Abstract
Main conclusion
The oxidant/antioxidant balance affects the ripening time of tomato fruit.
Abstract
Ripening of tomato fruit is associated with several modifications such as loss of cell wall firmness and transformation of chloroplasts to chromoplasts. Besides a peak in H2O2, reactive oxygen species (ROS) are observed at the transition stage. However, the role of different components of oxidative stress metabolism in fruit ripening has been scarcely addressed. Two GDP-l-galactose phosphorylase (GGP) Solanum lycopersicum L. cv Micro-Tom mutants which have fruit with low ascorbic acid content (30% of wild type) were used in this work to unravel the participation of ascorbic acid and H2O2 in fruit maturation. Both GGP mutants show delayed fruit maturation with no peak of H2O2; treatment with ascorbic acid increases its own concentration and accelerates ripening only in mutants to become like wild type plants. Unexpectedly, the treatment with ascorbic acid increases H2O2 synthesis in both mutants resembling what is observed in wild type fruit. Exogenous supplementation with H2O2 decreases its own synthesis delaying fruit maturation in plants with low ascorbic acid content. The site of ROS production is localized in the chloroplasts of fruit of all genotypes as determined by confocal microscopy analysis. The results presented here demonstrate that both ascorbic acid and H2O2 actively participate in tomato fruit ripening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit ripening is a complex phenomenon that involves several physiological and metabolic changes with a strong impact on fruit nutritional and sensory qualities (Kumar et al. 2016). The distinctive plastid differentiation taking place during ripening is a highly coordinated process involving programmed molecular and hormonal components (Giovannoni et al. 2017). This is particularly evident in chloroplasts of fruit undergoing the transformation to chromoplasts, an event characterized by the synthesis and accumulation of natural carotenoids (Egea et al. 2011). These changes are accompanied by the green to red color shift in tomato fruit (Klee and Giovannoni 2011).
Increments in the levels of reactive oxygen species (ROS) have been observed and are associated with the transition from chloroplasts to chromoplasts during ripening of tomato fruit (Jiménez et al. 2002). This could involve cellular damage where lipids and proteins seem to be a target for oxidation as observed in pepper chromoplasts (Martí et al. 2009; 2011). Changes in the activities of superoxide dismutase (the enzyme catalyzing the dismutation of the superoxide radical to H2O2) and in the components of the ascorbate–glutathione cycle scavenging H2O2 have been observed during fruit ripening of different species (Jiménez et al. 2002; Martí et al. 2009; Palma et al. 2015; Huan et al. 2016). While ROS could contribute to ripening, associated processes that control oxidative stress by enhancing antioxidants might improve tomato fruit quality and shelf life (Cocaliadis et al. 2014). Genetic and physiological evidence suggests that H2O2 acts as a signaling second messenger in plant cells, mediating the acquisition of tolerance to both biotic and abiotic stresses (Noctor et al. 2018). Bouvier et al. (1998) demonstrated that ROS act as a novel class of second messenger regulating the expression of chromoplast carotenoid biosynthesis genes.
As a direct scavenger of ROS, ascorbic acid protects carotenoids and regenerates tocopherols; while as the major redox buffer in plants it is present at high concentrations in most plant cell compartments, including the apoplast (Foyer and Noctor 2011). In addition, ascorbate serves as a co-factor for many enzymes such as violaxanthin de-epoxidase, which is required for the dissipation of excess excitation energy through the non-photochemical quenching of chlorophyll. It is also an electron donor in the reaction of the ascorbate–glutathione cycle where ascorbate peroxidase (APX) reduces H2O2 (Asada 1999). Analysis of leaf transcripts in the vtc1 mutant (vitamin C-deficient Arabidopsis) demonstrates that ascorbic acid is associated with proteins implicated in DNA-binding, cell cycle control, signaling and developmental processes, carbon, cell wall and lipid metabolism, and anthocyanin synthesis (Pastori et al. 2003). Moreover, ascorbic acid is also a regulator of cell division, cell elongation and growth (De Simone et al. 2017) and a signal transducing molecule; changes in ascorbic acid concentration can modulate PR gene expression and systemic acquired resistance (Pavet et al. 2005). Up to date, no study has been carried out regarding the consequence of ascorbic acid deficiency on the fruit ripening process, such as in tomato. Nevertheless, there is a close interaction between the metabolism of ascorbic acid and respiration and photosynthesis observed in leaves (Bartoli et al. 2006, 2009), cell wall synthesis in tomato fruit (Gilbert et al. 2009), hormones such as ethylene in rosette biomass accumulation in Arabidopsis (Caviglia et al. 2018) and tomato fruit yield (Alegre et al. 2020).
This work examines the hypothesis that ROS play an active role in the transition from green to red stages during the ripening of tomato fruit. With that aim, we used two independent lines of tomato mutants deficient in GDP-l-galactose phosphorylase (GGP), known to be the major controlling enzyme of the ascorbic acid biosynthesis pathway (Bulley and Laing 2016). As these GGP-deficient mutants display a strong decline in ascorbic acid content (30% of the wild type), their use could contribute to establish the role of ascorbic acid and oxidative stress during the ripening phase, especially during the chloroplast to chromoplast transition in tomato fruit.
