Abstract
Main conclusion
AS-3 line of Sorghum bicolor possesses functional components of apomixis—apospory, parthenogenesis and autonomous endospermogenesis. The data obtained indicate efficiency of selection for apomixis components in diploid species of cultivated crops.
Abstract
Apomixis (seed formation without fertilization) is one of most attractive phenomena in plant biology. In this paper, we provide the results of long-term selection for apomixis components in the progeny of grain sorghum (Sorghum bicolor (L.) Moench) hybrid plants with male sterility mutation. Selection was carried out for a high frequency of aposporous embryo sacs (ESs), autonomous pro-embryos, and the presence of maternal-type plants in test crosses with the line Volzhskoe-4v (V4v) homozygous for the Rs1 genes determining the red color of the leaves and stem of the hybrids. As a result of using this approach, the line, AS-3, was created, in which the frequency of ovaries with parthenogenetic embryos reached 42–45%. The autonomous development of embryos and endosperm was observed in the panicles of each of the 10 cytologically studied plants of this line. The frequency of parthenogenesis positively correlated with the high average daily air temperature during the first five out of 10 days preceding the onset of flowering (r = 0.75; P > 0.01). Genotyping of the plants from the progeny of hand-emasculated panicles of AS-3 pollinated with V4v performed using co-dominant SSR markers revealed that the F1 hybrids carrying the Rs1 gene (chromosome 6) possessed both paternal and maternal alleles of Sb1-10 (chromosome 4) and Xtxp320 (chromosome 10) markers, while in the maternal-type plants (rs1rs1), only the maternal alleles of these markers were present. In the endosperm of the kernels from which the maternal-type seedlings were obtained, only the maternal alleles were present, while in the endosperm of the kernels that produced hybrid seedlings, both the paternal and maternal alleles were observed. The data obtained indicate the presence of functional components of apomixis (apospory, parthenogenesis, autonomous endospermogenesis) in the grain sorghum line AS-3, and the efficiency of selection for apomixis in functionally diploid species of cultivated crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apomixis—seed formation without double fertilization—is one of the most intriguing biological phenomena, attracting attention of plant biologists for more than a century. Various forms of apomixis, or its embryological prerequisites, have been described in many species of wild flora, however, they are rarely found in cultivated crops created by multiple crosses and selections. The increased attention to the problem of apomixis is due to the hopes that the transfer of apomixis to cultivated plants will contribute to solving one of the main challenges of plant breeding—fixation of heterosis and clonal propagation of the best genotypes. In addition, the study of apomixis opens the way to understanding the development of female generative structures, genetic control of meiosis, development of the female gametophyte, double fertilization, embryo and endospermogenesis.
Numerous studies demonstrate that apomixis is a complex phenomenon depending on combination in one genotype the ability to form unreduced embryo sacs (either diplosporous, occurring as a result of meiosis disturbances, or aposporous, developing from somatic cells located nearby macrospore mother cell or meiotic embryo sac), parthenogenesis of the egg cell, and autonomous endospermogenesis (or formation of pseudogamous endosperm) (Barcaccia and Albertini 2013; Tavva et al. 2015; Fiaz et al. 2020; Hojsgaard 2020). Each of these processes is controlled by its own set of genes and is largely influenced by environmental factors that affect the expression of the corresponding genetic systems through different epigenetic mechanisms (Kumar 2017). The presence of each of these elements is a pre-requisite for apomixis, but not enough for autonomous seed development.
Over the past years due to intensive study of wild apomictic species, a number of candidate genes for different components of apomixis have been identified (Pupilli and Barcaccia 2012; Hand and Koltunow 2014; Brukhin 2017; Vijverberg et al. 2019; Worthington et al. 2019; Hojsgaard 2020). These studies contribute to the understanding of the molecular mechanisms of apomixis and open prospects for its construction using biotechnological methods, including genomic editing (Xie et al. 2019). However, significant progress has not been achieved yet.
Various breeding lines and accessions of Sorghum bicolor (L.) Moench have repeatedly served as objects for the study of apomixis. There are reports on identification of aposporous or diplosporous embryo sacs (ESs) (Hanna et al. 1970; Rao et al. 1978; Wu et al. 1994; Elkonin et al. 1995, 2012; Zhang et al. 1997; Ping et al. 2004; Carman et al. 2011). Autonomous development of embryo and endosperm was detected in these lines by cytological methods (Rao et al. 1978; Wu et al. 1994; Elkonin et al. 1995, 2012; Ping et al. 2004). Among sorghum lines that were reported to express any level of apomixis, the most known and extensively investigated was the line R473 (for review, see: Murty 1993). However, the presence of apomixis in this line, as well as in other lines and hybrids of functionally diploid species was questioned (Bala Ravi 1993; Sokolov et al. 2011). Nevertheless, these critical comments did not take into account variable nature of apomixis, its strong dependence on the environmental conditions of plant growing. For example, in sorghum line AS-1a, apomictic potencies manifested in hot and drought seasons, and almost did not express in moderate and wet conditions (Elkonin et al. 2012). In addition, apomixis has been identified in a number of diploid species from genus Boechera (Windham et al., 2015; Carman et al. 2019), Paspalum (Sienna et al. 2008), Ranunculus (Barke et al. 2018) and some others that encourage further studies of this phenomenon in other diploid species.
