Abstract
Main conclusion
We observed a close correlation between the inhibition of photosystem II and the oxidation of polyphenols during an acute oxidative stress in sunflower leaf discs.
Abstract
To assess the physiological significance of polyphenols as antioxidants in planta, we compared the kinetics of polyphenols oxidation with the inhibition of the photosynthetic apparatus in sunflower leaf discs exposed to an acute photooxidative stress. Illumination of leaf discs in the presence of methyl viologen induced a rapid and large non-photochemical quenching of chlorophyll-a fluorescence, which was reversed after 4 h of treatment as indicated by the ≈ 30% increases of the steady-state (Fs) and maximal (Fm′) levels of chlorophyll-a fluorescence relative to the first hour of treatment. This event coincided with the accelerated decreases of the maximum (Fv/Fm) and effective (∆F/Fm′) quantum yields of photosystem II, and also with the beginning of polyphenols oxidation, estimated by the UV absorbance of methanolic leaf extracts, and supported by the Folin–Ciocalteu method and cyclic voltammetry. The decreases of Fv/Fm and the concentrations of reducing polyphenols were highly correlated (R2 = 0.877) during the experiment. Coherent with the decrease of UV absorbance of methanolic extracts, polyphenol oxidation resulted in a marked decrease of UV absorbance of leaf epidermis. Also, polymerization of oxidized polyphenols caused the accumulation of brown pigments in the MV-treated leaf discs, decreasing leaf reflectance, especially at 550 and 740 nm. Fluorescence intensities were also decreased during the MV treatment. Interestingly, the emission fluorescence ratio F740/F684 (excitation at 550 nm) decreased similarly to Fv/Fm (R2 = 0.981) due to the brown pigments. Moreover, the excitation fluorescence ratio F484/F680 (emission at 740 nm) was linearly correlated (R2 = 0.957) to ∆F/Fm′, indicating a decrease of efficiency of energy transfer between the antenna pigments to the photosystem II reaction center during the oxidative stress. These results support the view that polyphenols can be effective antioxidants protecting the plants against reactive oxygen species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyphenols (PPs) are specialized metabolites in plants that are essential for their successful interactions with the environment. By their structural diversity and different localizations, PPs fulfil multiple functions such as deterrent against herbivores, barriers against pathogens, aid in pollen and seed dispersal, and molecule signals for symbiotic relations with microorganisms (Treutter 2005; Ghasemzadeh and Ghasemzadeh 2011; Mandal et al. 2010). PPs also contribute to plant tolerance against a wide range of abiotic stresses, like UV, high-light intensities, drought, salinity, heavy metals, air pollution, mechanical wounding, and nitrogen limitation (Dixon and Paiva 1995; Nakabayashi and Saito 2015; Treutter 2005). Besides their specific targets, all these stresses have in common to accelerate the production of reactive oxygen species (ROS) leading to oxidative stress.
Widely accepted but not fully demonstrated, the role of PPs as antioxidant in planta is inferred from several observations. PPs show antioxidant capacity in vitro, and their concentrations increase in plants exposed to the above-mentioned stresses (Dixon and Paiva 1995). Among the stress-induced PPs, there is a preferential accumulation of strong antioxidants notably quercetin, with its o-dihydroxy B-ring compared to the monohydroxy B-ring kaempferol (Agati et al. 2012; Edreva 2005). High flavonoid content was related to higher viability in senescent Vitis vinifera suspension cell cultures by quenching ROS, hence limiting the signalling cascade leading to programmed cell death (Bertolini et al. 2016). Moreover, the antioxidative role in planta of PPs is supported by Arabidopsis mutants that overaccumulate flavonoids with strong antioxidant capacity. Compared to the wild-type Col-0, these mutants showed higher tolerance to drought and methyl viologen-induced oxidative stress (Nakabayashi et al. 2014). Similar observations can be made with salicylic acid. This plant growth regulator improves plant tolerance to a wide range of abiotic stresses (Hayat et al. 2010), and it was shown to enhance PPs concentrations in plants (Kováčik et al. 2009), plant cell cultures (Dong et al. 2010), and algal cultures (Kováčik et al. 2010).
Under intense and transient stress conditions and under high-light intensities, excess of H2O2 generated in the chloroplasts that is not scavenged by the ascorbate–glutathione cycle diffuses to the vacuoles. There, class-III peroxidases can catalyse H2O2 reduction to H2O using PPs, mostly flavonoids and hydroxycinnamates, as electron donors. The resulting phenoxyl radicals are normally re-reduced by ascorbate, the latter being regenerated in the cytosol by glutathione and NADPH. This peroxidase/polyphenol/ascorbate system is efficient as long as the cytosolic ascorbate pool is not depleted: at this point, the phenoxy radicals accumulate and react with other radicals, amino acids or proteins, to polymerize into complex brown pigments (Pérez et al. 2002; Pourcel et al. 2007; Takahama 2004; Yamasaki et al. 1997).
One of the missing evidences required to confirm the physiological significance of PPs as antioxidants is the presence of a spatio-temporal correlation between the extent of oxidative stress and the oxidation of PPs (Agati et al. 2012; Hernández et al. 2009). Oxidation of PPs in planta and the accumulation of brown pigments are common during plant development, causing for instances the browning of seed coats, onion scales, heartwood (Pourcel et al. 2007; Takahama 2004), and aging tobacco leaves (Takahama 2004). However, during oxidative stress, there are only few reports of PPs oxidation in leaves (Agati et al. 2007; Hernández et al. 2006), in contrast with the numerous studies mentioned above showing their accumulation during plant acclimation to chronic environmental stress. Simultaneous decreases of the levels of phenolics and chlorophylls were observed during foliar senescence in Kalanchoe pinnata (Jaiswal et al. 2012). Meir et al. (1995) made similar observations during senescence of leaf discs from various edible herbs, i.e. similar decreases of chlorophyll contents and “aqueous reducing compounds”, which phenolic acids and flavonoids were considered as the main components. In another study, Arabidopsis leaves wild type and mutants with different anthocyanin contents were illuminated in the presence of methyl viologen (Shao et al. 2008). Photosynthetic reactions estimated from chlorophyll fluorescence parameters decreased in a triphasic pattern, to be nearly abolished after 3–4.5 h of illumination depending on the anthocyanin contents. In contrast, total phenolics and flavonoids, estimated from UV absorbance of methanolic extracts, decreased more or less linearly by 10–25% only after 12 h of treatments. In that experiment, vacuum infiltration of methyl viologen into the leaves may have lowered O2 concentrations, and the brown pigments resulting from the oxidation products of leaf phenolics were not monitored.
