transcripts in humans (Lareau et al. 2007), suggesting that this could be a crucial internal feedback loop that controls the abundance of functional SR proteins. Remarkably, current results indicate that these elements may arise independently in different SR members (Palusa and Reddy 2010; Hartmann et al. 2018a). However, the origin of these ultraconserved elements remains unclear. Similarly, plant SR genes have been reported to be extensively spliced to form PTC-containing isoforms, some of which can be subsequently degraded by the NMD pathway (Palusa and Reddy 2010; Kalyna et al. 2003; Hartmann et al. 2018b). However, the conserved sequences do not extend from animals to plants. Although these ultraconserved sequences are considered to affect splice site selection during spliceosome assembly, no regulators or binding proteins have been reported at the current stage. Thus, further investigation is needed to uncover the underlying mechanism of this autoregulatory feedback loop in the SR gene family. Furthermore, a group of RNA binding proteins, heterogeneous nuclear ribonucleoproteins (hnRNPs), can competitively bind to sequence motifs similar to those bound by SR proteins, forming a competitive binding inhibition of SR proteins (Fig. 3B) (Anko 2014).
In addition to regulation at the transcript level, SR proteins can recruit DROSHA to the basal junction in a CNNC-dependent manner, thereby enhancing the activity of the microprocessor and cleaving pri-miRNA to promote miRNA maturation (Fig. 3C) (Kim et al. 2018). Moreover, SR proteins can maintain their homeostasis at the translational level. Notably, animal SRSF1/ASF/SF2 can autoregulate its protein abundance through its 3′-UTR sequences by a set of microRNAs (Fig. 3C) (Sun et al. 2010; Meseguer et al. 2011; Wu et al. 2010). However, no related reports have been found in plant research.
In addition, posttranslational regulation of SR proteins has been documented in both animals and plants. For example, nucleocytoplasmic shuttling is mediated by transporters such as nuclear import factors transportin-SR (TRN-SR) and MOS14 and export factors NXF1 and CRM1/XPO1/exportin-1 in animals and plants, respectively. Depleting these transporter proteins results in the retention of corresponding SR proteins in specific subcellular compartments (Maertens et al. 2014; Tillemans et al. 2006; Stankovic et al. 2016). In addition, SR proteins are frequently modified by phosphorylation in their RS domain to modulate their protein activity, shuttling ability, and subcellular localization (Fig. 3D). In particular, several protein kinases, including SR protein-specific kinases (SRPKs) and Clk/Sty kinases, have been identified to be responsible for this process in animals (Velazquezdones et al. 2005). However, proteins responsible for the dephosphorylation of SR proteins have seldom been reported (Ma et al. 2010). In plants, phosphoproteomic profiling experiments have identified several potential regulators, such as SR protein kinase AFC2 in Arabidopsis (Golovkin and Reddy 1999), plant hormone-inducible PK12 (Savaldigoldstein et al. 2000), lammer domain-containing kinase (Savaldigoldstein et al. 2003), mitogen-activated protein kinases MPK3 and MPK6 (Feilner et al. 2005), and stress-responsive kinase SnRK2 (Wang et al. 2013), for SR protein phosphorylation, suggesting their roles during plant development and in response to biotic and abiotic stresses. However, the biological significance of these phosphorylation events has yet to be clarified. Interestingly, SR proteins have been demonstrated to interact with each other in both animals and plants (Lorkovic et al. 2008; Wu and Maniatis 1993). However, the biological function of this SR complex remains unclear.
Current working mode of plant SR protein
Based on the available literature, several working models for eliciting the biological function and potential regulation of plant SR proteins are proposed here for consideration (Fig. 4). We believe these models are worthy of further investigation in plants. First, AS is pervasive in eukaryotic cells, and whether the resulting transcript isoforms are functional at the protein level is still under debate (Blencowe 2017). The mainstream opinion suggests that most of this alternative or truncated isoforms are ultimately degraded by the RNA surveillance system, including the NMD pathway. As we have reviewed in the previous paragraphs, similar cases are found in the SR protein family. However, it has been demonstrated that some truncated isoforms can be translated (Jimenez et al. 2019). Reports suggest that truncated SR proteins could competitively interact with protein partners that initially bind to the primary protein isoform, thus performing an antagonizing role to attenuate biological processes regulated by the primary SR proteins (Fig. 4A).