Previously, studying expression of male sterility in sorghum hybrids with dominant mutation Mstc, derived from tissue culture (Elkonin 2005), we found the plants with complete male sterility capable of seed set. In this paper, we provide the results of years of research of the progeny of these hybrids resulted in obtaining the line, AS-3, capable of formation of aposporous embryo sacs, and autonomous embryo- and endospermogenesis that was proved by cytological observations and genetic analysis of the progeny using SSR markers and Rs1 marker gene expression.
Materials and methods
Plant materials and crosses
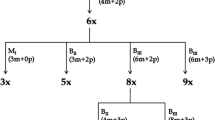
Plants from the grain sorghum (Sorghum bicolor (L.) Moench) hybrid combination (SK-723 (Mstc/mstc) × KVV-114) × KVV-114 (i.e., BC1 generation) were used as starting material. Male sterile plants from this combination carried dominant mutation Mstc derived from tissue culture (Elkonin 2005). Few male-sterile plants from this combination, in spite of complete male sterility, were capable of some seed setting. The progeny of these plants was subjected to further analysis. All panicles in each family were carefully bagged with a parchment bags before anthesis. Male sterile plants (with reduced non-dehiscent anthers and with fresh persistent stigmas on all panicle branches) from these families have been pollinated with pollen of the line Volzhskoe-4v (V4v) homozygous for a dominant genes Rs1, controlling anthocyanin pigmentation of the seedlings, or with the auto-diploid line Milo-145, that produces F1 hybrids with anthocyanin coloration of coleoptile. In the progeny of these crosses, diploid maternal-type plants lacking anthocyanin pigmentation were identified. The pollen of these plants was subjected to cytological analysis, and male-sterile individuals (with reduced anthers or with normal large anthers but lacking fertile pollen) were used for pollination with V4v pollen. Those plants, which had some percent of fertile pollen, were self-pollinated, and their progenies were studied in the next season. The scheme of crosses and selections used in this work is shown in Fig. 1.
All plants were grown in the experimental field of the Agricultural Research Institute of the South-East Region (Saratov, Russia) in the 2001–2020 years.
Cyto-embryological analysis of female gametophyte
Maternal-type plants from each generation of crosses with the tester-lines as well as the plants from self-pollinated progenies were subjected to cyto-embryological analysis of female gametophyte. The frequency of ovaries with aposporous embryo sacs (apo-ES), parthenogenetic embryos (PE) and autonomous endosperm, and the total frequency of apomixis elements including ovules with aposporous initial cells (AIC) were recorded. The plants with highest frequencies of these traits were selected and used in further test crosses.
In embryological analysis, branches of sorghum panicles were fixed with ethanol–acetic acid (3:1) for 3 days, washed with 70% ethanol for 3 days, and stored in 96% ethanol. Fixation was performed 3–4 days after the start of flowering, and branches to be fixed were collected from the central part of a panicle. Fixed ovaries were isolated from flowers, washed with distilled water for 20–30 min, treated with 4% ammonium—iron alum at 50 °C for 10 min, washed with distilled water at 50 °C for 15–20 min, and stained with 2% acetocarmine at 50 °C for 1.0–1.5 h. ESs were isolated from the stained ovaries with the use of microneedles under a stereomicroscope without squashing and maceration. Isolated ESs were transferred into a drop of glycerol on a slide, and covered by a slip, while their volume structure remained unchanged. For each plant, usually, 35–50 ovaries were studied. During selection for the frequency of apo-ESs, 5 plants were studied in each family. Preparations were examined with using Axioscope A1 microscope (Carl Zeiss, Germany) under transmitted light (magnification ×200, ×400). Figures were made by Axio Cam MRc digital camera using Axio Vision 4 computer program.
Cytological analysis of pollen
For pollen analysis, the anthers from 10 to 15 spikelets were placed on a glass slide and macerated. Pollen grains (PG) were stained with 1% iodine–potassium iodide stain for the estimation of starch accumulation. For each panicle, pollen analysis was performed in two replications (from the middle and from the lower layer of the panicle), each comprises 100 PGs. Pollen grains were classified as sterile (empty pollen grains), PGs with disturbances of starch development, poorly stained PGs, fertile PGs.
Plant genotyping
For genotyping plants from the progeny from the crosses AS-3/V4v, each mature kernel set on hand-emasculated panicles of the line AS-3 pollinated with V4v pollen was divided into two halves with a scalpel. The part with an endosperm without scutellum was kept in the tube until DNA isolation. The part with an embryo and scutellum was put in a Petri dish on a moisture filter paper to obtain plant. The developed plants were grown in a greenhouse and then transplanted to an experimental plot, where they were grown until full maturity. This step allows to analyze the DNA of the kernel endosperm and of the plant obtained from this kernel.