In this context, there is a need to further characterize the relation between the oxidation of PPs in leaves with the physiological damages occurring during an acute oxidative stress. To this end, we illuminated sunflower leaf discs in the presence of methyl viologen and correlated the inhibition of photosynthetic electron transport with the oxidation of PPs estimated from leaf methanolic extracts by three independent methods, notably cyclic voltammetry. This method is frequently used as a direct indicator of reducing capacity of PPs in food and beverage research, especially on wine and teas (Kilmartin and Hsu 2003; Kilmartin et al. 2001), but has not been used previously to characterise PPs in leaf extracts in relation to photosynthetic activity and photooxidative stress. We also monitored by reflectance and fluorescence spectroscopy the impacts of accumulating brown pigments on leaf optical properties. The results show a close temporal correlation between the oxidation of PPs and the loss of integrity of the photosynthetic electron chain.
Materials and methods
Plant material
Dwarf sunflower plants (Helianthus annuus L., var. Sunspot) were grown during 8 weeks in a growth chamber. The photoperiod was 16 h/8 h and the day-light intensity (PFD) was 150 µmol photons m−2 s−1. The day/night temperatures were 25 °C/17 °C. Plants received tap water as required and appropriate amount of NPK 20–20–20 fertilizer once a week.
Just before the experiments, discs (2.2 cm diam.) were cut with a cork borer from non-shaded and horizontally oriented areas of the 4th–6th leaves, and avoiding the midribs. Discs were kept between moist paper towels until the beginning of the treatments. PPs’ contents in leaf discs were estimated just before the experiments with a Dualex Scientific (Force-A, Orsay, France) leaf-clip sensor. To minimize the variations due to leaf development, only leaf discs with Dualex Flv values between 0.6 and 0.8 were used for the experiments.
Treatments
Leaf discs were floated with their abaxial side in contact with distilled water containing 0 or 0.4 mM methyl viologen (MV), and maintained in the growth chamber under the same PFD and temperature as the growth conditions. During the experiment, six discs were taken after 0, 3, and 7 h for the control, and after 1, 2, 4, 6, and 8 h for the MV treatment. At each hour, the following measurements described below were sequentially made within 30–40 min: modulated chlorophyll-a fluorescence, Multiplex 330 fluorescence, reflectance spectra, and fluorescence spectra. Then, the discs were stored at − 18 °C for less than 2 weeks for pigments and PPs analysis. The whole experiment was repeated twice.
Modulated chlorophyll-a fluorescence
Pulse amplitude modulated chlorophyll-a fluorescence was measured from leaf discs with a MINI-PAM fluorometer (Walz, Germany). For each disc, the stationary fluorescence level Fs was measured under the same ambient light intensity (150 µmol photons m−2 s−1), and then, the maximum Fm′ was induced by a 1 s saturating flash. From the Fs and Fm′, the effective quantum yield of photochemistry of Photosystem II (PSII) ∆F/Fm′ was calculated, where ∆F = Fm′ − Fs (Klughammer and Schreiber 2008).
To not interfere with the time course of the MV-induced photooxidative stress, a separate experiment was done to measure the changes of the maximum quantum yield of photochemistry of PSII during the experiment. At each hour during the treatments, leaf discs were placed in darkness for 40 min before the minimum Fo level was measured, and then, the maximum Fm was induced by a 1 s saturating flash. The maximum quantum yield of PSII photochemistry Fv/Fm was calculated, where Fv = Fm − Fo.
Reflectance
To follow the accumulation of brown pigments resulting from the oxidation of PPs, reflectance spectra from 360 to 740 nm (10 nm intervals) were measured on the adaxial side of leaf discs with a portable spectrophotometer CM-2600d (Konica Minolta, Roissy, France).
In a separate experiment, reflectance spectra were measured as described above on depigmented leaf discs, which had been exposed 8 h with 0 or 0.4 mM MV, and then incubated in methanol at 4 °C for 24 h.
Fluorescence spectroscopy
The consequences of PPs’ oxidation on leaf optical properties were also monitored during the experiment by recording the excitation and emission chlorophyll-a fluorescence spectra with a spectrofluorometer Cary Eclipse (Varian, Les Ulis, France). The discs were placed in a leaf holder positioned at 60° from the excitation source and 30° from the detector side. Fluorescence emission and excitation spectra were recorded with a bandwidth of 10 nm on both the excitation and the emission side, and a scan rate of 120 nm min−1. Fluorescence emission was recorded from 650 to 800 nm, excited at 550 nm, with the additional short-pass and long-pass protection filters, SP600 (Edmund Scientific) and RG630 (Schott), respectively. Fluorescence-excitation spectra from 390 to 680 nm, emitted at 740 nm, were recorded with the additional filters SP700 (Schott) and RG715 (Schott), on the excitation and emission side, respectively. Fluorescence-excitation spectra from 300 to 390 nm, emitted at 740 nm, were recorded with the DUG11 (Schott) and RG715 (Schott) filters, on the excitation and emission side, respectively. All spectra were corrected for the response of detectors and transmission of monochromators and filters as described in Louis et al. (2006). Throughout the manuscript, fluorescence excitation wavelengths are indicated by subscripts and fluorescence emission wavelengths, by superscripts.
The Multiplex 330 (FORCE-A, Orsay, France) proximal sensor was also used to measure instantaneously fluorescence from the whole surface of leaf discs. This photodiode- and filter-based sensor records chlorophyll-a fluorescence like the MINI-PAM, but simultaneously at two emission bands, centered at 684 and at 740 nm, with LED-excitation centered at 330, 450, and 630 nm.
Measurements of pigment and polyphenol concentrations
Leaf discs were cut into small pieces with a scalpel and incubated overnight in pure methanol at 4 °C in the dark. The extracts were filtered and the absorbance spectra from 220 to 800 nm were recorded with an Agilent/HP 8453 spectrophotometer (Hewlett Packard, Les Ulis, France). Chlorophyll-a + b concentrations were calculated according to Lichtenthaler (1987).
