Abstract
The photosynthetic apparatus of higher plants acclimates to irradiance. Among the features which are changing is the pool size of the pigments belonging to the violaxanthin cycle, in which zeaxanthin is formed. In high light grown leaves, the violaxanthin cycle pool size is up to five times larger than in low light. The changes are reversible on a time scale of several days. Since it has been published that violaxanthin cycle pigments do not transfer absorbed energy to chlorophyll, we hypothesized that excitation of chlorophyll fluorescence in the blue spectral region may be reduced in high light-acclimated leaves. Fluorescence excitation spectra of leaves of the Arabidopsis thaliana tt3 mutant showed strong differences between high and low light-acclimated plants from 430 to 520 nm. The resulting difference spectrum was similar to carotenoids but shifted by about 20 nm to higher wavelengths. A good correlation was observed between the fluorescence excitation ratio F 470/F 660 and the violaxanthin cycle pool size when leaves were acclimated to a range of irradiances. In parallel to the decline of F 470/F 660 with high light acclimation also the quantum yield of photosynthetic oxygen evolution in blue light decreased. The data confirm that violaxanthin cycle carotenoids do not transfer absorbed light to chlorophyll. It is proposed to use the ratio F 470/F 660 as an indicator for the light acclimation status of the chloroplasts in a leaf.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The xanthophyll zeaxanthin (Z) has been implied in the photoprotection of photosystem II (Demmig et al. 1987; Niyogi 1999; Jahns and Holzwarth 2012; Demmig-Adams et al. 2014). Through de-epoxidation of violaxanthin (V), Z is formed in leaves under conditions when the photosynthetic apparatus experiences excessive light (Demmig-Adams et al. 1990). Since Z is epoxidized again when the high light stress is relieved, the total reactions have been termed the xanthophyll, or more specifically, the violaxanthin cycle (V-cycle). Besides this short-term reaction, also an increase of the total pool size of the V-cycle during exposure to high growth irradiance has been observed (Thayer and Björkman 1990; Adams and Demmig-Adams 1992; Logan et al. 1996). In contrast to the relatively fast turnover of the V-cycle, where the half time of Z formation is in the minutes range, the adjustment of the V-cycle pool size takes several days (Thayer and Björkman 1990; Björkman and Demmig-Adams 1994; Bilger et al. 1995). The relationship between leaf content of xanthophylls and incident irradiance is relatively tight such that the V-cycle pool size can be taken as an indicator of the latter (Niinemets et al. 2003).
So far, the function of the V-cycle pool size adjustment is unclear. There have been correlations described between V-cycle pool size and the capacity for non-photochemical quenching (NPQ), which is considered to be photoprotective (Demmig-Adams and Adams 1992; Demmig-Adams et al. 2014). However, a potentially higher amount of zeaxanthin formed does not lead necessarily to a higher non-photochemical quenching of excitation energy (Bilger et al. 1995). It is also assumed that Z has a photoprotective role also as an antioxidant (Havaux and Niyogi 1999; Havaux et al. 2007). Hence, a larger V-cycle pool size would contribute to the antioxidative capacity in the chloroplasts.
Light-dependent changes of the pool size have practical consequences as well. Light-induced Z formation leads to an absorbance change in intact leaves around 505 nm (Bilger et al. 1989), which can also be detected as a reflectance change at about 530 nm (Gamon et al. 1990). This led to the formulation of the so-called photochemical reflectance index, PRI, which has been used since then as an indicator of the light-use efficiency of vegetation in remote sensing applications (Gamon et al. 1992; Garbulsky et al. 2011). However, not only the epoxidation state (EPS) of the V-cycle influences PRI, but also the total leaf carotenoid content has been shown to affect PRI (Sims and Gamon 2002; Gamon and Berry 2012; Wong and Gamon 2015a). Therefore, the effect of carotenoid contents on PRI may override the relationship to light-use efficiency (LUE) such that PRI may no longer function unambiguously as an indicator of LUE (Wong and Gamon 2015b).