DNA was extracted from leaves and endosperm using the modified STAB method of Doyle and Doyle (1987). Co-dominant SSR markers Sb1-10 (Brown et al. 1996) and Xtxp320 (Kong et al. 2000), differentiating lines AS-3 and V4v were used (Table 1). PCR analysis was performed using the MasterCycler Personal (Eppendorf). The reaction mixture contained 50 ng DNA, 0.01 U/μl SynTaq DNA polymerase (Synthol, Russia), 0.6 pmol of each primer, × onefold PCR buffer (Synthol, Russia), 2.5 mM MgCl2, 0.2 mM dNTP mixture (Synthol, Russia). The total volume of the reaction mixture was 25 μl. PCR for amplification of Xtxp320 SSR marker was performed using the following regime (Kong et al 2000): initial denaturation of 94 °C (4 min); 30 cycles: denaturation for 1 min at 94 °C, annealing for 30 s at 54 °C and extension at 72 °C for 1 min; the last PCR cycle was followed by a 7-min extension at 72 °C. For amplification of Sb1-10 SSR marker touchdown PCR was performed consisting of 94 °C for 4 min initial denaturation; 20 cycles of 94° for 30 s, 65°–56° for 30 s decreasing by 1 °C every 2 cycles, and 72 °C for 1 min; and 20 cycles of 94° for 15 s, 55° for 30 s, 72 °C for 1 min; followed by 72 °C for 10 min (Brown et al. 1996). The amplified fragments were fractionated in 3.5% agarose gels in 0.5-fold TAE buffer at 175 V (90 min). A 0.01% aqueous solution of ethidium bromide was used to visualize DNA fragments.
Study of the effects of environmental factors
The effects of the following environmental factors were recorded: the average daily air temperature during 10 days before the beginning of flowering and during flowering; the average daily air temperature for the first 5 days out of 10 days preceding the beginning of flowering; the amount of precipitation during flowering period. The hydrothermal coefficient (the ratio of the amount of precipitation to the amount of average daily temperatures for the period) was calculated. The meteorological data were kindly provided by the Laboratory of Meteorology of the Agricultural Research Institute of the South-East Region (Saratov, Russia).
Statistical methods
To evaluate the effect of selection upon the frequency of elements of apomixis, the variance analysis using the AGROS software package, version 2.09 (S.P. Martynov, Institute of General Genetics, RAS) with the Duncan’s Multiple Range Test was performed. In each generation, five plants from the progeny of selected individuals were studied.
To estimate the differences in frequency of parthenogenetic pro-embryos and aposporous structures at the different developmental stages, proportion comparisons were performed by Fisher’s method used in the case of small samples or samples differing in size (Zaitsev 1984). In this method, proportions are compared by Fisher’s test:
where F is the Fisher’s test, φ1 and φ2 are values resulting from a transformation of the proportions into radians, and N1 and N2 are the sizes of the samples under study.
In each experimental variant were studied 4–5 plants (independent biological replications).
The dependence of the manifestation of apomixis components of AS-3 line on the environmental factors was assessed using correlation analysis (Zaitsev 1984).
Results
Development of AS-3 line
Male sterile plants from the hybrid combination BC1 [(SK-723 × KVV-114) × KVV-114] had reduced shriveled anthers which did not contain any pollen grains (Fig. 2a). However, in some panicles the larger anthers also developed. Cytological analysis of pollen from such anthers revealed PGs that were either completely devoid of content or had significant disturbances in starch accumulation (weak coloration with a solution of potassium iodide, delamination of the content from the wall of the pollen grain). In some plants, however, a certain proportion of fertile PGs was present (Fig. 2b).
Anthers (a, upper part of the figure) and pollen (b) of semi-sterile plant from the BC1 hybrid combination [(SK-723 × KVV-114) × KVV-114]. Anthers of fertile line KVV-114 are shown at the bottom of Fig. 1a. Bar 100 µm
Cytological analysis of mega-gametophyte in male-sterile plants with small reduced anthers from this BC1 family showed that near the sexual embryo sac typical for Polygonum-type of sorghum, large cells (LCs) were present (Fig. 2a). These cells were located near the antipodals or in the chalazal part of the ovule and, apparently, were the initials of aposporous ESs (apo-ESs). In 5–7 days after the beginning of flowering alongside with LCs, the apo-ESs were observed (Fig. 2b, d). Such apo-ESs were more often located near the antipodal complex. The frequency of ovules with aposporous ESs varied in different plants from 2.4 to 12.5% (Table 2). In some plants, autonomously developing proembryos have been found in embryo sacs with intact (unfertilized) polar nuclei (Fig. 3e, f; Fig. 4); their frequency was 2.0–4.2% (Table 2).