The levels of soluble PPs were first estimated by the UV absorbance of the methanolic extracts after subtraction of chlorophylls contribution, calculated from spectra of pure chlorophyll-a and chlorophyll-b as described by Ounis et al. (2001). PPs concentrations were also estimated by the Folin–Ciocalteu method (Prior et al. 2005). In brief, 1 mL of the methanolic extract was added to 2.25 mL of diluted Folin–Ciocalteu reagent (0.2 N). After 6 min, 1.75 mL of saturated Na2CO3 was added, and the mixture was incubated for 2 h and then centrifuged. The absorbance of the supernatants was read at 760 nm. A calibration curve (R2 = 0.998) was made using the same procedure with nine concentrations of gallic acid (from 0.25 to 50 mg L−1).
Cyclic voltammetry
Cyclic voltammetry was used to clearly illustrate the oxidation of leaf PPs. For these measurements, higher PPs concentrations in leaf methanolic extracts were required. Therefore, a distinct experiment was done where discs were treated identically as described above in presence or absence of MV for 8 h. For both treatments, three groups of eight leaf discs were made, and each group was incubated in 40 mL of methanol at 65 °C for 20 min, followed by a second incubation in 15 min in 20 mL methanol. For each group, the two extracts were pooled and concentrated in a roto-evaporator to a final volume of 5 mL.
Methanolic extracts (1 mL) were introduced in a three-electrode cell containing 1 mL of 0.2 M potassium phosphate buffer (final pH of 7.4). A glassy carbon disc (BAS MF-2012, 3 mm diameter), an Ag/AgCl electrode, and a glassy carbon plate were used as working, reference, and counter electrodes, respectively. Argon was bubbled for several minutes in the solution to remove dissolved air. Cyclic voltammograms were recorded using a Voltalab PGZ 100 potentiostat, at a scanning rate of 50 mV/s, over the potential window from − 100 to 500 mV vs. Ag/AgCl. All electrode potentials were read against the reference electrode of Ag/AgCl. Before each measurement, the glassy carbon working electrode was gently polished with an aluminum powder solution on an abrasive surface for 2 min and then sonicated also for 2 min. The cell and the electrodes were washed with deionized water (milliQ grade, Millipore) between each experiment. Reducing equivalent contents expressed in microcoulomb (µC) were determined by first calculating the area under the curve “current vs. voltage”, and then dividing this surface area (in µA mV or µC s−1 mV) by the constant scanning rate (here 50 mV s−1) (Kilmartin and Hsu 2003).
Statistical analysis
For each dependent variable measured, descriptive statistics (means and standard errors of the mean) and analyses of variance were calculated with the SYSTAT software (SPSS, Chicago, IL, USA). The factors (independent variables) were “hours” (i.e., with or without MV) and “experiments”. For all dependent variables tested, highly significant effects (p < 0.01) of MV (hours) were observed except for chlorophylls and carotenoids contents (p > 0.05). Also, the interactions “hours × experiments” were non-significant for all dependent variables. This means that the same effects of MV on the different variables were observed in the two experiments.
Two measurements made with the Multiplex were considered as outliers by the SYSTAT software and discarded, since their difference with the mean was more than three times larger than the standard deviation.
Results
Characterization of methyl viologen effects on photosynthetic electron transport and photosynthetic pigments
Exposure of sunflower leaf discs to MV 0.4 mM under a white light of 150 µmol m−2 s−1 for 8 h inhibited the photosynthetic electron transport in three distinct phases. Within the first hour of treatment, ∆F/Fm′ decreased from about 0.7–0.3 and then remained stable for 2 h, before it further decreased close to 0 between 4 and 8 h of treatment (Fig. 1a). In contrast, the decrease of Fv/Fm due to the presence of MV was biphasic: it remained little affected during the first 2 h, and decreased afterward down to 0.2 (Fig. 1b).
Time course of the photooxidative damage to the photosynthetic electron transport in sunflower leaf discs monitored by the inhibition of the effective ∆F/Fm′ (a) and maximum (Fv/Fm) (b) quantum yields of photosystem II, and the changes of the maximum Fm′ (c) and the stationary Fs (d) fluorescence levels. Leaf discs were exposed to 150 µmol photons m−2 s−1 in absence (open circles) or in presence of 0.4 mM methyl viologen (filled circles)
The decrease of ∆F/Fm′ in the first half of the treatment could be related to the formation of a large non-photochemical quenching (NPQ), as Fm′ was strongly quenched (Fig. 1c), while Fs remained similar to the control values (Fig. 1d). The Fs and Fm′ values transiently increased at 4 h, indicating the collapse of the NPQ and the subsequent loss of integrity of the photosynthetic electron transport system.
Figure 2 shows that despite its strong effect on photochemical reactions, MV did not affect significantly the concentrations of chlorophyll-a + b and of carotenoids throughout the experiment.
Polyphenol oxidation during MV-induced oxidative stress estimated from leaf extracts
To determine if the losses of PSII photochemical efficiency were correlated to the oxidation of PPs in MV-treated leaf discs, we analysed the spectra of methanolic extracts from which chlorophylls a and b absorbance was subtracted as in Ounis et al. (2001) (Fig. 3a). The control spectrum shows the typical absorbance of carotenoids in the blue region, and the UV absorbance peak near 328 nm is characteristic of hydroxycinnamates (Stelzner et al. 2019). The MV treatment caused a decrease of the UV absorbance accompanied by a large increase in the violet region. These opposite changes are typical of phenolic oxidation (Ramešová et al. 2015; Sokolová et al. 2012). Also, the increase of absorbance at 420 nm is a common indicator of phenolic oxidation causing the browning of plant tissues and processed plant products (Takahama et al. 1999; Tomás-Barberán et al. 1997).
Polyphenol oxidation estimated from leaf methanolic extracts. a Chlorophyll-less absorbance spectra of extracts from leaf treated or not with methyl viologen for different times. b Absorbance values at 328 nm of the methanolic extracts from control (open circles) and MV-treated (filled circles) leaf discs. c Concentrations of reducing polyphenols in the methanolic extracts by the Folin–Ciocalteu method, expressed in gallic acid equivalents
The absorbance at 328 nm decreased in a biphasic manner during the MV treatment: A328 decreased slightly in the first 2 h, before a more pronounced decline between 4 and 8 h. After 8 h of MV treatment, A328 accounted for 56% of the control values (Fig. 3b). The increase of absorbance near 420–440 nm is prominent, but the spectra are noisy due to the large absorbance of chlorophylls in that region, close to the sensitivity limit of the spectrophotometer (see original spectra in Fig S1).