Materials and methods
Plant material and treatments
The experiments were performed with wild type (Solanum lycopersicum L. cv Micro-Tom) and two GDP-l-galactose phosphorylase-deficient mutant lines (Slggp1, named GGP-5261 and GGP-49C12, respectively). GGP-5261 and GGP-49C12 lines were selected from the NBRP-Tomato population (Tsukuba-Japan) and TILLING-Tomato collection (Bordeaux-France), respectively (Baldet et al. 2013). The experiments were carried out in the greenhouses of the Institute of Plant Physiology, National University of La Plata, Argentina. Plants were grown in 3-L pots filled with fertilized soil under normal greenhouse irradiance (photosynthetic photon flux density of about 700 µmol m−2 s−1 at midday) and temperatures of 25 ± 2 and 20 ± 3 °C, day and night, respectively. For the different treatments, fruits were sprayed three times a week before nightfall with 5 mL per plant of either 10 mM H2O2 or 20 mM ascorbic acid at pH 5.7 for 20 days following a mature green (MG) fruit formation on trusses. Controls were sprayed with distilled water and 0.01% Tween 20 was used as surfactant in all treatments. The effect of ascorbic acid and H2O2 treatments on “in vine” ripening was determined at MG, breaker (BR), orange (OR) and red ripe (RR) fruit collected 24 h after the last treatment. Ripening stages were selected according to the United States Department of Agriculture standard grades of fresh market tomatoes (Tu et al. 2000). The fruits were washed with distilled water after harvest. A batch of samples were frozen in liquid nitrogen and stored at −80 °C until use.
Three independent experiments were performed using at least three plants for each treatment.
Ripening time
The effect of ascorbic acid and H2O2 treatments on ripening time was determined by counting the days from MG to RR stage by analyzing the color change of 20 fruit per genotype from different plants. For each treatment, the number of days for “in vine” ripening was taken when 50% plus one fruit reached the RR stage.
Ascorbic acid concentration
The effect of ascorbic acid and H2O2 treatments on total and reduced ascorbic acid content was measured in approximately 150 mg of frozen fruit pericarp powder. The extraction of total ascorbic acid was carried out with a 3% (v/v) trifluoroacetic acid solution and centrifuged at 16,000×g for 10 min. The content was measured with a HPLC system (Shimadzu LC-10ATvp solvent delivery module) fitted with a Zorbax MicroSpher C-18 column (100 mm × 4.6 mm, Agilent Technologies) and detected at 265 nm (Shimadzu UV–Vis SPD-10Avp detector) as described in Bartoli et al. (2006). Dehydroascorbic acid (the oxidized form of ascorbate) was estimated as the difference between the contents of total and reduced forms (Bartoli et al. 2006). To calculate the redox state of the ascorbic acid, the following formula was used: % of dehydroascorbic acid = [(dehydroascorbic acid content)/(ascorbic acid content + dehydroascorbic acid content)−1] × 100.
Glutathione concentration
The same homogenate used for ascorbic acid was used for glutathione measurements. Contents of total and oxidized glutathione were determined spectrophotometrically (spectrophotometer AV-160A Shimadzu, Japan) as described in Griffith (1980) in a reaction mixture containing 100 mM potassium phosphate (pH 7.5), 5 mM EDTA, 0.3 mM NADPH, 6 mM DTNB and approximately 50 units of glutathione reductase/mL. Oxidized glutathione was measured after incubating 100 µL of each sample with 2 µL of vinylpyridine. The redox state was calculated with the same formula used for ascorbate.
Hydrogen peroxide production
The effect of ascorbic acid and H2O2 treatments on fruit H2O2 production was determined by immersing the entire fruit (fresh weight of about 3 g) immediately after harvest in 2 mL of a solution containing 10,000 U/L horseradish peroxidase, 10 µmol/L Amplex Red (N-acetyl-3,7-dihydroxyphenoxazine) and 50 mmol/L Tris–HCl buffer, pH 7 (Amplex Red assay kit, Molecular Probes). The reaction was linear for several hours. The change in the fluorescence of the solution was recorded 100 min after beginning the incubation with a CLARIOstar® Plus Multi-mode Microplate Reader (Ortenberg, Germany) in fluorescence mode at 560 nm excitation wavelengths (Zhou et al. 1997).
Subcellular ROS detection
Immediately after harvesting, fruits were washed and their pericarps cut in thin slices with a hand microtome for the subcellular location of ROS according to Ramos-Artuso et al. (2019). The segments were incubated in 50 mM Tris/HCl solution, pH 7.4, containing 0.025 mM 2′,7′-dichlorofluorescein diacetate (DCFDA, Sigma-Aldrich®) for 30 min in the darkness. Then they were rinsed with the same solution without the fluorescent probe. Tissues were observed using Confocal Spectral Laser Microscope (Leica TCS SP5, ex = 488 nm and em = 525 nm). Fruit slices incubated in the solution without DCFDA were used as a target to rule out the existence of autofluorescence.