Proposed working models of plant SR proteins in response to internal or external stimuli. Several research questions remain to be addressed. A Whether the resulting transcript isoforms are degraded or translated into a truncated isoform? B Plant splicing-associated proteins respond to abiotic stress and generate different splice variants for spliceosome assembly, providing a possible explanation for the differential mechanism of alternative splice site selection. C Is that possible to generate several functional isoforms to alter the splicing patterns of downstream target genes under various stimuli? The mechanism to sense the changing environment and maintain the equilibrium of each functional isoform remains unclear
Furthermore, emerging evidence suggests a critical link between the SR family of plant proteins and the role of alternative splicing in plant stress responses (Duque 2011). Plant splice-related proteins can respond to abiotic stresses such as salt and cadmium and generate different splice variants for spliceosome assembly, providing a possible explanation for the differential mechanisms of alternative splice site selection (Fig. 4B) (Laloum et al. 2017; Zhu et al. 2017). Sequencing of the transcriptome suggests that PtSCL10 affects cold stress by regulating the splicing of the cold stress-related genes ICE1, LYY, COR2A, but the mechanism of splicing is not yet clear (Zhao et al. 2021). Some serine/arginine (SR) proteins in Lepidium meyenii can potentially regulate AS under cold conditions (Shi et al. 2019). Overexpression of the RS domain in transgenic Arabidopsis also enhanced salt tolerance (Forment et al. 2002). Some studies have also shown that chemical molecules regulate SR proteins’ response. For example, in a survey of agricultural herbicides, GEX1A has weed control activity and can inhibit pre-mRNA splicing by affecting SR proteins (Chen et al. 2019a). But is it possible for SR genes to generate several functional isoforms under abiotic stress to alter the splicing patterns of downstream target genes in response to various stimuli? The mechanism for sensing the changing environment and maintaining the balance of each functional isoform is unclear (Fig. 4C).
Moreover, in plants, some PTC-containing transcripts of SR genes accumulate substantially (Palusa et al. 2007), suggesting that an underlying mechanism other than NMD may determine the fate of these splice variants. The SR proteins Gbp2 and Hrb1 in yeast play a role in the nonsense-mediated decay of mRNAs containing spliced premature termination codons (PTC)(Grosse et al. 2021).The classical NMD pathway could also explain how the primary splice isoform could attenuate its transcript abundance by producing PTC-containing nonfunctional alternative splice variants for NMD degradation. However, this system becomes more complex by introducing the second or third functional isoform produced under stress treatments that the NMD pathway cannot degrade. The underlying mechanism that maintains the equilibrium of these functional isoforms urgently needs to be identified (Fig. 4). This raises the question of whether the equilibrium of these functional splice isoforms is regulated at the RNA level or by trans-acting components or both. How does this regulatory mechanism sense the changing environment and attenuate the composition of each functional isoform? Last but not least, a recent study indicated that splicing factors could be recruited by transcription factors, further strengthening the notion that transcription and splicing are highly coordinated processes (Xiao et al. 2019). This finding also opens a new research area for plant SR proteins.
Concluding remarks and future perspectives
In the past thirty years, scientists have made substantial efforts to understand the function of SR gene family proteins. From their evolutionary perspective to their molecular functions, this RNA binding protein family has been extensively investigated and often regarded as a universal regulator in RNA processing in multiple cellular compartments. However, several research questions have yet to be addressed in plants, including mapping their evolutionary trajectory across the green lineage, molecular functions during various processes of RNA metabolism, and regulatory mechanisms that lead to differential accumulation across different cellular compartments. Thus, modern cutting-edge technology and a proper experimental system must be combined in plant SR protein research by taking cues from studies investigating animal SR proteins. Establishing an in vitro splicing system can provide more detailed insights into the mechanism of spliceosome assembly and pre-mRNA splicing. However, the lack of an in vitro splicing system in plants has become a significant limitation in the further study of plant splicing (Albaqami and Reddy 2018). In addition, mass spectrometry analysis is also critical in the study of RNA-binding proteins (Hernandez et al. 2009). Therefore, constructing in vitro splicing systems in plants and applying mass spectrometry will facilitate the analysis of individual SR proteins and their corresponding splicing variants in well-controlled environments. Given the higher number of SR genes present in plants, multiple knockout mutants and single knockout mutants of a particular isoform using CRISPR‒Cas9 and CRISPR‒Cas13 systems (Morton et al. 2019b), respectively, would be required to counter this redundancy issue, allowing independent genetic analysis of each SR member and their splice variants. In addition, high-throughput profiling techniques combined with biochemical analysis, such as affinity purification-linked quantitative proteomic identification and CLIP or RIP sequencing (Terzi and Simpson 2009), will accelerate the research progress to study the interactome and binding targets of these RNA-binding proteins, providing novel insights into plant SR members during plant developmental processes or stress responses.