The oxidation of PPs monitored by the decrease of A328 was supported by the contents of reducing PPs (gallic acid equivalents, GAE) estimated with the Folin–Ciocalteu method: we observed a small decrease of GAE in the first 2 h, followed by a larger decline after 4 h (Fig. 3c). At the end of the treatment, the GAE in MV-treated discs represented 67% of the control values. There is a close correlation (R2 = 0.934) between the estimations of PPs contents by the Folin–Ciocalteu method and the absorbance at 328 nm.
The Folin–Ciocalteu method estimates the overall capacity of PPs (and other antioxidants) to reduce the molybdo-tungsto-phosphoric heteropolyanion (Prior et al. 2005). Therefore, we used cyclic voltammetry to provide a more detailed illustration of the loss of the reducing capacity of PPs in extracts from MV-treated leaf discs. Cyclic voltammograms shown in Fig. 4 are plots of the cell current as a function of the potential (in mV vs. Ag/AgCl) of the working electrode, measured on the methanolic extracts from control and MV-treated leaf discs. Peak currents (y-axis) are proportional to the concentration of reducing-active components in the extracts. Peak potentials (along the x-axis) indicate the potential at which the species donate their electrons. The curve of MV-treated leaf discs is markedly lower than the control curve above 100 mV. The antioxidant capacity of the samples can be estimated by the area under the curve, from which we calculated the number of charges passed during the oxidative scanning from − 100 to 500 mV. A value of 1285 µC was obtained for the control, and a value of 778 µC for leaf discs treated with MV during 8 h, i.e., 60% of the control value.
Polyphenol oxidation monitored in situ by alterations of leaf discs optical properties
Reflectance
The treatment of leaf discs with MV caused their browning that became visually apparent in the second half of the experiment. To characterise these changes, reflectance spectra of leaf discs were measured throughout the experiment. The most noticeable effect of the MV treatment is the flattening of the spectra, with decreased reflectance that is more evident in the far-red and green regions, i.e., where chlorophylls absorb less (Fig. 5a). The red edge, i.e., the position of the sharp reflectance increase, remained unchanged. Reflectance at 740 nm was little affected until the fourth hour of treatments, and then, it decreased to 52% of the control value (Fig. 5b). In the blue and red regions of the spectra, reflectance decreased between 0 and 4 h, but then increased slightly.
Reflectance spectra of leaf discs depigmented after their incubation in methanol were also measured (Fig. 6). Much higher reflectance values are seen in control than MV-treated depigmented leaf discs, showing that that the browning of MV-treated leaf discs is caused by the accumulation of insoluble brown pigments following the polymerization of oxidized PPs (Takahama 2004; Yamasaki et al. 1997).
Fluorescence emission spectra
Considering the loss of non-photochemical quenching, the decrease of UV-absorbing PPs, and the accumulation of brown pigments during MV treatment, fluorescence spectroscopy can be a powerful tool to detect oxidative stress in leaves. Therefore, we first measured emission spectra of chlorophyll-a fluorescence excited at 550 nm (Fig. 7a). In the first 2 h, the intensity of the fluorescence peak at 740 nm was slightly decreased by the MV treatment (Fig. 7b). Then, the F740 intensity increased at 4 h, reminiscent of the enhanced Fs level observed at that time (Fig. 1c), before it decreased by more than 50% after 6 and 8 h.
a Emission spectra of chlorophyll fluorescence excited at 550 nm measured from leaf discs treated or not with methyl viologen for different times. b Fluorescence intensities emitted at 740 nm and c ratio of fluorescence intensities emitted at 740 and 684 nm from control (open circles) and methyl viologen-treated (filled circles) leaf discs during the experiment
Not only the intensity but also the shape of the spectra was affected by the MV treatment, as seen by the fluorescence ratio F740/F684 (Fig. 7c). The MV effect on the F740/F684 ratio was biphasic: it remained unaffected during the first 2 h, and then decreased from 4.30 to a value of 2.28, 53% relative to the control. Interestingly, the changes of Fv/Fm (Fig. 1c) and F740/F684 during the MV treatment were highly correlated (R2 = 0.981).
Fluorescence-excitation spectra
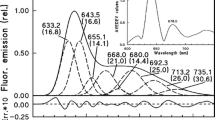
Fluorescence-excitation spectra can provide useful information about light absorption and energy transfer by the antenna pigments, processes that could be affected by the PPs oxidation, and the losses of PSII photochemical efficiency during the MV-oxidative stress. The excitation spectrum of control leaf discs shows two pronounced peaks, one at 420 nm that can be assigned to excitation of chlorophyll-a, and the other at 484 nm, associated with the energy transfer from the accessory pigments chlorophyll-b and xanthophylls to chlorophyll-a (Zeng et al. 2016) (Fig. 8a). There are also minor Qx bands visible between 500 and 600 nm. In spectra from leaf discs’ exposed MV, the averaged fluorescence intensity F740 excited over 390 and 680 nm decreased similarly to Fs and F740: a small decrease in the first 2 h, and then an increase at the fourth hour before a pronounced decrease beyond that time. More interestingly, the shape of the F740 excitation spectra was significantly altered: larger decreases occurred in the blue, especially the peaks at 420 and 484 nm, compared to the red region of the spectrum. The Qx bands between 500 and 600 nm were strongly suppressed in MV-treated leaf discs.
a Excitation spectra of chlorophyll fluorescence emitted at 740 nm measured from leaf discs treated or not with methyl viologen for different times. b Ratio of fluorescence intensities excited at 484 and 680 nm measured from control (open circles) and MV-treated (filled circles) leaf discs during the experiment. c Correlation between the F484/F680 excitation ratio and the quantum yield of photosystem II ∆F/Fm′. d Intensities of fluorescence at 740 nm excited at 350 nm during the 8-h experiment in control (open circles) and methyl viologen-treated (filled circles) leaf discs
Since the fluorescence peak at 484 nm can be related to the energy transfer between the antenna pigments (Zeng et al. 2016), we calculated the ratio of the fluorescence intensities at 484 nm over 680 nm as a potential indicator of the efficiency of energy transfer from the antenna pigments to the reaction center of PSII. The results show that during the MV treatment, the F484/F680 decreased strongly during the first hour, less between 1 and 4 h, and then more rapidly after the fourth hour (Fig. 8b). Interestingly, the changes of F484/F680 are closely correlated (R2 = 0.957) to the values of ∆F/Fm′ measured after different times of treatment with MV (Fig. 8c).