Malondialdehyde (MDA) concentration
Two hundred milligram of frozen pericarp fruit was ground in 1 mL of 50 mM potassium phosphate buffer, pH 7.0, and 100 μL of a 0.2% (w/v) ethanol solution of butylated hydroxytoluene for the measurement of MDA. Homogenates were centrifuged at 16,000×g for 10 min. Supernatants were mixed with 1 M HClO (1:1), vigorously shaken and centrifuged again at 16,000×g. Then one volume of 0.8% (w/v) thiobarbituric acid was added to two volumes of the supernatant and incubated at 90 °C for 1 h. Samples were filtered and injected onto a HPLC system previously described and MDA detected at 590 nm. The HPLC conditions consisted in a running buffer of 70% 100 mM potassium phosphate buffer pH 7.0 and 30% methanol as described by Templar et al. (1999).
Enzyme assays
The activity of different enzymes was measured by grinding approximately 500 mg of frozen fruit powder in 1 mL of 100 mM Bicine buffer pH 7.5 containing 10% (v/v) glycerol and protease inhibitors (1 nM of phenylmethylsulfonyl fluoride; 1 µM E-64 and 1 µM of EDTA, from Sigma-Aldrich®). The homogenates were centrifuged at 16,000×g for 10 min and the supernatant was used for the enzymatic assays performed with a UV–visible recording spectrophotometer (AV-160A Shimadzu, Japan). Catalase (CAT) activity was measured according to Bartoli et al. (1999), monitoring H2O2 decomposition at 240 nm in a reaction medium consisting of 50 mM potassium phosphate buffer (pH 7.0), 15 mM H2O2, up to 100 µL of homogenate (1 mg/mL protein) and 0.1% (v/v) Triton X-100. Ascorbate peroxidase (APX) activity was measured by a modification of the method described by Amako et al. (1994). Soluble and membrane fractions were extracted with 50 mM phosphate buffer, pH 7.0, 1 mM EDTA and 1 mM ascorbic acid. The reaction mixture contained 50 mM phosphate buffer pH 7.0, 0.5 mM ascorbate, and 0.1 mM H2O2 and oxidation of ascorbic acid was followed by a decrease at 265 nm. The activity was calculated using an extinction coefficient of 2.8 m/mol cm for ascorbic acid. Peroxidase (PX) activity was measured spectrophotometrically at 430 nm according to Bartoli et al. (1995), in a reaction medium consisting of 50 mM potassium phosphate buffer (pH 6), 5 mM pyrogallol and 8 mM H2O2. The activity was calculated using an extinction coefficient of 2.47 m/mol cm. Superoxide dismutase (SOD) activity was determined due to its capacity to inhibit the reduction on nitroblue tetrazolium generated by the xanthine–xanthine oxidase reaction (Beauchamp and Fridovich 1971). The reaction mixture contained 50 mM potassium phosphate (pH 7.8), 0.83 µM xanthine and 0.015 mM EDTA. An aliquot of the crude extract including around 0.2–1 units of SOD was added. The reaction was started by the addition of an aliquot of the xanthine oxidase producing a change of 0.025 U of absorbance at 560 nm/min in each reaction. The activity was followed for 40 s at 560 nm. One unit of SOD is defined as the amount of enzyme that inhibits the reduction by 50%. NADPH oxidase (NOX) was measured by a modification of the method described by Li et al. (2004) by lucigenin-enhanced chemiluminescence in a reaction medium containing 50 mM potassium phosphate (pH 7.4), 5 μM lucigenin and 40 μM NADPH. Light emission was measured every 5 s for 40 s using the microplate reader in luminescence mode. Total soluble protein content was determined in the samples according to Bradford (1976) using bovine serum albumin (Sigma-Aldrich®) as standard.
Ripening parameters
The respiration rate was determined with an infrared CO2 sensor (Lutron Electronic Enterprise Co. L.T.D., Model GC-2028) at 23 °C. Four intact fruits (typically 15 g in total) were placed in a 1.74-L tight glass chamber and the atmosphere concentration of CO2 was recorded every minute for at least 20 min. Results were expressed in mmol of CO2 kg−1 FW h−1. To determine the fruit firmness, a texture analyzer (TA. XT. PLUS, Micro Systems TM Goldalming, Surrey, UK) was used with a 2.5-mm-diameter flat probe. The deformation tests were set with the following parameters: distance 0.5 mm, speed test 0.25 mm/s and 0.058 N of trigger force. The maximum force of the test was recorded to analyze the fruit firmness. Results were expressed in N. Total soluble solids (TSS) content, total titratable acidity (TTA), pH and TSS/TTA ratio were measured according to Steelheart et al. (2019a). Ten fruits were analyzed for each treatment.
Statistical analysis
Data represent an average of the results obtained from at least three independent experiments (spring/summer 2017–2019) with a minimum of three plants for each treatment. The results were analyzed by means of ANOVA and the means were compared by the Tukey test at a 0.05 significance level.