One of the primary functions of carotenoids in the light harvesting complex (LHC) is to serve as antenna pigments (Siefermann-Harms 1987). This function, however, is dependent on a specific steric relationship between carotenoid and chlorophyll pigment in order to allow Förster resonance energy transfer (Caffari et al. 2014) and for a long time it has been recognized that not all carotenoids may take part in energy transfer (Haxo 1960). While lutein and neoxanthin very efficiently transfer energy to chlorophyll (chl) a or chl b, respectively, violaxanthin and, very probably, also the other pigments in the V-cycle are very inefficient in this function (Caffari et al. 2001; Martiskainen et al. 2011). Also β-carotene has been attributed a rather low energy transfer efficiency of about 35 % (de Weerd et al. 2003). Furthermore, a large part of the V-cycle pigments, presumably all which are formed when irradiance increases over the lowest levels, are probably not part of the LHC structure as described by Liu et al. (2004). Their exact location remains unclear. Accordingly, these pigments would be unable to transfer energy to chlorophyll.
Research into the function of irradiance dependent pool size changes as well as into their role in remote sensing applications may profit from a quick and non-destructive optical indicator of V-cycle pool size. It has been speculated that fluorescence excitation in intact leaves within the blue spectral range may be affected by variations of the carotenoid content of leaves (Bilger et al. 1997). If this was true there may exist a systematic relationship between fluorescence excitation efficiency by blue light and the V-cycle pool size, which may allow the detection of its changes by chlorophyll fluorescence measurements.
To test if changes in the pool size would affect the excitation efficiency of chlorophyll fluorescence with blue light, Arabidopsis thaliana plants were grown in a climate chamber at varying irradiance to obtain a large variation in the V-cycle pool size. To avoid an interfering effect of anthocyanins on the optical properties of the leaves we used mutants, tt3 and tt5, which are deficient in the formation of anthocyanins. Furthermore, we tested if observed reductions in fluorescence excitation efficiency would have also consequences for photosynthetic light-use efficiency.
Materials and methods
Plant material
Plants of the Arabidopsis thaliana (L.) Heynh. tt3 and tt5 mutant (background ecotype ler-0, Nottingham Arabidopsis Stock Centre, ID N84, N86) were grown in peat soil substrate (TKS 2, Floragard Vertriebs-GmbH, Oldenburg, Germany). The tt3 mutant lacks an enzyme dedicated to anthocyanin biosynthesis, the dihydroflavonol-4-reductase (DFR). These mutants were used in order to avoid anthocyanin biosynthesis in the high light acclimation, which would have confounded the fluorescence excitation spectra. tt5 mutants are unable to synthesize flavonols due to the lack of chalcone flavanone isomerase (CHI) and were used to explore the effect of flavonol biosynthesis on the excitation spectra. The plants were cultivated at 21 °C under short day conditions with a photoperiod of 8 h in a climate chamber (Johnson Controls, Mannheim, Germany) for 6 weeks. The photosynthetic photon flux densities (PPFD) provided by metal halide lamps (CMT360LS W BH, Iwasaki Electric, Tokyo, Japan) were 50 and 90–110 µmol m−2 s−1. Irradiation of plants with UV radiation was excluded by the placement of perspex sheets (5 mm) between lamps and the remainder of the chamber. After 6 weeks 32 plants of the tt3 mutant grown at 90–110 µmol m−2 s−1 were placed at 8 different heights on a custom-built stair to adjust the PPFD in a range from 50 to 420 µmol m−2 s−1 in order to induce the light acclimation of V-cycle pool size. Irradiance was determined with a quantum meter (LI-250, LI-COR Biosciences, Lincoln, U.S.A.). At the same time, the temperature was lowered to 9 °C to further enhance the reaction of the plants and the photoperiod was extended to 16 h per day. Some plants of the tt3 and tt5 mutants remained at the cultivation conditions as a control. Additionally, plants of the tt5 mutant were simply irradiated with a PPFD of 420 µmol m−2 s−1 to induce acclimation to high light. After 16 days of light acclimation, plants were used for measurements of photosynthetic gas exchange, chlorophyll fluorescence and pigment analysis. The 5th–7th youngest leaves were sampled, which were initialised prior to change of light regimes but which developed to mature leaves during the high light acclimation. Leaves of Cornus sanguinea L. and Fagus sylvatica L. were sampled in the Botanical Garden of Kiel University, Germany, over a wide light gradient from the outer crown into deep shade. Sampling was in the months of June, July and August, respectively, when leaves were fully matured. Leaves of Vincetoxicum hirundinaria Medik. were collected during field trips in the vicinity of Bad Staffelstein (Bavaria, Germany) at the end of May and early June in 2013 and 2014, respectively, over a wide gradient of habitats, ranging from a rather dense forest stand to the open field.