Compared to the chlorophyll-a fluorescence (F740) excited by visible light, the intensity of UV-excited chlorophyll-a fluorescence was relatively weak (Fig. 8a). However, in a marked contrast, its intensity increased during the MV treatment (Fig. 8d) presumably due to a loss of epidermal PPs.
Multiplex 330 fluorescence measurements
To better document the increase of UV-induced chlorophyll-a fluorescence during the MV-treatment, far-red fluorescence (FRF) intensities were measured from control and MV-treated leaf discs with a portable fluorometer Multiplex 330. This sensor has an additional excitation wavelength 330 nm close to the absorption maximum of hydroxycinnamates. The intensities of the FRF induced by blue excitation (Fig. 9a) followed the same patterns during the experiment as for the Fs (Fig. 1c) and the F740 excited at 550 nm (Fig. 7b) measurements made with different fluorometers. Also, the multiplex measurements of FRF induced by UV (330 nm) markedly increased during the MV treatment (Fig. 9b), strongly supporting the measurements made with the Cary spectrofluorometer (Fig. 8d).
Blue-excited (a) and UV-excited (b) far-red fluorescence measured with a Multiplex portable fluorometer from control (open circles) and methyl viologen-treated (filled circles) leaf discs during the 8-h experiment. c LogFER (log(FRFBLUE/FRFUV)) as an estimation of the epidermal UV absorbance of control (open circles) and methyl viologen-treated (filled circles) leaf discs during the experiment
The logarithm of the ratio of the FRF intensities induced by blue and UV excitations (log(FRFBLUE/FRFUV330) or LogFER) is a good estimate of the epidermal UV absorbance due to the presence of soluble PPs (Cerovic et al. 2002). The LogFER values remained stable during the first 4 h, and then declined to 53% of the control value after 8 h of MV treatment (Fig. 9c).
Discussion
The role of PPs as antioxidants in planta is inferred from several circumstantial evidences, but direct observations of their oxidation co-occurring with physiological damages during oxidative stress are still lacking. The difficulty to demonstrate such correlation arises in part from the variations of reducing PPs concentrations during normal plant development (Louis et al. 2009, 2012) and during plant acclimation to chronic environmental stress (Dixon and Paiva 1995; Treutter 2005). Therefore, we induced an acute oxidative stress in sunflower leaf discs that were exposed to MV (0.4 mM) under their growth light intensity for up to 8 h. Near the fourth hour of the MV-treatment, there was a collapse of the non-photochemical quenching (NPQ) and an accelerated decline of the photochemical efficiencies of PSII. This loss of integrity of the photosynthetic reactions concurred with the beginning of PPs oxidation monitored by different methods in leaf methanolic extracts, and supported in situ by changes in leaf optical properties.
Under our experimental conditions, the effects of MV on photosynthetic electron transport in sunflower leaf discs can be described in three distinct phases. First, MV caused a rapid decline of the effective PSII quantum yield (occurring within 5 min, results not shown), followed by a second phase where ∆F/Fm′ remained stable or even slightly increased for up to 2 h. This second phase is characterised by the presence of a large NPQ as seen by the marked decrease of Fm′, which could recover after a dark period of 40 min (Fv/Fm remained unaffected). This large NPQ would result from the acidification of the thylakoid lumen (Derks et al. 2015). Indeed, lower production of NADPH relative ATP due to the diversion by MV of electrons from PSI to O2 restricts the Calvin Cycle, decreases ADP + Pi concentrations due to lower ATP utilization, and thereby impedes photophosphorylation. Then, the third phase of the MV effects began around the fourth hour by transient increases of Fs and Fm′, followed by their quenching below their initial values. These transient increases suggest the collapse of the NPQ as if the thylakoid membranes became suddenly permeable to H+ due to oxidative damages. This effect accelerated the declines of the PSII quantum yields Fv/Fm and ∆F/Fm′. It is noteworthy that the inhibition of the photosynthetic electron transport during the 8 h of MV-induced oxidative stress occurred before any significant decrease in the concentrations of the photosynthetic pigments.
Similar triphasic patterns of MV effects on photosynthetic electron transport were reported previously by Shao et al. (2008). They observed, however, in some phenotypes of Arabidopsis leaves, a large recovery of ∆F/Fm′ during the second phase of the MV effects. The origin of this difference is unknown, but such increase could be explained notably by the actinic PFD during the MV treatment in their experiments, which was six times higher than the growth PFD, as well as the absorption of MV into the leaves by vacuum infiltration.
The turning point of the MV treatment in sunflower leaf discs could be the loss of integrity of the photosynthetic apparatus at the fourth hour, indicated by the collapse of NPQ. This collapse coincides with the accelerating rates of PPs oxidation in sunflower leaf discs. Indeed, the values of Fv/Fm (Fig. 1b) and of the A328 (Fig. 3b) values during the MV treatment are highly correlated (R2 = 0.877). The 4-h delay before the acceleration of PPs oxidation is coherent with the model where H2O2 generated in chloroplasts, mitochondria or peroxisomes diffuses into the vacuoles, even those of epidermal cells, to be scavenged in a reaction catalysed by class-III peroxidases using PPs as electron donors. The oxidized PPs would be re-reduced by ascorbate, as long as ascorbate is regenerated from dehydroascorbate in the cytosol by glutathione and NADPH (Pérez et al. 2002; Takahama 2004). According to this model, it appears that under our experimental conditions, this H2O2 peroxidase/polyphenol/ascorbate scavenging cycle could operate during 4 h before the reducing capacity to regenerate ascorbate become exhausted, overwhelmed by the high rate of ROS production, and then, the oxidized PPs started to accumulate.