Results
Effects of ascorbic acid and H2O2 treatments on ripening time
The two Slggp mutants displayed a delayed ripening time since they took about three more days than the wild type to reach the RR stage from the MG stage (Table 1). When they were treated with 20 mM ascorbic acid, the fruit ripening time was equivalent to wild type, while on the contrary, the treatment with H2O2 extended the ripening time in both mutants but not in wild type (Table 1).
Ascorbic acid and glutathione concentration and redox state during fruit ripening
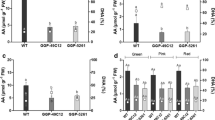
Ascorbic acid concentrations remained constant during fruit ripening in wild type and both Slggp lines, though in the mutant fruits the ascorbate content is around 30% of the wild type (Fig. 1a). When ascorbate was exogenously supplied, AA fruit concentration increased by twofold in the two mutants whereas it remained unchanged and comparable to that of the untreated plants in the wild type fruit (Fig. 1b). H2O2 treatment had no effect on ascorbic acid concentration in wild type and mutant fruits (Fig. 1c). The redox state of ascorbate decreased (i.e. the concentration of the oxidized form decreases), in wild type fruit during ripening whereas it remained constant in both mutant fruits (Fig. 1a). Ascorbic acid supplementation had no significant effect on the redox state in the wild type, while it decreased in Slggp plants at BR, OR and RR stages thus abolishing the differences between the genotypes (Fig. 1b). After H2O2 treatment, ascorbic acid redox state increased at BR and OR stages in wild type fruit while it tended to decrease in mutant fruits, especially at the BR stage (Fig. 1c). The glutathione content remained constant during fruit ripening in all the genotypes but the mutants showed increased glutathione concentration at MG and BR stages (Fig. 1d). Ascorbic acid and H2O2 treatments had no effect on glutathione content in fruit of both mutants and wild type (Fig. 1e, f). In wild type, glutathione was more highly oxidized than in mutants fruit (Fig. 1d). Ascorbic acid treatment reduced the redox state at OR and RR in mutants but not in wild type fruit (Fig. 1e). The treatment with H2O2 decreased the glutathione redox state in wild type fruits at all ripening stages and in mutants for OR and RR fruits (Fig. 1f).
Ascorbic acid content and redox state (%) in control (a), 20 mM ascorbic acid (b) and 10 mM H2O2 (c) treatments and glutathione content and redox state (%) in control (d), 20 mM ascorbic acid (e) and 10 mM H2O2 (f) treatments. Values are the mean of three-independent experiments with two replicates each. Data are expressed based on fresh weight. Asterisk denotes statistical differences between different genotypes for the same ripening stage and treatment, open circle denotes statistical differences between the same genotype and ripening stage for different treatments and closed square denotes statistical differences compared to the MG stage for the same genotype (ANOVA, p ≤ 0.05)
H2O2 production and lipid peroxidation during fruit ripening
The production of H2O2 increased progressively in wild type fruit from MG up to OR stage and then declined at RR. Such a pattern was not observed in the mutants; on the contrary, in the GGP-49C12, fruits it tended to decrease during ripening (Fig. 2a). Ascorbic acid treatment had no effect on the wild type fruits, while unexpectedly it induced a significant increase in H2O2 production (three- to fourfold compared to control) at MG and BR stages in both Slggp lines (Fig. 2b). H2O2 treatment produced a small reduction in its synthesis in BR and OR wild type fruits; in mutant plants, it induced an increase at the MG stage followed by a strong decrease at the BR stage (Fig. 2c).
H2O2 production by tomato fruit in control (a), 20 mM ascorbic acid (b) and 10 mM H2O2 (c) treatments and lipid peroxidation in control (d), 20 mM ascorbic acid (e) and 10 mM H2O2 (f) treatments. Fruit were washed with distilled water and H2O2 production measured for the following 100 min. Values are the mean of three-independent experiments with two replicates each. Asterisk denotes statistical differences between different genotypes in the same ripening stage and treatment, open circle denotes statistical differences between same genotype and ripening stage for different treatments and closed square denotes statistical differences compared to the MG stage for the same genotype (ANOVA, p ≤ 0.05)
Under control conditions, the lipid peroxidation level remained constant at all ripening stages for all genotypes; however, the concentration of MDA was always higher in wild type compared to the mutants (Fig. 2d). Ascorbic acid treatment significantly reduced the lipid peroxidation level in wild type, mainly at MG and BR stages (ten- and fivefold, respectively). The reverse effect was observed in the mutant plants for which MDA content increased gradually by twofold up to the RR stage (Fig. 2e). The lipid peroxidation pattern was similar under H2O2 treatment increasing in all genotypes during ripening (Fig. 2f).
H2O2 metabolism during fruit ripening.
To assay the capacity of H2O2 removal, the APX, PX and CAT activities were measured (Fig. 3). CAT activity showed a peak at BR and OR stages only in the wild type tomatoes. In contrast, mutants presented similar activities throughout ripening with higher values than wild type at MG and RR (Fig. 3a). While ascorbic acid and H2O2 treatments reduced CAT activity in mutants at the MG stage, H2O2 increased CAT activity in the wild type (Fig. 3b, c).