Author contributions statement
Conceptualization, J.Z. and M.C; writing original draft preparation, Z.J., D.D. and Y.Z.; Conceptualisation and drawing of the figure, Z.J. and Y.Z.; Proofreading and editing of the content of chapters 1, 2 and 3, D.D. and M.C.; Proofreading and editing of the content of chapters 4, 5 and 6, A.F.; Revision and refinement of figures, Y.L.; funding, J.Z. and M.C. The version of the manuscript was agreed by all authors.
Data availability
Not applicable.
References
Albaqami M, Reddy ASN (2018) Development of an in vitro pre-mRNA splicing assay using plant nuclear extract. Plant Methods 14(1):1–12
Ali GS, Reddy AS (2006) ATP, phosphorylation and transcription regulate the mobility of plant splicing factors. J Cell Sci 119(Pt 17):3527–3538
Anko M (2014) Regulation of gene expression programmes by serine–arginine rich splicing factors. Semin Cell Dev Biol 32:11–21
Anko M, Mullermcnicoll M, Brandl H, Curk T, Gorup C, Henry I, Ule J, Neugebauer KM (2012) The RNA-binding landscapes of two SR proteins reveal unique functions and binding to diverse RNA classes. Genome Biol 13(3):1–17
Aravind L, Watanabe H, Lipman DJ, Koonin EV (2000) Lineage-specific loss and divergence of functionally linked genes in eukaryotes. Proc Natl Acad Sci USA 97(21):11319–11324
Aznarez I, Nomakuchi TT, Tetenbaumnovatt J, Rahman MA, Fregoso O, Rees H, Krainer AR (2018) Mechanism of nonsense-mediated mRNA decay stimulation by splicing factor SRSF1. Cell Rep 23(7):2186–2198
Barta A, Kalyna M, Lorković ZJ (2008) Plant SR proteins and their functions. Curr Top Microbiol Immunol 326:83–102
Barta A, Kalyna M, Reddy AS (2010a) Implementing a rational and consistent nomenclature for serine/arginine-rich protein splicing factors (SR proteins) in plants. Plant Cell 22(9):2926–2929
Bedard KM, Daijogo S, Semler BL (2007) A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J 26(2):459–467
Blencowe BJ (2017) The relationship between alternative splicing and proteomic complexity. Trends Biochem Sci 42(6):407–408
Botti V, Mcnicoll F, Steiner MC, Richter FM, Solovyeva A, Wegener M, Schwich OD, Poser I, Zarnack K, Wittig I, Neugebauer KM, Müller-Mcnicoll M (2017) Cellular differentiation state modulates the mRNA export activity of SR proteins. J Cell Biol 216(7):1993–2009
Brugiolo M, Botti V, Liu N, Mullermcnicoll M, Neugebauer KM (2017) Fractionation iCLIP detects persistent SR protein binding to conserved, retained introns in chromatin, nucleoplasm and cytoplasm. Nucleic Acids Res 45(18):10452–10465
Busch A, Hertel KJ (2012) Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip Rev Rna 3(1):1–12
Butt H, Bazin J, Prasad K, Awad N, Crespi M, Reddy ASN, Mahfouz MM (2022) The rice serine/arginine splicing factor RS33 regulates pre-mrna splicing during abiotic stress responses. Cells 11(11):1796
Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR (1997) Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol 138(2):225–238
Califice S, Baurain D, Hanikenne M, Motte P (2012a) A single ancient origin for prototypical serine/arginine-rich splicing factors. Plant Physiol 158(2):546–560
Chen T, Cui P, Xiong L (2015) The RNA-binding protein HOS5 and serine/arginine-rich proteins RS40 and RS41 participate in miRNA biogenesis in arabidopsis. Nucleic Acids Res 43(17):8283–8298
Chen Q, Han Y, Liu H, Wang X, Sun J, Zhao B, Li W, Tian J, Liang Y, Yan J, Yang X, Tian F (2018) Genome-wide association analyses reveal the importance of alternative splicing in diversifying gene function and regulating phenotypic variation in maize. Plant Cell 30(7):1404–1423