Photosynthesis
The maximal quantum yield of photosynthesis based on incident light was determined by measuring light-dependent oxygen evolution of leaves using two O2-electrode systems (LD2, Hansatech Instruments Ltd, Kings Lynn, Norfolk, UK) equipped with a Clark-type electrode (S1, Hansatech). Photosynthetically active radiation was provided by a halogen light source (LS2, Hansatech) equipped with a 100 W tungsten filament spotlight (Xenophot, Osram GmbH, Augsburg, Germany) in combination with a short pass filter having a cut off at 732 nm (Optics Balzers AG, Liechtenstein) for heat protection. Dichroitic filters (DtBlue and DtRed, Balzers) were combined with various neutral density filters (NG series, Schott AG, Mainz, Germany) to define increasing blue and red irradiances. Spectra of the provided irradiances with all filter combinations were recorded using a spectroradiometer (DM 150, Bentham Instruments Ltd, Reading, U.K.) with a resolution of 1 nm (Fig. I, Supplemental). The spectra were used to calculate the photon flux densities of blue and red light.
O2 evolution measurements were made with plants acclimated at a range of irradiances at 9 °C. 24 h prior to the measurements all plants were exposed to a low PPFD of 120 µmol m−2 s−1 at 9 °C for 16 h in order to minimize potential photoinhibitory effects on photosynthetic quantum yield measurements. Determination of F v/F m with an Imaging-PAM fluorometer (Heinz Walz GmbH, Effeltrich, Germany) was used to estimate the PS II efficiency of selected leaves. Prior to oxygen evolution measurements, plants were exposed to white light with a PPFD of 120 µmol m−2 s−1 provided by a LED-panel (Stairville Floodpanel 150, Musikhaus Thomann e.K., Burgebrach, Germany) to maintain photosynthesis. In the electrode system, leaves were at first irradiated again with white light of a PPFD of 120 µmol m−2 s−1. Subsequently, dark respiration was recorded. In the following step, blue and red light were applied consecutively with 4 irradiances ranging from 10 to 58 µmol m−2 s−1 (blue light) and 9 to 52 µmol m−2 s−1 (red light). The order of blue or red illumination was alternated on the succeeding leaf. After the measurements, leaves were scanned and their areas were determined using the software SigmaScan Pro 5 (Systat Software, Point Richmond, U.S.A.). Subsequently, leaves were kept on moistened filter paper for 20–30 min at dim room light for measurements of chlorophyll fluorescence.
Chlorophyll fluorescence measurements
Following determination of photosynthetic quantum yield, the same leaves were used for chlorophyll fluorescence measurements to assess the optical effects of an enhanced V-cycle pool size. Leaves were fixed in a leaf clip (Walz) and chlorophyll fluorescence was determined by blue measuring light with a wavelength of 470 nm provided by an UVA-PAM fluorometer (Gademann Instruments, Würzburg, Germany). Subsequently, leaves were exposed to the excitation beam of a Mini-PAM fluorometer (Walz) to measure chlorophyll fluorescence induced by red light (660 nm). The spectra of the two excitation beams are shown in Fig. I (Supplemental). The fluorescence levels, F470 and F660, were normalized to the level obtained with a blue plastic foil (Walz). To test if the chlorophyll fluorescence levels were affected by the epoxidation state of the V-cycle, F 470/F 660 of pre-darkened or shortly irradiated leaves was determined. Leaves of the tt3 mutant acclimated to low light (LL, 90–110 µmol m−2 s−1) or high light (HL, 420 µmol m−2 s−1) and sun and shade leaves of F. sylvatica were either pre-darkened for 1 h or irradiated for 15 min with strong light provided by a metal halide lamp (PPFD of 1200 mmol m−2 s−1). Following predarkening leaf discs (0.85 cm2) were punched out and immediately frozen in liquid nitrogen. Fluorescence was measured at the adjacent part of the sample areas. Two more adjacent discs were punched out and were placed on wet paper towels. Since the leaves of the tt3 mutant were rather small two leaves of the same age were sampled per plant. Subsequently, leaf discs were irradiated with white light. Leaf temperature during illumination was kept in a range of 15–17 °C by placing them on an aluminium plate, which was cooled by a thermostated water bath (RK8 CP, Lauda Dr. R. Wobser GmbH & Co KG, Lauda-Königshofen, Germany). At the end of the illumination, one disc was immediately frozen in liquid nitrogen for pigment determination and the remaining leaf disc was used for fluorescence measurements.