Male sterile plants, in which no fertile PGs were found by cytological analysis, were pollinated with pollen of the V4v line, homozygous for the dominant marker genes Rs1 determining the anthocyanin color of seedlings. In the next generation from such a crossing, both hybrid plants with anthocyanin coloration of leaves and stem and green maternal-type plants devoid of anthocyanin coloration were found (Table 3). Hybrid plants at the next stages of development also clearly differed from maternal-type plants by heterosis for the plant height, leaf length, panicle size and grain yield. Maternal-type plants were either completely male-sterile or had some fertile pollen and set seeds upon self-pollination (semi-sterile plants). Plants with complete male sterility confirmed by cytological analysis were again pollinated with pollen from the V4v line. In the offspring from this crossing, green maternal-type plants were again observed, which were sterile or semi-sterile.
The procedure for selection of male-sterile maternal-type plants from the progeny of panicles pollinated with V4v, their pollination with pollen from the V4v line, and selection in the progeny for green plants for repeated crossing with the V4v line was carried out for 5 generations (Fig. 1, Table 2). In each generation, along with selection for plant phenotype and cytological analysis of pollen, cytological analysis of female gametophytes was carried out, which indicated the presence of apo-ESs in the ovules of the studied plants, as well as autonomously developing pro-embryos (Table 2, Fig. 4). In some ESs, there were cases of autonomously developing endosperm (Figs. 4e, f), and there were no signs of pollen tubes penetration into the ES with developing pro-embryo or endosperm. To obtain the next generation, the plants with the highest frequency of apo-ESs and autonomous pro-embryos, which were identified by cyto-embryological analysis were used.
In the course of selection, in one family, 15/06 (Fig. 1), a plant (#5) was identified with a relatively high frequency of autonomous pro-embryos (10.4%) (Table 2), which, when crossed with V4v, gave 96% green plants in its progeny (family 13/07, Fig. 1, Table 3). These plants included both sterile and partially sterile individuals with a high frequency of apo-ESs (up to 25%, Table 2). Analysis of the progeny of partially sterile plants showed the inheritance of the ability for aposporous structure formation and parthenogenetic proembryos (family 21/08, Table 2). Later, to obtain the next generation, self-pollinated progeny of plants with partial male fertility and the maximum frequency of aposporous structures and autonomous development of pro-embryo or endosperm were selected. This selection was carried out over the next 8 generations (Fig. 1). The plants with complete male sterility from different generations were pollinated with pollen of the V4v to test their ability to formation of seeds with maternal-type embryos. The results of such testing (Table 3) show that in the progeny of many panicles, green maternal-type plants appeared with variable frequency. The appearance of such plants could hardly have been a consequence of self-pollination, since the maternal panicles had no fertile pollen. As a result of this approach, we created a line, AS-3, in which the frequency of ovaries with parthenogenetic embryos in individual plants reached 42–45% in the 2020 season (Fig. 1; Table 2). The autonomous development of embryos and endosperm was observed in the panicle of each of 10 cytologically studied plants, whereas in the early generations of selection, the ability for autonomous development was observed maximum in 2–4 plants (p < 0.01). These data indicate the efficiency of selection for apomixis.
Manifestation of the ability for parthenogenesis
Temporal fixation of ovaries from the middle layer of panicles, carried out starting from the second day after the beginning of flowering, showed that 2–3 days after the flowering onset, the development of parthenogenetic embryos in the unfertilized ESs (with intact polar nuclei) was observed (Fig. 4). With an increase in the time after the beginning of flowering, the proportion of ESs with parthenogenetic development considerably increased (Fig. 5). The frequencies of parthenogenetic embryos on days 4, 5, and 6 differ significantly (p > 0.01). These data indicate a tendency towards autonomous parthenogenesis in the AS-3 line, which is realized in the absence of fertilization. In addition, with an increase in the time after the beginning of flowering, the proportion of ovules with aposporous structures (apo-ES and large cells-initials of apo-ES) significantly increased (Fig. 5).
Frequency of ovules with aposporous structures (aposporous embryo sacs (apoES), and large cells—initials of apoES), and parthenogenetic proembryos at different time intervals after the beginning of flowering of panicles of the AS-3 line. Each datum is a mean of 35–50 investigated ovaries in five plants. Bars represent ± standard error (SE)
To clarify the existence of parthenogenesis ability in plants of the AS-3 line, crosses of emasculated panicles of this line with lines V4v and Milo-145 were carried out (Table 4). It was found that the seeds in such crosses had a low germination. Nevertheless, green maternal-type plants were found in the progeny of emasculated panicles, which had complete phenotypic similarity to the maternal line. Their frequency in different crosses varied from 0 up to 66.7% (Table 4). These data confirm the results of cytological observations and indicate that AS-3 line has the ability to form seeds on the basis of apomixis.
Genotyping of plants from the progeny of crossings AS-3 line with the V4v line using SSR markers
To verify the apomictic origin of plants, which were found in the progeny from the crosses of emasculated panicles of AS-3 line with the line V4v, genotyping of the offspring was undertaken using molecular markers. In a preliminary study of the AS-3 and V-4v lines using seven SSR markers, we found that SSR markers Sb1-10 (chromosome 4) and Xtxp320 (chromosome 10) clearly differentiate these lines. These markers have co-dominant mode of expression in F1 hybrids of these lines. PCR analysis of DNA isolated from the leaves of green seedlings identified in the AS-3 (7-6-1/19) × V4v progeny showed the presence of the maternal alleles and the absence of the paternal alleles of the markers Sb1-10 and Xtxp320 (Fig. 6). At the same time, both alleles were present in anthocyanin (hybrid) seedlings obtained from the same progeny.