Changes in catalase (CAT), ascorbate peroxidase (APX) and peroxidase (PX) activity in control, 20 mM ascorbic acid and 10 mM H2O2 treatments. Values are the mean of three independent experiments with two replicates each. Asterisk denotes statistical differences between different genotypes for the same ripening stage and treatment, open circle denotes statistical differences between same genotype and ripening stage for different treatments and closed square denotes statistical differences compared to the MG stage for the same genotype (ANOVA, p ≤ 0.05)
APX activity increased in wild type at BR and OR stages but not in mutants in untreated fruit (Fig. 3d). This trend was not observed in ascorbic acid and H2O2 supplemented tomatoes and showed in general lower activity in all genotypes (Fig. 3e, f). In particular, H2O2 treatment decreased the APX activity but activity increased in both mutants at the RR stage.
PX activity did not change during normal ripening in wild type but both mutants showed higher activities than wild type from BR to RR stages and particularly a peak at the OR stage (Fig. 3g). Ascorbic acid application reduced PX activity avoiding this peak in one of the mutants (Fig. 3h). H2O2 treatment produced an increase in PX activity in both mutants at the BR stage (Fig. 3i).
Besides the in vivo synthesis of H2O2 analyzed with Amplex Red, ROS production was assayed by measuring the activities of NADPH oxidase and SOD.
NADPH oxidase activity showed a peak in fruit at BR stage in wild type with twofold higher activity than in the mutant tomatoes (Fig. 4a). Ascorbic acid application produced an increase in NADPH oxidase activity in wild type at MG stage, but no change was observed in Slggp plants (Fig. 4b). H2O2 treatments provoked increases in NADPH oxidase activity in both mutants at BR stage which were higher than in wild type (Fig. 4c). Similar to NADPH oxidase, SOD activity showed a peak in wild type only at BR stage (Fig. 4d). Ascorbic acid stimulated this enzyme activity in mutants reaching similar values to those observed in wild type at BR (Fig. 4e). H2O2 treatment also produced an increase in SOD activity in both mutants at BR stage but in all genotypes at OR stage (Fig. 4f).
Changes in superoxide dismutase (SOD) and NADPH oxidase (NOX) activity in control, 20 mM ascorbic acid and 10 mM H2O2 treatments. Values are the mean of three-independent experiments with two replicates each. Asterisk denotes statistical differences between different genotypes for the same ripening stage and treatment, open circle denotes statistical differences between same genotype and ripening stage for different treatments and closed square denotes statistical differences compared to the MG stage for the same genotype (ANOVA, p ≤ 0.05)
Subcellular localization of H2O2 production
Although DCFDA oxidation visualized by confocal microscopy cannot be used to quantify ROS production it is useful to determine in which subcellular compartment H2O2 is produced. Figure 5 shows DCFDA oxidation in all genotypes at BR stage. Chloroplasts were the only organelles where H2O2 was detected in cells of the wild type and both mutant fruits. Similar effects were observed in ascorbic acid and H2O2 treated fruit (Supplementary Fig. S1 and Supplementary Fig. S2, respectively).
Tomato pericarp tissues at BR stage analyzed by confocal microscopy. DCFDA probe was used for the detection of the sub-cellular H2O2 localization. Bright field images, 0,025 mM DCFDA fluorescence, chlorophyll autofluorescence, for wild type, GGP-5261 and GGP-49C12 are shown. Colocalization of the DCFDA signal and chlorophyll autofluorescence was observed in all cases
Ascorbic acid and H2O2 effects in the fruit quality parameters during ripening
Respiration decreased throughout the ripening of the fruit of all genotypes. A decrease in respiration in wild type fruit was observed between MG and BR stages but in both mutants between OR and RR (Fig. 6a–c). Firmness decreased with maturation, both mutants having higher values than wild type at RR stage in untreated fruit (Fig. 6d–f). None of the genotypes showed changes in the pH of the fruit (Suppl. Table S1) but the total soluble solids/total titratable acidity ratio that decreased with maturation remained higher in both mutants than in wild type at BR, OR and RR stages in untreated plants (Suppl. Table S1).
Respiration and firmness in control, 20 mM ascorbic acid and 10 mM H2O2 treatments. Values are the mean of three-independent experiments with two replicates each. Respiration data are expressed based on fresh weight. Asterisk denotes statistical differences between different genotypes for the same ripening stage and treatment, open circle denotes statistical differences between the same genotype and ripening stage for different treatments and closed square denotes statistical differences compared to the MG stage for the same genotype (ANOVA, p ≤ 0.05)
Discussion
Changes in the production or concentration of ROS and antioxidants are associated with fruit ripening; however, their specific roles are still barely addressed. Tomato Slggp1 mutants, with low ascorbic acid content, and exogenous chemical treatments were used in this work to unravel the functions of this antioxidant as well that of H2O2 during tomato fruit maturation.