Chen MX, Wijethunge B, Zhou SM, Yang JF, Dai L, Wang SS, Chen C, Fu LJ, Zhang J, Hao GF, Yang GF (2019a) Chemical modulation of alternative splicing for molecular-target identification by potential genetic control in agrochemical research. J Agric Food Chem 67(18):5072–5084
Chen S, Li J, Liu Y, Li H (2019b) Genome-wide analysis of serine/arginine-rich protein family in wheat and brachypodium distachyon. Plants (basel) 8(7):188
Chen MX, Zhang KL, Zhang M, Das D, Fang YM, Dai L, Zhang J, Zhu FY (2020) Alternative splicing and its regulatory role in woody plants. Tree Physiol 40(11):1475–1486
Chen MX, Mei LC, Wang F, Boyagane Dewayalage IKW, Yang JF, Dai L, Yang GF, Gao B, Cheng CL, Liu YG, Zhang J, Hao GF (2021) PlantSPEAD: a web resource towards comparatively analysing stress-responsive expression of splicing-related proteins in plant. Plant Biotechnol J 19(2):227–229
Cho S, Hoang A, Sinha R, Zhong X, Fu X, Krainer AR, Ghosh G (2011) Interaction between the RNA binding domains of Ser-Arg splicing factor 1 and U1–70K snRNP protein determines early spliceosome assembly. Proc Natl Acad Sci USA 108(20):8233–8238
Choi N, Liu Y, Oh J, Ha J, Ghigna C, Zheng X, Shen H (2021) Relative strength of 5’ splice-site strength defines functions of SRSF2 and SRSF6 in alternative splicing of Bcl-x pre-mRNA. BMB Rep 54(3):176–181
Dreyfuss G, Swanson MS, Piñol-Roma S (1988) Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci 13(3):86–91
Duque P (2011) A role for SR proteins in plant stress responses. Plant Signal Behav 6(1):49–54
Fededa JP, Kornblihtt AR (2008) A splicing regulator promotes transcriptional elongation. Nat Struct Mol Biol 15(8):779–781
Feilner T, Hultschig C, Lee J, Meyer S, Immink RGH, Koenig A, Possling A, Seitz H, Beveridge A, Scheel D (2005) High throughput identification of potential arabidopsis mitogen-activated protein kinases substrates. Mol Cell Proteomics 4(10):1558–1568
Forment J, Naranjo MA, Roldán M, Serrano R, Vicente O (2002) Expression of arabidopsis SR-like splicing proteins confers salt tolerance to yeast and transgenic plants. Plant J 30(5):511–519
Fu X, Maniatis T (1990) Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343(6257):437–441
Giannakouros T, Nikolakaki E, Mylonis I, Georgatsou E (2011) Serine-arginine protein kinases: a small protein kinase family with a large cellular presence. FEBS J 278(4):570–586
Golovkin M, Reddy AS (1999) An SC35-like protein and a novel serine/arginine-rich protein interact with arabidopsis U1–70K protein. J Biol Chem 274(51):36428–36438
Graveley BR, Hertel KJ, Maniatis T (2001) The role of U2AF35 and U2AF65 in enhancer-dependent splicing. RNA 7(6):806–818
Grosse S, Lu YY, Coban I, Neumann B, Krebber H (2021) Nuclear SR-protein mediated mRNA quality control is continued in cytoplasmic nonsense-mediated decay. RNA Biol 18(10):1390–1407
Gu J, Ma S, Zhang Y, Wang D, Cao S, Wang ZY (2020) Genome-wide identification of cassava serine/arginine-rich proteins: insights into alternative splicing of pre-mrnas and response to abiotic stress. Plant Cell Physiol 61(1):178–191
Hartmann L, Wiesner T, Wachter A (2018a) Subcellular compartmentation of alternatively spliced transcripts defines SERINE/ARGININE-RICH PROTEIN30 expression. Plant Physiol 176(4):2886–2903
Hartmann L, Wießner T, Wachter A (2018b) Subcellular compartmentation of alternatively spliced transcripts defines SERINE/ARGININE-RICH PROTEIN30 expression. Plant Physiol 176(4):2886–2903