PCR analysis with primers to the SSR marker Sb1-10 (a) and Xtxp320 (b). a DNA from the leaves of plants of the sorghum line AS-3 (♀) (1, 2), leaves (L) of green maternal-type seedlings (m) (3, 5) from the offspring of the emasculated panicle of the line AS-3, pollinated with pollen from the line Volzhskoe-4v (V4v) and endosperm (E) of kernel, from which maternal-type seedlings were obtained (4, 6), the paternal line V4v (♂) (7, 8), leaf (L) of F1 hybrid seedling (9) and endosperm (E) from which this seedling developed (10); 11—markers of the length of DNA fragments; 12—negative control (no DNA). Tracks 9 and 10 clearly show the co-dominant nature of the marker. b DNA from the leaves of the AS-3 plants (♀) (1–4), leaves of green maternal-type seedlings (m) (5, 7) from the offspring of the cross AS-3 × V4v and endosperm from the kernels, from which maternal-type seedlings were obtained (E) (6, 8), the paternal line V4v (♂) (9, 10), leaves of hybrid seedlings (11, 12), and endosperm of the kernels, from which they were obtained (13–16) (each in duplicate); 17—markers of the length of DNA fragments. Tracks 11–16 clearly show the co-dominant nature of the marker
It is noteworthy that the paternal allele was also absent in the DNA extracted from the endosperm of the kernels, from which the green seedlings were obtained, while DNA from the endosperm of the kernels, from which the hybrid plants developed, contained both the maternal and the paternal alleles (Fig. 6). These data clearly agree with the results of cyto-embryological analysis and confirm the autonomous nature of endosperm in apomictic kernels of the AS-3 line.
Thus, genotyping of seedlings using two SSR markers and a marker gene Rs1 localized in three different chromosomes—Sb1-10 in chromosome 4, Xtxp320 in chromosome 10, and Rs1 in chromosome 6 (Mace and Jordan 2010)—indicates the matroclinal nature of some seedlings developing in crosses of AS-3 and V4v sorghum lines. The results of genotyping of seedlings using PCR analysis were fully confirmed at later stages of development by phenotypic differences for numerous traits between plants carrying only maternal alleles of Sb1-10 and Xtxp320, and maternal and paternal alleles of these markers (F1 hybrids AS-3/V4v). Therefore, genetic differences between these plants are due to numerous loci and cannot be explained by elimination of paternal alleles of these SSR markers.
Environmental effects on expression of apomictic potentials in AS-3
During the course of selection of the AS-3 line, a significant variation in the frequency of aposporous structures (apo-ESs and initial cells) and parthenogenetic pro-embryos was found in different seasons. Assuming the variability of weather conditions in different seasons during the sorghum flowering period, we studied the influence of different meteorological factors on the manifestation of apomictic potencies in the AS-3 line. A careful analysis showed that the frequency of the aposporous structures depends on the air temperature. High average daily temperatures during the 10 days prior to beginning of flowering significantly increase their frequency (r = 0.72, p > 0.01) (Fig. 7a).
At the same time, precipitation during the flowering period resulted in an increase in the hydro-thermal coefficient (HTC), decreased the frequency of apo-ESs (Fig. 7c, r = − 0.82; p > 0.01), but did not affect the frequency of LC—aposporous initial cells. It was also found that high average daily temperatures during the first five of 10 days preceding the onset of flowering significantly increased the frequency of parthenogenesis (r = 0.75; p > 0.01) (Fig. 7b). Consequently, hot and dry weather on the eve and during the flowering period promotes the manifestation of apomictic potentials of the AS-3 line, while cooler and wetter weather suppresses their realization.
Discussion
The experimental creation of lines with gametophytic apomixis is an extremely difficult task, the successful solution of which is impossible without combining in one genotype genetic factors that control a variety of embryological processes. The data presented above show that sequential selection over many generations for a high frequency of apospory, combined with selection for the ability to form maternal-type plants in crosses with a genetically marked line, can be one of the effective approaches for creating lines with facultative apomixis.
Photographs of embryo sacs, without signs of pollen tube penetration, with intact polar nuclei, but containing multicellular embryos, irrefutably indicate that AS-3 has the ability to parthenogenesis. The presence of maternal-type plants, which have clear genetic differences from hybrids, in the progeny of panicles with cytologically identified parthenogenetic embryos, indicates the formation of a part of the kernels on the basis of apomixis. Variation in the frequency of parthenogenesis observed in different seasons in the AS-3 line is a characteristic feature of many apomicts, in which the environmental factors, i.e., drought stress, temperature, and photoperiod regulate the manifestation of apomictic potencies (Saran and de Wet 1976; Elkonin et al. 2012; Quarin 1986; Klatt et al. 2016, 2018; Rodrigo et al. 2017; Ulum et al. 2020).