Excitation spectra of chlorophyll fluorescence were recorded from leaf discs of tt3 and tt5 control plants grown at a PPFD of 50 µmol m−2 s−1 at 21 °C and of plants acclimated at 9 °C to high light of a PPFD of 420 µmol m−2 s−1. Before excitation of fluorescence, leaf discs were irradiated for at least 10 min on wet filter paper with a PPFD of 120 µmol m−2 s−1 provided by a halogen lamp (KL 2500, Schott) to reach a steady state of photochemical and non-photochemical fluorescence quenching. Less than 1 min passed between the end of the pre-illumination and the start of the measurement of the fluorescence excitation spectra. Leaf discs were fixed with double adhesive tape (Scotch Double Sided 12.7 mm, No. 655, 3 M, St. Paul, U.S.A.) on a black non-fluorescing cardboard strip, which matched the cuvette holder of the fluorescence spectrophotometer (F 4500, Hitachi Ltd. Corporation, Tokyo, Japan). Chlorophyll fluorescence was excited in steps of 1 nm from 380 to 710 nm and detected at 720 nm. The time required for one spectrum was about 30 s. The detector was protected by a long pass filter (RG 715, Schott) against higher order maxima of the excitation beam. Chlorophyll fluorescence excitation spectra were normalized to the average fluorescence level at 672–678 nm. The spectra were not corrected for the relative sensitivity of the fluorescence spectrophotometer. Fluorescence excitation ratios (FER) were calculated by dividing the spectra obtained from HL acclimated leaves by those of tt5 LL leaves. To get apparent absorbance spectra, the negative decadic logarithm of FER was calculated. Use of the tt5 plants as reference ensured the absence of flavonols even in plants which were acclimated to high light.
Pigment analysis
Chlorophylls and carotenoids
After measurements of chlorophyll fluorescence, discs (0.85 cm2) were punched out of the measured part of the lamina using a cork borer and placed into 2-ml reaction tubes containing five glass beads (2 and 4 mm diameter; Roth GmbH & Co. KG, Karlsruhe, Germany). The tubes were frozen in liquid nitrogen and stored at −86 °C until extraction for pigment analysis. The frozen leaf discs were homogenized for 5 min in their reaction tubes in a cell mill (MM 2, Retsch GmbH, Haan, Germany) at 90 % of stroke capacity. Then 500 µl of the ice cold extraction buffer (30 % (v/v) 30 mM Tris buffer at pH 7.8, 70 % (v/v) acetone) was added and the samples were extracted for 2 min on a thermoshaker (TSC, Biometra GmbH, Jena, Germany) at 1400 rpm and 4 °C. After centrifugation for 5 min at 12,000 g the supernatant was collected. The extraction procedure was repeated with 500 µl 70 % acetone. Finally, in a last step, the pellet was resuspended with 500 µl of 100 % acetone, extracted on the thermoshaker and centrifuged at 12,000 g for 5 min. The unified supernatant was filtered (0.45 µm, F 2504-3, Thermo Scientific, Rockwood, U.S.A.) into brown HPLC vials. Quantification of the photosynthesis pigments was carried out with an HPLC-system of the Agilent 1100-series (Agilent Technologies, Santa Clara, U.S.A.). Pigments were separated with a Hypersil ODS-column (4.6 × 250 mm, 5 µm particle size, Thermo Fisher Scientific Inc., Waltham, U.S.A.) in combination with a mobile phase defined by a gradient of 10 mM Tris buffer (Roth) at pH 7.8 and 100 % acetone (Roth). The gradient started with 75 % acetone for 7.5 min. During the following 9.5 min, the concentration of acetone was increased to 100 % and remained there for 3 min. During the post-run the acetone was decreased within 2 min to 75 %. Finally, the column was flushed for 8 min before injection of the next sample. Chl a and b as well as the carotenoids were identified by their absorption spectra monitored by a photodiode array detector (Agilent) and their retention time. The detector was calibrated using pure pigment extracts for all carotenoids (except antheraxanthin) prepared by thin layer chromatography (Lichtenthaler and Pfister 1978, modified) and spectrophotometric determination using the extinction coefficients of Davies (1976). Chlorophyll was calibrated using a leaf extract and the equations provided by Porra et al. (1989). Epoxidation state of the V-cycle pigments (EPS) was calculated as described in Thayer and Björkman (1990).