In the third phase of the MV-induced oxidative stress, about 40% of the soluble pool of PPs found in methanolic extracts of sunflower leaf discs was oxidized. This oxidation was characterised by a concomitant decrease of the absorbance that was maximal near 328 nm, and a large increase of absorbance near 420 nm, changes that are typical of phenolic oxidation (Ramešová et al. 2015; Sokolová et al. 2012). Specifically, the maximum near 328 nm is characteristic for hydroxycinnamic acids and their ester chlorogenic acid, which are the dominant type of PPs in sunflower leaves (Stelzner et al. 2019). The estimations of polyphenol oxidation by A328 decrease were corroborated by their close correlation with the reducing PPs contents estimated by the Folin–Ciocalteu method.
Cyclic voltammetry is frequently used to estimate the antioxidant capacity of plant extracts (Neill et al. 2002) and beverages such as wines and teas (Kilmartin and Hsu 2003; Kilmartin et al. 2001), but has not been used yet to characterise PPs in leaf extracts in a physiological context related to photosynthesis and photooxidative stress. Here, we used this method to obtain a descriptive illustration of the loss of reducing PPs in methanolic extracts from MV-treated leaf discs. Compared to the control leaf discs, the main effect of the MV treatment is the loss of the oxidative wave near 240 mV. Different phenolic compounds such as gallic acid, caffeic acid, quercetin, and catechin show similar voltammograms, with the same main oxidation wave near 240 mV measured at pH 7.0 (Hernanz-Vila et al. 2017). Voltammograms from green and black teas also show a main oxidation wave near 230 mV (pH 7.0), which was lower for the black tea, consistent with the oxidation of black tea leaves (Kilmartin and Hsu 2003; Sava et al. 2001).
The presence of oxidized PPs was detected by the absorbance increase near 420 nm in methanolic extracts of leaf discs treated with for 4–8 h (Fig. 3a). However, most of the oxidized PPs may have polymerised in situ or react with proteins to form insoluble brown pigments or melanin-brown pigments (Pourcel et al. 2007; Takahama 2004; Yamasaki et al. 1997). Their presence affected leaf optical properties, notably by decreased reflectance in the green and far-red regions, less dominated by chlorophylls. The decrease was significant after 6 h of treatment, i.e., 2 h later than the absorbance increase at 420 nm in the methanolic extracts was detected. This suggests that concentration of oxidized PPs must reach some level to initiate their polymerization leading to the accumulation of brown pigments in significant amount to affect leaf reflectance. The presence of insoluble brown pigments in MV-treated leaves was confirmed by measuring reflectance spectra of depigmented leaf discs after incubation in methanol: the reflectance was much lower for MV-treated leaf discs than control leaf discs.
Fluorescence spectroscopy provides a comprehensive view of the effects induced by the MV treatment described above, i.e., the transient changes of the fluorescence levels Fs, the losses of PSII photochemical efficiencies, the accumulation of brown pigments, and the decrease of UV-absorbing compounds. Variations of the Fs levels are the results of two opposite effects. A closure of PSII reactions centers causes Fs to increase, whereas an opposite effect on Fs results from the presence of a non-photochemical quenching, either due to the presence of the light-induced acidification of the thylakoid lumen (leading to the formation of zeaxanthin) or to the presence of damaged PSII (Klughammer and Schreiber 2008). Therefore, the Fs increase seen at the fourth hour indicates a higher proportion of closed PSII, likely due to the loss of the regulated NPQ. This transient Fs increase is followed by Fs quenching below the initial values, indicating damages PSII at 6 and 8 h. This Fs pattern is evident in the averaged intensity of the chlorophyll-a emission spectrum of fluorescence excited at 550 nm (Fig. 7), of the excitation spectrum of fluorescence emitted at 740 nm (Fig. 8), and also in the intensities of the far-red fluorescence induced by blue excitation measured by the Multiplex 330 portable fluorometer (Fig. 9a).
Besides the variations of the overall intensity of the fluorescence emission spectrum, it is interesting to note the preferential decrease of the F740 relative to F684, so the F740/F684 ratio decreased closely to Fv/Fm (R2 = 0.981) during the MV treatment. This correlation does not indicate a causal relation between the two variables. Indeed, specific inhibition of PSII due to photoinhibition or other stress (heat, chilling…) decreases preferentially the F684 band (Agati et al. 1995). Thereby, the F740/F684 ratio should increase during the MV treatment rather than decrease. However, the chlorophyll-a fluorescence emission spectra from our sunflower leaf discs are characterised by a low F684 relative to F740 intensities. This is due to the large chlorophylls concentrations in sunflower leaves and the excitation at 550 nm, two factors causing the preferential re-absorption of red fluorescence and then high F740/F684 (Agati et al. 1995, 1996; Buschmann and Lichtenthaler 1998; Gitelson et al. 1999). Here, the low F684 intensity relative to F740 makes the F740/F684 less sensitive to lower PSII quantum yield. The observed correlation F740/F684 and Fv/Fm rather indicates that both variables are similarly affected by a common factor, here the oxidative stress, causing the decrease of Fv/Fm, and the accumulation of brown pigments. The stronger decrease of reflectance at 740 nm than at 684 nm seen in MV-treated leaf discs (see Fig. 5a) would cause preferential absorption of F740, and, therefore, the decrease of the F740/F684 ratio.
Complementary information about the MV-induced oxidative damaged in sunflower leaf discs was provided by the excitation spectra of the chlorophyll-a fluorescence emitted at 740 nm (the emission band not reabsorbed by chlorophyll). Besides the averaged fluorescence intensity that varied similarly to Fs (a transient increase at 4 h before declining afterward), there is a specific decrease of the main fluorescence-excitation peak at 484 nm during the MV treatment. Zeng et al. (2016) showed that illumination of isolated chloroplasts caused a preferential decrease of fluorescence excitation near 477 nm, which was interpreted as a decrease of energy transfer between the antenna pigments, both chlorophyll-b and carotenoids. In contrast, Nichelmann et al. (2016) observed that a strong illumination (1200 µmol m−2 s−1 for 15 min) barely decreased the fluorescence-excitation ratio F470/F660 measured in Arabidopsis thaliana leaves. They rather demonstrated the presence of a good correlation between this F470/F660 ratio and the violaxanthin cycle pool size in A. thaliana leaves acclimated to different growth irradiances. Since, during our experiment, the total carotenoid contents remained constant, the similar decreases of F484/F680 and ∆F/Fm′, therefore, indicates a loss of energy transfer between the antenna pigments and the reaction center of PSII caused by the MV treatment. It will be interesting to determine the relative importance of energy transfer between the antenna pigments and xanthophylls pool size on the F484/F680 ratio in different species and conditions, as the chlorophyll-a fluorescence intensities induced at these two excitation wavelengths could potentially provide an estimate of photosynthetic efficiency in proximal fluorosensing.