It is known that drought and high ambient temperatures affect the pattern of DNA (Lukens and Zhan 2007; Tan 2010; Verhoevan et al. 2010; Wang et al. 2011; Liu et al. 2015) and histone (Granot et al. 2009) methylation, and, thereby, modify the expression of plant genes. A number of studies have clearly shown that it is the changes at the epigenetic level, in particular, the change in the nature of DNA methylation in the cells of the female generative structures that regulate the manifestation of apomictic potentials (Kumar 2017). Perhaps, hot and dry weather on the eve and during the flowering period causes any changes in DNA methylation in the loci regulating mega-gametophyte development in AS-3 line that promotes the manifestation of its apomictic potentials. A similar conclusion was made by us earlier when observing the manifestation of apomixis in another line of sorghum, AS-1a (Elkonin et al. 2012). Perhaps, drought stress is a strong trigger of manifestation of apomixis components in sorghum. In this regard, it becomes clear that the maintenance of the AS-3 line must be carried out under strictly defined environmental conditions, namely, under conditions of drought and high temperatures during the panicle development and flowering that are favorable for the formation of apo-ESs and parthenogenetic embryos. Otherwise, it is highly likely to lose apomictic potentials in this line.
At the same time, it should be noted that the embryological mechanisms of the realization of apomictic potencies in the AS-3 line remain not fully understood. The presence of several ESs in the ovule, including an abnormal one, as well as the large additional cells, possibly the initials of the apo-ESs, may indicate an aposporous origin of the embryo sacs. Such pictures are often observed in apomicts, in which gametophytic apomixis is realized on the basis of apospory (Koltunow et al. 1998; Bicknell and Koltunow 2004; Siena et al. 2008). However, in the absence of clear experimental data on the course of female meiosis, the possibility of the development of diploid embryo sacs by diplospory, as a result of disorders in meiosis in the macrospore mother cells, cannot be ruled out. In this regard, one should stress that parthenogenetic embryos in AS-3 have diploid nature, since we did not observe haploid plants in any family or generation of selection. Detailed studies of female meiosis and early stages of mega-gametophytogenesis in the AS-3 line should clarify this issue.
The key question of apomixis is the development of endosperm in the seeds containing apomictic embryo. It is believed that a normal endosperm development in diploid species is possible only at a certain proportion of the maternal and paternal genomes (2:1) because of the imprinting of the maternal genes that control endospermogenesis (Spielman et al. 2003; Gutierrez-Marcos et al. 2003; Birchler 2014). As shown by PCR analysis with primers for two SSR markers, Sb1-10 and Xtxp320, the co-dominant nature of which is clearly visible in the analysis of DNA isolated from leaves and endosperm of the F1 hybrids, the endosperm in the kernels carrying maternal-type embryos lacks the paternal alleles of these markers. This fact might indicate the origin of the endosperm in such kernels without the participation of the paternal genome, i.e., autonomously. Cytological pictures of the autonomous development of the endosperm in the ESs without signs of pollen tube penetration, also indicate the possibility of autonomous endospermogenesis. It is possible that such an autonomous development of endosperm in the AS-3 line is due to a violation of the mechanism of imprinting of genes that control endospermogenesis. The existence of such a mechanism in species capable of apomixis was postulated earlier (Vinkenoog and Scott 2001). A significant clarification of the genetic nature of endosperm can be obtained in future experiments using flow cytometry, which will clarify its ploidy and, accordingly, its origin. Remarkably, our data on autonomous origin of endosperm in apomictic kernels of AS-3 line also agree with published results on the autonomous development of the endosperm in other sorghum lines with apomictic potentials (Rao et al. 1978; Wu et al. 1994; Ping et al. 2004).
Conclusion
The results of our investigations show that sorghum line AS-3 possesses functional components of apomixis: aposporous embryo sac formation, parthenogenesis and autonomous endospermogenesis. The frequency of ovules with aposporous embryo sacs and parthenogenetic pro-embryos in AS-3 line significantly increases under high average daily air temperature and drought stress before the onset of flowering. The data obtained indicate that lines with facultative apomixis can be created in functionally diploid species of cultivated crops.
Author contribution statement
LE and EB conceived and designed research. EB performed cytological investigations. AV and VP conducted PCR experiments. EB and LE analyzed data. LE wrote the manuscript. All authors read and approved the manuscript.
Data availability
The germplasms described in this paper are available upon requests.