Flavonoids
Leaf discs of the tt3 and the tt5 mutant were ground in reaction tubes with a cell mill (Retsch) for 5 min. Subsequently, 250 µl of the ice cold extraction medium consisting of 50 % methanol (v/v) and 1 % of 10.2 M hydrochloric acid (v/v) was added and the cell debris was extracted for 2 min at 4 °C and 1400 rpm with a thermoshaker (Biometra). The suspension was then centrifuged at 14,200 g and 4 °C for 5 min and the supernatant was collected. This extraction was repeated. Finally, the pellet was resuspended with 250 µl of 100 % methanol and extracted a third time before the supernatants were unified. Following a last centrifugation at 14,200 g and 4 °C, HPLC vials were filled with 100 µl of the extract. Flavonoids were analysed with a HPLC-system (10-series, Shimadzu Corporation, Kyoto, Japan). The compounds were separated on a Licrosphere RP 18-column (4 × 250 mm, 5 µm particle size, Merck KGaA, Darmstadt, Germany) using a gradient of 0.01 % of 14.8 M phosphoric acid (solvent A, v/v) and 0.1 % of 14.8 M phosphoric acid (v/v) in 90 % methanol (solvent B, v/v). The gradient started with 20 % of solvent B and within 12 min the concentration was increased to 45 % which was constantly applied for 13 min. In a period of 15 min, the concentration of solvent B was further increased to 75 % and subsequently within 2 min raised to 100 %. During the post-run the concentration of solvent B was reduced to 20 % in a time span of 2 min. Absorption was recorded with a photodiode array detector (SPD M 10A, Shimadzu) at 330 and 360 nm. Flavonols and hydroxycinnamic acids (HCA’s) were identified according to their retention times and absorption spectra. Leaf content of polyphenolics was expressed as sinapic acid (HCA), kaempferol and quercetin equivalents according to the calibration of the system with pure substances. For the calibration known concentrations of sinapic acid, kaempferol and quercetin dihydrate (all from Roth) were injected and chromatograms were recorded at the wavelengths of 330 and 360 nm, respectively.
Measurements with field grown plants
Leaves of F. sylvatica and C. sanguinea were collected from single plants over a broad light gradient from the outer crown into deep shade. Leaves of C. sanguinea with apparent anthocyanin content were not used for our analysis. Discs with a diameter of 1.3 cm were punched and fluorescence excitation efficiency was determined as described above after a few minutes of dark adaptation. After the fluorescence measurements, the leaf discs were dried at 50 °C overnight in a cabinet dryer (U 30, Memmert GmbH & Co KG, Schwabach, Germany). Samples of V. hirundinaria were collected as whole leaves. Chlorophyll fluorescence measurements were executed as described above. Leaf area was determined using Sigmascan Pro5 (Systat Software Inc., Point Richmond, U.S.A) from photographic images taken from the leaves in the field. The leaves were dried in a household oven set at 80 °C for 3 h and stored in paper bags. At the lab they were dried again in a cabinet dryer overnight. The dry weight of all leaves was determined with an analytical balance (A200S, Sartorius AG, Göttingen, Germany) with 0.1 mg resolution. Leaf mass per area (LMA) was calculated by division of dry weight and the area of the leaf samples and is indicated in g m−2.
Statistics
Correlation of data was tested by regression analysis for statistical significance. Analysis was performed with GraphPad Prism (Version 5.0, GraphPad Software, La Jolla, U.S.A.). A probability value (p) of p < 0.05 was defined to verify statistical relevance. Leaf contents of polyphenolics were tested for normality and equal variances and were analysed with a One-way ANOVA in combination with a Tukey’s post hoc test (GraphPad Prism). The ratios of F 470/F 660 determined on pre-darkened and irradiated leaf samples of F. sylvatica and the A. thaliana tt3 mutant, respectively, were, after ensuring normality and equal variances, tested with a Two-way ANOVA followed by a Bonferroni post hoc test (GraphPad Prism).