Concerning the second main excitation peak, at 422 nm, its larger attenuation relative to the red part of the spectrum can be attributed to the accumulation of brown pigments, which absorb maximally at 420 nm (Takahama et al. 1999; see Fig. 3a).
In contrast of the decreases of averaged fluorescence intensities mentioned above, excitation spectra showed higher UV-excited chlorophyll-a fluorescence intensities in MV-treated leaf discs compared to control discs. Intensities of UV-excited chlorophyll-a fluorescence are naturally weak due to the lower absorbance of chlorophylls in that spectral region, and also due to the blocking of UV excitation by the presence of hydroxycinnamates in the epidermis of sunflower leaves (Cerovic et al. 2002; Stelzner et al. 2019). These increases of UV-excited fluorescence in MV-treated leaf discs are coherent with the oxidation of PPs seen by the decrease of UV absorbance of the methanolic leaf extracts. They are also well supported by the measurements made with the portable fluorometer Multiplex 330. Whereas blue-excited far-red fluorescence followed the same patterns as Fs, UV-excited (330 nm) far-red fluorescence markedly increased after 4 h of MV treatment. This corresponds to a 53% decrease of LogFER, which estimates leaf epidermal UV absorbance, coherent with the loss of UV-absorbing compounds with an absorbance maximum near 328 nm (Fig. 3b).
Globally, our study provides one of the missing evidences required to confirm that PPs act as effective antioxidants protecting the plants against ROS, by showing the presence of a temporal correlation between the inhibition of Fv/Fm and the oxidation of PPs during an acute oxidative stress in sunflower leaf discs. We showed that fluorescence and reflectance spectroscopy as well as cyclic voltammetry could be useful in future studies to test the hypothesis that in leaves with higher PPs contents, Fv/Fm could either persist longer or decline slower during acute oxidative stress.
References
Agati G, Mazzinghi P, Fusi F, Ambrosini I (1995) The F685/F730 chlorophyll fluorescence ratio as a tool in plant physiology: response to physiological and environmental factors. J Plant Physiol 145(3):228–238. https://doi.org/10.1016/S0176-1617(11)81882-1
Agati G, Mazzinghi P, Lipucci Di Paola M, Fusi F, Cecchi G (1996) The F685/F730 chlorophyll fluorescence ratio as indicator of chilling stress in plants. J Plant Physiol 148(3–4):384–390. https://doi.org/10.1016/S0176-1617(96)80270-7
Agati G, Matteini P, Goti A, Tattini M (2007) Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol 174(1):77–89. https://doi.org/10.1111/j.1469-8137.2007.01986.x
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. https://doi.org/10.1016/j.plantsci.2012.07.014
Bertolini A, Petrussa E, Patui S, Zancani M, Peresson C, Casolo V, Vianello A, Braidot E (2016) Flavonoids and darkness lower PCD in senescing Vitis vinifera suspension cell cultures. BMC Plant Biol 16(1):233. https://doi.org/10.1186/s12870-016-0917-y
Buschmann C, Lichtenthaler HK (1998) Principles and characteristics of multi-colour fluorescence imaging of plants. J Plant Physiol 152(2–3):297–314. https://doi.org/10.1016/S0176-1617(98)80144-2
Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I (2002) The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ 25(12):1663–1676. https://doi.org/10.1046/j.1365-3040.2002.00942.x
Derks A, Schaven K, Bruce D (2015) Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta Bioenerg 1847(4–5):468–485. https://doi.org/10.1016/j.bbabio.2015.02.008
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7(7):1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148(2–3):99–104. https://doi.org/10.1016/j.jbiotec.2010.05.009
Edreva A (2005) The importance of non-photosynthetic pigments and cinnamic acid derivatives in photoprotection. Agric Ecosyst Environ 106:135–146. https://doi.org/10.1016/j.agee.2004.10.002
Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: role and biochemical activity in plants and human. J Med Plant Res 5(31):6697–6703. https://doi.org/10.5897/JMPR11.1404
Gitelson AA, Buschmann C, Lichtenthaler HK (1999) The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens Environ 69(3):296–302. https://doi.org/10.1016/S0034-4257(99)00023-1
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68(1):14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
Hernández I, Alegre L, Munné-Bosch S (2006) Enhanced oxidation of flavan-3-ols and proanthocyanidin accumulation in water-stressed tea plants. Phytochemistry 67(11):1120–1126. https://doi.org/10.1016/j.phytochem.2006.04.002
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14(3):125–132. https://doi.org/10.1016/j.tplants.2008.12.003
Hernanz-Vila D, Jara-Palacios MJ, Escudero-Gilete ML, Heredia FJ (2017) Applications of voltammetric analysis to wine products. In: Stoytcheva M, Zlatev R (eds) Applications of the voltammetry. IntechOpen Limited, London, pp 109–127. https://doi.org/10.5772/67696
Jaiswal S, Chawla R, Sawhney S (2012) Correlations among attributes of senescence and antioxidative status of leaf discs during epiphyllous bud differentiation in Kalanchoe pinnata Lam. (Pers.). Z Naturforsch Sect C J Biosci 67 C(7–8):418–428. https://doi.org/10.1515/znc-2012-7-810
Kilmartin PA, Hsu CF (2003) Characterisation of polyphenols in green, oolong, and black teas, and in coffee, using cyclic voltammetry. Food Chem 82(4):501–512. https://doi.org/10.1016/S0308-8146(03)00066-9
Kilmartin PA, Zou H, Waterhouse AL (2001) A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J Agric Food Chem 49(4):1957–1965. https://doi.org/10.1021/jf001044u
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:27–35
Kováčik J, Grúz J, Bačkor M, Strnad M, Repčák M (2009) Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep 28(1):135–143. https://doi.org/10.1007/s00299-008-0627-5
Kováčik J, Klejdus B, Hedbavny J, Bačkor M (2010) Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci 178(3):307–311. https://doi.org/10.1016/j.plantsci.2010.01.009