The effect of ascorbic acid concentration on fruit ripening
Many functions are established for ascorbic acid during the growth and development of plants. It is considered as the central water-soluble antioxidant, with specific roles mainly in photosynthesis and in cell expansion and division as well as acting as a cofactor for many enzymes (Foyer et al. 2020). Among these enzymes, 1-aminocyclopropane-1-carboxylic acid oxidase, which catalyzes ethylene formation, requires ascorbic acid (Dong et al. 1992); however, we have previously observed that fruits of both Slggp1 mutants show increased ethylene production (Alegre et al. 2020). Most studies dealing with ascorbic acid deficiency effects in plants have focused on leaves and a few in fruit. Several tomato plants have been generated with down- or up-regulation of gene expression for enzymes of ascorbate synthesis pathway and also those of the recycling pathways (Amaya et al. 2014; Gilbert et al. 2009; Bulley et al. 2012; Cronje et al. 2012; Cai et al. 2015; Alhagdow et al. 2007; Zhang et al. 2013). However, there is a lack of information regarding the consequences of ascorbic acid content modification on the timing of fruit ripening. To address this question, we used two Slggp1 mutant plants producing fruits whose ascorbic acid content is around 3 times lower than the wild type. This deficiency is associated with delayed ripening from green to red ripe stages of the fruit (Table 1). The return to wild type traits observed in both mutants after ascorbate treatment demonstrates the participation of this metabolite in fruit ripening.
During ripening, firmness of tomato fruits is characterized by a progressive decline, but firmness remains higher in both Slggp1 mutants at RR stage as previously observed (Alegre et al. 2020); however, this change in firmness was not reverted by ascorbic acid nor H2O2 treatments (Fig. 6). These results suggest that the deficiency of SlGGP1 activity results in changes of some fruit ripening processes that are not associated with ROS or antioxidant concentrations. This effect may be due to an accumulation of precursors destined for cell wall formation in Slggp1 mutant tissues. The low ascorbate content in GDP-D-mannose 3,5-epimerase (SlGME)-silenced plants (for both SlGME1 and SlGME2 genes) is linked with firmness reduction of the tomato fruit (Gilbert et al. 2009). Together these results suggest that silencing either GME or GGP, in addition to reducing ascorbic acid biosynthesis flux, also impairs processes related to fruit softening, and this may depend on the precise impairment which could occur before or after the delivery of sugars for cell wall formation. This conclusion must be confirmed by future experiments.
H2O2 participation in fruit ripening
The increase in the ROS steady state at the beginning of tomato fruit ripening is well demonstrated by several studies. Jimenez et al. (2002) observed an increase in H2O2 and oxidative damage at the chloroplast-chromoplast transition stage. Similarly, Kumar et al. (2016) observed a peak of H2O2 at BR stage during tomato ripening. Brennan and Frenkel (1977) demonstrated the participation of H2O2 in the initiation and promotion of fruit ripening in pears. In our work, in vivo H2O2 production increased during the transition from MG to OR stages only in wild type fruit (Fig. 2). Barsan et al. (2012) in a proteomic study observed an increase of stress responsive proteins associated with redox pathways such as components of the ascorbate–glutathione cycle or heat-shock proteins during the green to red transition in tomato. However, ROS production may be a secondary associated event, or in contrast, may be an active signal promoting ripening associated processes (Decros et al. 2019). Unexpectedly, the ascorbic acid supplementation provoked an increase in H2O2 production. Although ascorbic acid has a central function as antioxidant, it may have also a prooxidant function. This ROS generating activity depends on its concentration and the presence of transition metals such as Fe3+ and Cu2+ (Buettner and Jurkiewicz 1996). Associated with this, increase in H2O2 production the ripening time in both Slggp1 mutants was shortened. In concordance with this observation, the treatment with H2O2 provokes decreases in its own production at BR stage delaying fruit ripening in plants of both Slggp1 lines. Taken together, these results highlight the participation of H2O2 in the tomato chloroplast-chromoplast transition.
Increased oxidation may be a key component of ROS signaling; however, the absence of a link between MDA content and redox states of antioxidants (i.e. glutathione and ascorbate) with changes in ripening time suggests that oxidative damage is not a key determinant for fruit maturation and reinforces the evidence that H2O2 itself represents a signal involved in fruit ripening (Steelheart et al. 2019b). The relative high stability of H2O2 in cells is crucial for its accumulation and function in different plant cell compartments (Foyer and Noctor 2016). The high stability allows the diffusion of H2O2 from different organelles to provide retrograde signaling by impacting gene expression (Galvez-Valdivieso and Mullineaux 2010).
Chloroplasts, mitochondria, peroxisomes and the apoplast represent the main subcellular sites of ROS production (Noctor et al. 2018). Photosynthetic and respiratory electron transport chains are considered as the main sources of ROS in chloroplast and mitochondria, respectively. In peroxisomes, H2O2 is produced by enzymes associated with photorespiratory metabolism. In addition, the associated NADPH oxidase and SOD activities may also contribute to the accumulation of H2O2 in the apoplast of plant cells. To determine the subcellular sites of H2O2 production we performed confocal microscopy in fruit tissues. This analysis demonstrates that the production of ROS in control, ascorbic acid or H2O2-treated fruit matches with chlorophyll, indicating that the main H2O2 production takes place in chloroplasts. The dismantling of thylakoid membranes observed during the chloroplast–chromoplast transition (Barsan et al. 2012) may cause an increase in ROS accumulation. In addition, the increase in the activities of NADPH oxidase and SOD in wild type fruit indicates they could participate in H2O2 production at BR stage (Fig. 4). However, the absence of DCFDA oxidation in the apoplast suggests that the H2O2 production in this compartment may be very low; furthermore, it does not mean is absent during fruit ripening. New experiments using more accurate methods must be carried out to determine the participation of ROS produced in the apoplast on fruit ripening.