Hernandez H, Makarova OV, Makarov EM, Morgner N, Muto Y, Krummel DP, Robinson CV (2009) Isoforms of U1–70k control subunit dynamics in the human spliceosomal U1 snRNP. PLoS ONE 4(9):e7202
Holmes RK, Tuck AC, Zhu C, Dunn-Davies HR, Kudla G, Clauder-Munster S, Granneman S, Steinmetz LM, Guthrie C, Tollervey D (2015) Loss of the yeast SR protein Npl3 alters gene expression due to transcription readthrough. PLoS Genet 11(12):e1005735
Isshiki M, Tsumoto A, Shimamoto K (2006a) The serine/arginine-rich protein family in rice plays important roles in constitutive and alternative splicing of pre-mRNA. Plant Cell 18(1):146–158
Jeong S (2017) SR Proteins: binders, regulators, and connectors of RNA. Mol Cells 40(1):1–9
Ji X, Zhou Y, Pandit S, Huang J, Li H, Lin CY, Xiao R, Burge CB, Fu X (2013) SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 153(4):855–868
Jimenez M, Urtasun R, Elizalde M, Azkona M, Latasa MU, Uriarte I, Arechederra M, Alignani D, Barcenavarela M, Alvarezsola G (2019) Splicing events in the control of genome integrity: role of SLU7 and truncated SRSF3 proteins. Nucleic Acids Res 47(7):3450–3466
Kalyna M, Lopato S, Barta A (2003) Ectopic expression of atRSZ33 reveals its function in splicing and causes pleiotropic changes in development. Mol Biol Cell 14(9):3565–3577
Kenan DJ, Query CC, Keene JD (1991) RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci 16(6):214–220
Kim K, Nguyen TD, Li S, Nguyen TA (2018) SRSF3 recruits DROSHA to the basal junction of primary microRNAs. RNA 24(7):892–898
Krainer AR, Maniatis T (1985) Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for Pre-mRNA splicing in vitro. Cell 42(3):725–736
Laloum T, Martin G, Duque P (2017) Alternative splicing control of abiotic stress responses. Trends Plant Sci 23(2):140–150
Lareau LF, Inada M, Green RE, Wengrod J, Brenner SE (2007a) Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature 446(7138):926–929
Lazar G, Schaal TD, Maniatis T, Goodman HM (1995) Identification of a plant serine-arginine-rich protein similar to the mammalian splicing factor SF2/ASF. Proc Natl Acad Sci USA 92(17):7672–7676
Lemaire R, Prasad J, Kashima T, Gustafson J, Manley JL, Lafyatis R (2002) Stability of a PKCI-1-related mRNA is controlled by the splicing factor ASF/SF2: a novel function for SR proteins. Genes Dev 16(5):594–607
Li X, Manley JL (2005) Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 122(3):365–378
Lin S, Coutinhomansfield G, Wang D, Pandit S, Fu X (2008) The splicing factor SC35 has an active role in transcriptional elongation. Nat Struct Mol Biol 15(8):819–826
Lopato S, Waigmann E, Barta A (1996) Characterization of a novel arginine/serine-rich splicing factor in arabidopsis. Plant Cell 8(12):2255–2264
Lorkovic ZJ, Hilscher J, Barta A (2008) Co-localisation studies of arabidopsis SR splicing factors reveal different types of speckles in plant cell nuclei. Exp Cell Res 314(17):3175–3186
Ma C, Ghosh G, Fu X, Adams JA (2010) Mechanism of dephosphorylation of the SR protein ASF/SF2 by protein phosphatase 1. J Mol Biol 403(3):386–404
Maertens GN, Cook N, Wang W, Hare S, Gupta SS, Oztop I, Lee K, Pye VE, Cosnefroy O, Snijders APL (2014) Structural basis for nuclear import of splicing factors by human transportin 3. Proc Natl Acad Sci USA 111(7):2728–2733
Manley JL, Krainer AR (2010) A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins). Genes Dev 24(11):1073–1074
Maruyama K, Sato N, Ohta N (1999) Conservation of structure and cold-regulation of RNA-binding proteins in cyanobacteria: probable convergent evolution with eukaryotic glycine-rich RNA-binding proteins. Nucleic Acids Res 27(9):2029–2036
Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, Barettino D (2011) MicroRNAs-10a and 10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF). J Biol Chem 286(6):4150–4164
Michlewski G, Sanford JR, Caceres JF (2008) The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell 30(2):179–189
Morton M, Altamimi N, Butt H, Reddy ASN, Mahfouz M (2019a) Serine/Arginine-rich protein family of splicing regulators: new approaches to study splice isoform functions. Plant Sci 283:127–134
Mullermcnicoll M, Botti V, Domingues AMDJ, Brandl H, Schwich OD, Steiner MC, Curk T, Poser I, Zarnack K, Neugebauer KM (2016) SR proteins are NXF1 adaptors that link alternative RNA processing to mRNA export. Genes Dev 30(5):553–566
Ni JZ, Grate L, Donohue JP, Preston C, Nobida N, Obrien G, Shiue L, Clark TA, Blume JE, Ares M (2007) Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev 21(6):708–718
Palusa SG, Reddy ASN (2010) Extensive coupling of alternative splicing of pre-mRNAs of serine/arginine (SR) genes with nonsense-mediated decay. New Phytol 185(1):83–89
Palusa SG, Ali GS, Reddy AS (2007) Alternative splicing of pre-mRNAs of arabidopsis serine/arginine-rich proteins: regulation by hormones and stresses. Plant J 49(6):1091–1107
Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40(12):1413–1415
Reed R, Cheng H (2005) TREX, SR proteins and export of mRNA. Curr Opin Cell Biol 17(3):269–273
Richardson DN, Rogers MF, Labadorf A, Benhur A, Guo H, Paterson AH, Reddy ASN (2011) Comparative analysis of serine/arginine-rich proteins across 27 Eukaryotes: insights into sub-family classification and extent of alternative splicing. PLoS ONE 6(9):e24542
Rosenkranz RRE, Bachiri S, Vraggalas S, Keller M, Simm S, Schleiff E, Fragkostefanakis S (2021) Identification and regulation of tomato serine/arginine-rich proteins under high temperatures. Front Plant Sci 12:645689
Ruwe H, Kupsch C, Teubner M, Schmitz-Linneweber C (2011) The RNA-recognition motif in chloroplasts. J Plant Physiol 168(12):1361–1371
Sanford JR, Coutinho P, Hackett JA, Wang X, Ranahan WP, Caceres JF (2008a) Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF. PLoS ONE 3(10):e3369
Sanford JR, Wang X, Mort M, Vanduyn N, Cooper DN, Mooney SD, Edenberg HJ, Liu Y (2008b) Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome Res 19(3):381–394
Savaldigoldstein S, Sessa G, Fluhr R (2000) The ethylene-inducible PK12 kinase mediates the phosphorylation of SR splicing factors. Plant J 21(1):91–96
Savaldigoldstein S, Aviv D, Davydov O, Fluhr R (2003) Alternative splicing modulation by a LAMMER kinase impinges on developmental and transcriptome expression. Plant Cell 15(4):926–938
Shi Y, Su Z, Yang H, Wang W, Jin G, He G, Siddique AN, Zhang L, Zhu A, Xue R, Zhang C (2019) Alternative splicing coupled to nonsense-mediated mRNA decay contributes to the high-altitude adaptation of maca (Lepidium meyenii). Gene 694:7–18
Stankovic N, Schloesser M, Joris M, Sauvage E, Hanikenne M, Motte P (2016) Dynamic distribution and interaction of the arabidopsis SRSF1 subfamily splicing factors. Plant Physiol 170(2):1000–1013
Sun S, Zhang Z, Sinha R, Karni R, Krainer AR (2010) SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nat Struct Mol Biol 17(3):306–312
Terzi LC, Simpson GG (2009) Arabidopsis RNA immunoprecipitation. Plant J 59(1):163–168
Tillemans V, Leponce I, Rausin G, Dispa L, Motte P (2006) Insights into nuclear organization in plants as revealed by the dynamic distribution of arabidopsis SR splicing factors. Plant Cell 18(11):3218–3234