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Louis J, Cerovic ZG, Moya I (2006) Quantitative study of fluorescence excitation and emission spectra of bean leaves. J Photochem Photobiol B Biol 85(1):65–71. https://doi.org/10.1016/j.jphotobiol.2006.03.009
Louis J, Meyer S, Maunoury-Danger F, Fresneau C, Meudec E, Cerovic ZG (2009) Seasonal changes in optically assessed epidermal phenolic compounds and chlorophyll contents in leaves of sessile oak (Quercus petraea): towards signatures of phenological stage. Funct Plant Biol 36(8):732–741. https://doi.org/10.1071/FP09010
Louis J, Genet H, Meyer S, Soudani K, Montpied P, Legout A, Dreyer E, Cerovic ZG, Dufrêne E (2012) Tree age-related effects on sun acclimated leaves in a chronosequence of beech (Fagus sylvatica) stands. Funct Plant Biol 39(4):323–331. https://doi.org/10.1071/FP11248
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5(4):359–368. https://doi.org/10.4161/psb.5.4.10871
Meir S, Kanner J, Akiri B, Philosoph-Hadas S (1995) Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 43(7):1813–1819. https://doi.org/10.1021/jf00055a012
Nakabayashi R, Saito K (2015) Integrated metabolomics for abiotic stress responses in plants. Curr Opin Plant Biol 24:10–16. https://doi.org/10.1016/j.pbi.2015.01.003
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77(3):367–379. https://doi.org/10.1111/tpj.12388
Neill SO, Gould KS, Kilmartin PA, Mitchell KA, Markham KR (2002) Antioxidant capacities of green and cyanic leaves in the sun species, Quintinia serrata. Funct Plant Biol 29(12):1437–1443. https://doi.org/10.1071/FP02100
Nichelmann L, Schulze M, Herppich WB, Bilger W (2016) A simple indicator for non-destructive estimation of the violaxanthin cycle pigment content in leaves. Photosynth Res 128(2):183–193. https://doi.org/10.1007/s11120-016-0218-1
Ounis A, Cerovic Z, Briantais J-M, Moya I (2001) Dual-excitation FLIDAR for the estimation of epidermal UV absorption in leaves and canopies. Remote Sens Environ 76:33–48. https://doi.org/10.1016/S0034-4257(00)00190-5
Pérez FJ, Villegas D, Mejia N (2002) Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry 60(6):573–580. https://doi.org/10.1016/S0031-9422(02)00146-2
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12(1):29–36. https://doi.org/10.1016/j.tplants.2006.11.006
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53(10):4290–4302. https://doi.org/10.1021/jf0502698
Ramešová Š, Sokolová R, Degano I (2015) The study of the oxidation of the natural flavonol fisetin confirmed quercetin oxidation mechanism. Electrochim Acta 182:544–549. https://doi.org/10.1016/j.electacta.2015.09.144
Sava VM, Galkin BN, Hong MY, Yang PC, Huang GS (2001) A novel melanin-like pigment derived from black tea leaves with immuno-stimulating activity. Food Res Int 34(4):337–343. https://doi.org/10.1016/S0963-9969(00)00173-3
Shao L, Shu Z, Peng CL, Lin ZF, Yang CW, Gu Q (2008) Enhanced sensitivity of Arabidopsis anthocyanin mutants to photooxidation: a study with fluorescence imaging. Funct Plant Biol 35(8):714–724. https://doi.org/10.1071/FP08069
Sokolová R, Ramešová S, Degano I, Hromadová M, Gál M, Abka J (2012) The oxidation of natural flavonoid quercetin. Chem Commun 48(28):3433–3435. https://doi.org/10.1039/c2cc18018a
Stelzner J, Roemhild R, Garibay-Hernández A, Harbaum-Piayda B, Mock HP, Bilger W (2019) Hydroxycinnamic acids in sunflower leaves serve as UV-A screening pigments. Photochem Photobiol Sci 18(7):1649–1659. https://doi.org/10.1039/c8pp00440d
Takahama U (2004) Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: physiological significance of the oxidation reactions. Phytochem Rev 3(1–2):207–219. https://doi.org/10.1023/B:PHYT.0000047805.08470.e3
Takahama U, Hirotsu M, Oniki T (1999) Age-dependent changes in levels of ascorbic acid and chlorogenic acid, and activities of peroxidase and superoxide dismutase in the apoplast of tobacco leaves: mechanism of the oxidation of chlorogenic acid in the apoplast. Plant Cell Physiol 40(7):716–724. https://doi.org/10.1093/oxfordjournals.pcp.a029598
Tomás-Barberán FA, Gil MI, Castañer M, Artés F, Saltveit ME (1997) Effect of selected browning inhibitors on phenolic metabolism in stem tissue of harvested lettuce. J Agric Food Chem 45(3):583–589. https://doi.org/10.1021/jf960478f
Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7(6):581–591. https://doi.org/10.1055/s-2005-873009
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115(4):1405–1412. https://doi.org/10.1104/pp.115.4.1405
Zeng L, Wang Y, Zhou J (2016) Spectral analysis on origination of the bands at 437 nm and 475.5 nm of chlorophyll fluorescence excitation spectrum in Arabidopsis chloroplasts. Lumin 31(3):769–774. https://doi.org/10.1002/bio.3022
Acknowledgements
We are grateful to Lionel Saunois and Amandine Dubois from the greenhouse facility for taking care of the sunflower plants, and to Gwendal Latouche for his technical assistance. GS acknowledges his sabbatical leave from the Université du Québec à Trois-Rivières.
Author information
Authors and Affiliations
Contributions
GS and ZGC conceived research, conducted most experiments and analyzed the data. WMAER and PM conducted experiments with voltammetry and analyzed the data. GS prepared the figures and wrote most of the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
ZGC declares a link to the company FORCE-A as one of the co-authors of the Multiplex patent that the company exploits. Other authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2019_3316_MOESM1_ESM.docx
Fig. 1S Polyphenol oxidation estimated from leaf methanolic extracts. Absorbance spectra of extracts from leaf treated or not with methyl viologen for different times (DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Samson, G., Cerovic, Z.G., El Rouby, W.M.A. et al. Oxidation of polyphenols and inhibition of photosystem II under acute photooxidative stress. Planta 251, 16 (2020). https://doi.org/10.1007/s00425-019-03316-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-019-03316-x