The concentration of H2O2 in plant tissues depends on the balance of its production and removal. Arabidopsis plants with low ascorbic acid show altered antioxidant activity (Colville and Smirnoff 2008). Similarly, in this work, tomato Slggp1 plants present higher GSH content as well as greater catalase and peroxidase activities that may avoid the peak in H2O2 accumulation observed in non-treated wild type fruit. Ascorbic acid and H2O2 treatments increased or decreased H2O2 production, respectively, at the BR stage (Fig. 2). These modifications in ROS production cannot be explained by the change in the activity of a particular antioxidant but may result from the balance of them, such as CAT, APX or PX activities and glutathione content measured in this work.
Taken as a whole, the results presented here demonstrate that changes in the H2O2 and ascorbic acid levels actively participate in the development of fruit ripening.
Author contribution statement
CS, MLA, IG, GEGG, DJ, CB, YO and HE performed the experiments and made the statistical analysis of the data. PB, CR, CS and CGB planned different aspects of the research and wrote de manuscript. All authors read and approved the manuscript.
References
Alegre ML, Steelheart C, Baldet P, Rothan C, Just D, Okabe Y, Ezura H, Smirnoff N, Gergoff Grozeff GE, Bartoli CG (2020) Deficiency of GDP-l-galactose phosphorylase, an enzyme required for ascorbic acid synthesis, reduces tomato fruit yield. Planta 251:54
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Amako K, Chen G, Asada K (1994) Separate assay specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Amaya I, Osorio S, Martinez-Ferri E, Lima-Silva V, Doblas VG, Fernández-Muñoz R, Fernie AR, Botella MA, Valpuesta V (2014) Increased antioxidant capacity in tomato by ectopic expression of the strawberry d-galacturonate reductase gene. Biotechnol J. https://doi.org/10.1002/biot.201400279
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Baldet P, Bres C, Okabe Y, Mauxion JP, Just D, Bournonville C, Ferrand C, Mori K, Ezure H, Rothan C (2013) Investigating the role of vitamin C in tomato through TILLING identification of ascorbate-deficient tomato mutants. Plant Biotechnol 30:308–314
Barsan C, Zouine M, Maza E, Bian W, Egea I, Rossignol M, Bouyssie D, Pichereaux C, Purgatto E, Bouzayen M, Latché A, Pech J-C (2012) Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol 160:708–725
Bartoli CG, Simontachi M, Guiamet JJ, Montaldi E, Puntarulo S (1995) Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium flowers. Plant Sci 104:161–168
Bartoli CG, Simontacchi M, Tambussi E, Beltrano J, Montaldi E, Puntarulo S (1999) Drought and watering-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L leaves. J Exp Bot 50:373–381
Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Bartoli CG, Tambussi EA, Fanello DD, Foyer CH (2009) Control of ascorbic acid synthesis and accumulation by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett 583:118–122
Beauchamp CH, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bouvier F, Backhaus RA, Camara B (1998) Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem 273:30651–30659
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 71:248–254
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59:411–416
Buettner GR, Jurkiewicz BA (1996) Chemistry and biochemistry of ascorbic acid. In: Cadenas E, Paker L (eds) Handbook of antioxidants. Marcel Dekker Inc, New York, pp 91–115
Bulley S, Laing W (2016) The regulation of ascorbate biosynthesis. Curr Opin Plant Biol 33:15–22
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Andrew A, Laing WA (2012) Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J 10:390–397
Cai X, Zhang C, Ye J, Hu T, Ye Z, Li H, Zhang Y (2015) Ectopic expression of FaGalUR leads to ascorbate accumulationwith enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Growth Regul 76:187–197
Caviglia M, Mazorra Morales LM, Concellón A, Gergoff Grozeff GE, Wilson M, Foyer CH, Bartoli CG (2018) Ethylene signaling triggered by low concentrations of ascorbic acid regulates biomass accumulation in Arabidopsis thaliana. Free Radical Bio Med 122:130–136
Cocaliadis MF, Fernández-Muñoz R, Pons C, Orzaez D, Granell A (2014) Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J Exp Bot 65:4589–4598
Colville L, Smirnoff N (2008) Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. J Exp Bot 59:3857–3868
Cronje C, George GM, Fernie AR, Bekker J, Kossmann J, Bauer R (2012) Manipulation of l-ascorbic acid biosynthesis pathways in Solanum lycopersicum: elevated GDP-mannose pyrophosphorylase activity enhances l-ascorbate levels in red fruit. Planta 235:553–564
Decros G, Baldet P, Beauvoit B, Stevens R, Flandin A, Colombie S, Gibon Y, Pétriacq P (2019) Get the balance right: ROS homeostasis and redox signalling in fruit. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01091
De Simone A, Hubbard R, Viñegra de la Torre N, Velappan Y, Wilson M, Considine MJ, Soppe WJJ, Foyer CH (2017) Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana. Antioxid Redox Signaling 27:1505–1519