References
Bala Ravi S (1993) Apomixis in sorghum line R473—truth or myth? A critical analysis of published work. Curr Sci 64:306–315
Barcaccia G, Albertini E (2013) Apomixis in plant reproduction: a novel perspective on an old dilemma. Plant Reprod 26(3):159–179. https://doi.org/10.1007/s00497-013-0222-y
Barke BH, Daubert M, Hörandl E (2018) Establishment of apomixis in diploid F2 hybrids and inheritance of apospory from F1 to F2 hybrids of the Ranunculus auricomus complex. Front Plant Sci 9:1111. https://doi.org/10.3389/fpls.2018.01111
Bicknell RA, Koltunow AM (2004) Understanding apomixis: recent advances and remaining conundrums. Plant Cell 16:S228–S245. https://doi.org/10.1105/tpc.017921
Birchler JA (2014) Interploidy hybridization barrier of endosperm as a dosage interaction. Front Plant Sci 5:281. https://doi.org/10.3389/fpls.2014.00281
Brown SM, Hopkins MS, Mitchell SE et al (1996) Multiple methods for the identification of polymorphic simple sequence repeats (SSRs) in sorghum [Sorghum bicolor (L.) Moench]. Theor Appl Genet 93:190–198. https://doi.org/10.1007/BF00225745
Brukhin V (2017) Molecular and genetic regulation of apomixis. Russ J Genet 53:943–964. https://doi.org/10.1134/S1022795417090046
Carman J, Jamison M, Elliott E et al. (2011) Apospory appears to accelerate onset of meiosis and sexual embryo sac formation in sorghum ovules. BMC Plant Biol 11(9) http://www.biomedcentral.com/1471-2229/11/9.
Carman JG, Mateo de Arias M, Gao L et al (2019) Apospory and diplospory in diploid Boechera (Brassicaceae) may facilitate speciation by recombination-driven apomixis-to-sex reversals. Front Plant Sci 10:724. https://doi.org/10.3389/fpls.2019.00724/full
Doyle J, Doyle J (1987) A rapid procedure for DNA purification from small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Elkonin LA (2005) Dominant male sterility in sorghum: effect of nuclear background on inheritance of tissue-culture-induced mutation. Theor Appl Genet 111(7):1377–1384. https://doi.org/10.1007/s00122-005-0069-1
Elkonin LA, Enaleeva NK, Belyaeva EV et al (1995) Partially fertile line with apospory obtained from tissue culture of male sterile plant of sorghum. Ann Bot 76(4):359–364. https://doi.org/10.1006/anbo.1995.1108
Elkonin LA, Belyaeva EV, Fadeeva IYu (2012) Expression of the apomictic potential and selection for apomixis in sorghum line AS-1a. Russ J Genet 48(1):32–40. https://doi.org/10.1134/S102279541111007X
Fiaz S, Wang X, Younas A et al (2020) Apomixis and strategies to induce apomixis to preserve hybrid vigor for multiple generations. GM Crops Food 12(1):57–70. https://doi.org/10.1080/21645698.2020.1808423
Granot G, Sikron N, Gaspan O et al (2009) Histone modifications associated with drought tolerance in the desert plant Zygophyllum dumosum Boiss. Planta 231(1):27–34. https://doi.org/10.1007/s00425-009-1026-z
Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG (2003) Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos Trans R Soc B Biol Sci 4:21–27. https://doi.org/10.1098/rstb.2003.1292
Hand ML, Koltunow AMG (2014) The genetic control of apomixis: asexual seed formation. Genetics 197(2):441–450. https://doi.org/10.1534/genetics.114.163105
Hanna WW, Schertz KF, Bashaw EC (1970) Apospory in Sorghum bicolor (L) Moench. Science 170(3955):338–339. https://doi.org/10.1126/science.170.3955.338
Hojsgaard D (2020) Apomixis technology: separating the wheat from the chaff. Genes. https://doi.org/10.3390/genes11040411
Klatt S, Hadacek F, Hodač L et al (2016) Photoperiod extension enhances sexual megaspore formation and triggers metabolic reprogramming in facultative apomictic Ranunculus auricomus. Fronts Plant Sci 7:278. https://doi.org/10.3389/fpls.2016.00278
Klatt S, Schinkel CC, Kirchheimer B et al (2018) Effects of cold treatments on fitness and mode of reproduction in the diploid and polyploid alpine plant Ranunculus kuepferi (Ranunculaceae). Ann Bot 121(7):1287–1298. https://doi.org/10.1093/aob/mcy017
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–230. https://doi.org/10.1007/s004970050144
Kong L, Dong J, Hart GE (2000) Characteristics, linkage-map positions, and allelic differentiation of Sorghum bicolor (L.) Moench DNA simple-sequence repeats (SSRs) Theor Appl Genet 101:438–448 https://doi.org/10.1007/s001220051501
Kumar S (2017) Epigenetic control of apomixis: a new perspective of an old enigma. Adv Plants Agric Res 7(1):00243. https://doi.org/10.15406/apar.2017.07.00243
Liu J, Feng L, Li J, He Z (2015) Genetic and epigenetic control of plant heat responses. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00267
Lukens LN, Zhan SH (2007) The plant genome’s methylation status and response to stress, implications for plant improvement. Curr Opin Plant Biol 10(3):317–322. https://doi.org/10.1016/j.pbi.2007.04.012