Velazquezdones A, Hagopian JC, Ma C, Zhong X, Zhou H, Ghosh G, Fu X, Adams JA (2005) Mass spectrometric and kinetic analysis of ASF/SF2 phosphorylation by SRPK1 and Clk/Sty. J Biol Chem 280(50):41761–41768
Wan L, Deng M, Zhang H (2022) SR splicing factors promote cancer via multiple regulatory mechanisms. Genes (basel) 13(9):1659
Wang H, Jiang Y (2021) SRp20: A potential therapeutic target for human tumors. Pathol Res Pract 224:153444
Wang P, Xue L, Batelli G, Lee S, Hou Y, Van Oosten MJ, Zhang H, Tao WA, Zhu J (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci USA 110(27):11205–11210
Wu JY, Maniatis T (1993) Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75(6):1061–1070
Wu H, Sun S, Tu K, Gao Y, Xie B, Krainer AR, Zhu J (2010) A splicing-independent function of SF2/ASF in microRNA processing. Mol Cell 38(1):67–77
Wu J, Liu H, Lu S, Hua J, Zou B (2021) Identification and expression analysis of chloroplast ribonucleoproteins (cpRNPs) in arabidopsis and rice. Genome 64(5):515–524
Xiao R, Sun Y, Ding J, Lin S, Rose DW, Rosenfeld MG, Fu X, Li X (2007) Splicing regulator sc35 is essential for genomic stability and cell proliferation during mammalian organogenesis. Mol Cell Biol 27(15):5393–5402
Xiao R, Chen J, Liang Z, Luo D, Chen G, Lu ZJ, Chen Y, Zhou B, Li H, Du X (2019) Pervasive chromatin-RNA binding protein interactions enable RNA-based regulation of transcription. Cell 178(1):107
Xing D, Wang Y, Hamilton M, Ben-Hur A, Reddy AS (2015) Transcriptome-wide identification of RNA targets of arabidopsis SERINE/ARGININE-RICH45 uncovers the unexpected roles of this RNA binding protein in RNA processing. Plant Cell 27(12):3294–3308
Xu S, Zhang Z, Jing B, Gannon P, Ding J, Xu F, Li X, Zhang Y (2011) Transportin-SR is required for proper splicing of resistance genes and plant immunity. PLoS Genet 7(6):e1002159
Yan Q, Xia X, Sun Z, Fang Y (2017) Depletion of Arabidopsis SC35 and SC35-like serine/arginine-rich proteins affects the transcription and splicing of a subset of genes. PLoS Genet 13(3):e1006663
Zhang Z, Krainer AR (2004) Involvement of SR proteins in mRNA Surveillance. Mol Cell 16(4):597–607
Zhang X, Shi Y, Powers JJ, Gowda NB, Zhang C, Ibrahim HM, Ball HB, Chen SL, Lu H, Mount SM (2017) Transcriptome analyses reveal SR45 to be a neutral splicing regulator and a suppressor of innate immunity in Arabidopsis thaliana. BMC Genomics 18(1):772–772
Zhao X, Tan L, Wang S, Shen Y, Guo L, Ye X, Liu S, Feng Y, Wu W (2021) The SR splicing factors: providing perspectives on their evolution, expression, alternative splicing, and function in populus trichocarpa. Int J Mol Sci 22(21):11369
Zhu FY, Chen MX, Ye NH, Shi L, Ma KL, Yang JF, Cao YY, Zhang Y, Yoshida T, Fernie AR, Fan GY, Wen B, Zhou R, Liu TY, Fan T, Gao B, Zhang D, Hao GF, Xiao S, Liu YG, Zhang J (2017a) Proteogenomic analysis reveals alternative splicing and translation as part of the abscisic acid response in arabidopsis seedlings. Plant J 91(3):518–533
Acknowledgements
This work was supported by the Guizhou Provincial Basic Research Program (Natural Science)-ZK[2023]-099, the National Natural Science Foundation of China (NSFC81401561, 91535109, 32001452), and the Hong Kong Research Grant Council (AoE/M-05/12, AoE/M-403/16, GRF14160516, 14177617, 12100318).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, ZC., Das, D., Zhang, Y. et al. Plant serine/arginine-rich proteins: versatile players in RNA processing. Planta 257, 109 (2023). https://doi.org/10.1007/s00425-023-04132-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04132-0