Dong JG, Fernández-Maculet JC, Yang SF (1992) Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA 89:9789–9793
Egea I, Bian W, Barsan C, Jauneau A, Pech JC, Latche A, Li Z, Chervin C (2011) Chloroplast to chromoplast transition in tomato fruit: spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann Bot 108:291–297
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Foyer CH, Noctor G (2016) Stress-triggered redox signaling: what´s in pROSpect? Plant Cell Environ 39:951–964
Foyer CH, Kyndt T, Hancock RD (2020) Vitamin C in plants: novel concepts, new perspectives and outstanding issues. Antioxid Redox Signaling. https://doi.org/10.1089/ars.2019.7819
Galvez-Valdivieso G, Mullineaux PM (2010) The role of reactive oxygen species in signaling from chloroplasts to the nucleus. Physiol Plant 138:430–439
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, Petit J, Stevens R, Causse M, Fernie AR, Lahaye M, Rothan C, Baldet P (2009) GDP-d-mannose epimerase (GME) plays a key role at the intersection of ascorbate and non cellulosic cell wall biosynthesis in tomato. Plant J 60:499–508
Giovannoni J, Nguyen C, Ampofo B, Zhong S, Fei Z (2017) The epigenome and transcriptional dynamics of fruit ripening. Annu Rev Plant Biol 68:61–84
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Huan C, Jiang L, An X, Yu M, Xu Y, Ma R, Yu Z (2016) Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol Biochem 104:294–303
Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P (2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214:751–758
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Kumar V, Irfan M, Ghosh S, Chakraborty N, Chakraborty S, Datta A (2016) Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma 253:581–594
Li JM, Wheatcroft S, Fan LM, Kearney T, Shah AM (2004) Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109:1307–1313
Martí MC, Camejo D, Olmos E, Sandalio LM, Fernández-García N, Jiménez A, Sevilla F (2009) Characterisation and changes in the antioxidant system of chloroplasts and chromoplasts isolated from green and mature pepper fruits. Plant Biol 11:613–624
Martí MC, Camejo D, Vallejo F, Romojaro F, Bacrizo S, Palma JM, Sevilla F, Jiménez A (2011) Influence of fruit ripening stage and harvest period on the antioxidant content of sweet pepper cultivars. Plant Foods Hum Nutr 66:416–423
Noctor G, Reichheld J-P, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol 80:3–12
Palma JM, Sevilla F, Jiménez A, del Río LA, Corpas FJ, Álvarez de Morales P, Camejo DM (2015) Physiology of pepper fruit and the metabolism of antioxidants: chloroplasts, mitochondria and peroxisomes. Ann Bot 116:627–636
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in arabidopsis. Plant Physiol 139:1291–1303
Ramos-Artuso F, Galatro A, Lima A, Batthyány C, Simontacchia M (2019) Early events following phosphorus restriction involve changes in proteome and affects nitric oxide metabolism in soybean leaves. Environ Exp Bot 161:203–217
Steelheart C, Alegre M, Vera Bahima J, Senn ME, Simontacchi M, Bartoli CG, Gergoff Grozeff GE (2019a) Nitric oxide improves the effect of 1-methylcyclopropene extending the tomato (Lycopersicum esculentum L.) fruit postharvest life. Sci Hortic 255:193–201
Steelheart C, Galatro A, Bartoli CG, Gergoff Grozeff GE (2019b) Nitric oxide and hydrogen peroxide: signals in fruit ripening. In: Gupta DK, Palma JM, Corpas FJ (eds) Nitric oxide and hydrogen peroxide: signalling in higher plants. Springer Nature Switzerland AG, Cham, pp 175–200
Templar J, Kon SP, Milligan TP, Newman DJ, Raftery MJ (1999) Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated HPLC method. Nephrol Dial Transplant 14:946–951
Tu K, Jancsók P, Nicolaï B, De Baerdemaeker J (2000) Use of laser-scattering imaging to study tomato-fruit quality in relation to acoustic and compression measurements. Int J Food Sci Technol 35:503–510
Zhang C, Ouyang B, Yang C, Zhang X, Liu H, ZhangY ZJ, Li H, Ye Z (2013) Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-d-Mannose pyrophosphorylase gene in tomato plant. PLoS ONE 8(4):e61987
Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253:162–168
Acknowledgements
We are really grateful to Dr Rebecca Stevens for the English edition of the manuscript and to Dr Diana Lauff for her technical assistance. GGG and CGB are career members from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). CS, MA and IG are fellows at CONICET. The experiments were supported by grants PICT 2015-0103 (Agencia Nacional de Promoción Científica y Técnica, Argentina) and A322 and A337 (Universidad Nacional de La Plata, Argentina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mee-Len Chye.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steelheart, C., Alegre, M.L., Baldet, P. et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta 252, 36 (2020). https://doi.org/10.1007/s00425-020-03440-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03440-z