Mace ES, Jordan DR (2010) Location of major effect genes in sorghum (Sorghum bicolor (L.) Moench). Theor Appl Genet 121:1339–1356. https://doi.org/10.1007/s00122-010-1392-8
Murty UR (1993) Appraisal on the present status of research on apomixis in sorghum. Curr Sci 64(5):315–318
Ping J-A, Zhang F-Y, Cui G-M et al (2004) A study of the properties of autonomous seed setting and embryology in Sorghum apomictic line 2083. Acta Agron Sinica 30(7):714–718
Pupilli F, Barcaccia G (2012) Cloning plants by seeds: Inheritance models and candidate genes to increase fundamental knowledge for engineering apomixis in sexual crops. J Biotechnol 159(4):291–311. https://doi.org/10.1016/j.jbiotec.2011.08.028
Quarin CL (1986) Seasonal changes in the incidence of apomixis of diploid, triploid, and tetraploid plants of Paspalum cromyorrhizon. Euphytica 35(2):515–522. https://doi.org/10.1007/bf00021860
Rao NGP, Narayana LL, Reddy VR (1978) Apomixis and its utilization in grain Sorghum. I. Embryology of two apomictic parents. Caryologia 31(4):427–433. https://doi.org/10.1080/00087114.1978.10796762
Rodrigo JM, Zappacosta DC, Selva JP et al (2017) Apomixis frequency under stress conditions in weeping lovegrass (Eragrostis curvula). PLoS ONE 12:e0175852. https://doi.org/10.1371/journal.pone.0175852
Saran S, de Wet J (1976) Environmental-control of reproduction in Dichanthium intermedium. J Cytol Genet 11:22–28
Siena LA, Sartor ME, Espinoza F et al (2008) Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sex Plant Reprod 21:205–215. https://doi.org/10.1007/s00497-008-0080-1
Sokolov VA, Panikhin PA, Tarakanova TK (2011) Is there a gametophytic apomixis in diploid flowering plants? Vavilov J Genet Plant Breed 15(1):80–101
Spielman M, Vinkenoog R, Scott RJ (2003) Genetic mechanisms of apomixis. Philos Trans R Soc Lond Ser B 358:1095–1103. https://doi.org/10.1098/rstb.2003.1298
Tan M-P (2010) Analysis of DNA methylation of maize in response to osmotic and salt stress based on methylation-sensitive amplified polymorphism. Plant Physiol Biochem 48(1):21–26. https://doi.org/10.1016/j.plaphy.2009.10.005
Tavva SS, Mohan Dev, Venkateswara Rao Y et al. (2015) Apomixis in crop improvement. In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV (eds) Plant biology and biotechnology: Vol. I: plant diversity, organization, function and improvement. Springer, pp 656−669 https://doi.org/10.1007/978-81-322-2286-6
Ulum FB, Costa Castro C, Hörandl E (2020) Ploidy-dependent effects of light stress on the mode of reproduction in the Ranunculus auricomus complex (Ranunculaceae). Front Plant Sci 11:104. https://doi.org/10.3389/fpls.2020.001
Verhoeven KJ, Jansen JJ, van Dijk PJ, Biere A (2010) Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol 185(4):1108–1118. https://doi.org/10.1111/j.1469-8137.2009.03121.x
Vijverberg K, Ozias-Akins P, Schranz ME (2019) Identifying and engineering genes for parthenogenesis in plants. Front Plant Sci 10:128. https://doi.org/10.3389/fpls.2019.00128
Vinkenoog R, Scott RJ (2001) Autonomous endosperm development in flowering plants: how to overcome the imprinting problem? Sex Plant Reprod 14:189–194. https://doi.org/10.1007/s00497-001-0106-4
Wang W-S, Pan YJ, Zhao X-Q et al (2011) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62(6):1951–1960. https://doi.org/10.1093/jxb/erq391
Windham MD, Beck JB, Li F-W et al (2015) Searching for diamonds in the apomictic rough: a case study involving Boechera lignifera (Brassicaceae). Syst Bot. https://doi.org/10.1600/036364415X690076
Worthington M, Ebina M, Yamanaka N et al (2019) Translocation of a parthenogenesis gene candidate to an alternate carrier chromosome in apomictic Brachiaria humidicola. Genomics 20(1):41–58. https://doi.org/10.1186/s12864-018-5392-4
Wu S-B, Shang Y-J, Han X-M et al (1994) Embryological study on apomixis in a sorghum line SSA-1. Acta Bot Sinica 36(11):833–837
Xie E, Li Y, Tang D et al (2019) A strategy for generating rice apomixis by gene editing. J Integr Plant Biol 61(8):911–916. https://doi.org/10.1111/jipb.12785
Zaitsev GN (1984) Matematicheskaya statistika v eksperimental’noi botanike (Mathematical Statistics in Experimental Botany). Nauka, Moscow
Zhang F, Meng C, Yan X et al (1997) Studies on the apomixis property and genetic behavior of the sorghum apomixis line SSA-1. Acta Agron Sinica 23:89–94
Funding
This study was funded partly by the Ministry of Science and Higher Education of Russian Federation, research theme 0751-2019-0004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest to report regarding the present study.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belyaeva, E.V., Elkonin, L.A., Vladimirova, A.A. et al. Manifestation of apomictic potentials in the line AS-3 of Sorghum bicolor (L.) Moench. Planta 254, 37 (2021). https://doi.org/10.1007/s00425-021-03681-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03681-6