Results
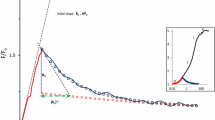
Chlorophyll fluorescence excitation spectra recorded on leaves acclimated to low light (LL) and high light (HL) (50 and 420 µmol m−2 s−1, respectively) are shown in Fig. 1. The spectra of all leaf samples show consistent relative maxima at 435, 485, 650 and 675 nm roughly corresponding to the absorption bands of chlorophylls a and b. High light acclimation of the tt3 and tt5 mutants caused a strong reduction of the excitation efficiency at wavelengths below 520 nm, whereas at higher wavelengths all spectra were more or less identical. Significant differences indicated by not overlapping 95 % confidence intervals (n = 6, data not shown) were observed between 250 and 520 nm. While both mutants showed identical spectra when grown at low light, the spectra of the high light acclimated plants diverged from 335 to 430 nm. This effect was presumably caused by the flavonol deficiency in the tt5 mutant. High PPFD and low temperature are inducing flavonol biosynthesis (Kirakosyan et al. 2004; Olsen et al. 2009; Løvdal et al. 2010). Epidermally located polyphenolics are screening the underlying chloroplasts in the mesophyll against fluorescence excitation (Markstädter et al. 2001; Cerovic et al. 2002). The differences between LL and HL spectra in the tt5 mutant, which lacks chalcone isomerase and where accordingly flavonol formation is missing, may be caused by hydroxycinnamic acids, which were still formed in these plants (Supplemental Fig. II) and which also have a screening function (Li et al. 1993; Sheahan 1996). The difference in the excitation spectra between tt3 HL and tt5 HL leaves below 440 nm is presumably entirely due to the strong accumulation of flavonols in the tt3 plants (Fig. II Supplemental). While the reduction of fluorescence excitation in the high light acclimated plants at wavelengths below 430 nm can be ascribed to polyphenolics, this is not possible for the high light-induced changes at longer wavelengths. Here, high light-induced carotenoid biosynthesis may be responsible since these pigments have their main absorption bands in this wavelength range. This is supported by the comparison of −log(FER) of the leaf samples and the summarized V-cycle absorbance (Fig. 1, inset). The two difference spectra show some similarity, but display also dissimilarities as compared to the carotenoid absorption spectrum. The difference spectra are bathochromically shifted by about 14 nm relative to the carotenoid spectrum in the major peak. The latter shows also a peak at 448 nm, which may relate to the shoulder in the difference spectrum at 466 nm. The absence of a peak at this wavelength in the spectrum obtained by the fluorescence measurements may be caused by spectral flattening due to the high concentration of chlorophyll present in the leaves. At shorter wavelengths the absorption of polyphenolics which were produced upon high light acclimation probably dominates the spectra.
Normalized chlorophyll fluorescence excitation spectra of leaf discs of the A. thaliana tt3 (dashed lines) and tt5 mutants (continuous lines). Plants were either cultivated at a photosynthetic photon flux density (PPFD) of 50 µmol m−2 s−1 (low light, LL, thin lines) or acclimated to a PPFD of about 420 µmol m−2 s−1 at 9 °C (high light, HL, thick lines) for 16 days. Chlorophyll fluorescence was detected at wavelengths >715 nm. Means of n = 6. The inset shows the absorbance spectrum of the sum of the violaxanthin cycle carotenoids (thin line) and the negative decadic logarithm of fluorescence excitation ratios (FER) calculated for high light acclimated leaves of tt3 (dashed line) and tt5 mutant (continuous line) with fluorescence signals of low light acclimated leaves of the tt5 mutant as a reference
In order to assess the influence of carotenoid content on fluorescence excitation efficiency, A. thaliana tt3 plants were acclimated for 16 days to a range of PPFDs. The pool of the V-cycle pigments linearly increased with growth irradiance by almost a factor of 4, whereas lutein and β-carotene increased only by a factor of less than 1.2 (Fig. 2). The regression coefficient for the V-cycle was 0.81, whereas those for lutein and β-carotene were with 0.37 and 0.24, respectively, rather weak. A similar acclimation of V-cycle pool size has been observed before (Thayer and Björkman 1990; Björkman and Demmig-Adams 1994). To obtain the strong change of a factor 4, it was necessary to grow the plants at low temperature in order to make the light more excessive (Huner et al. 1996). For a similar response at room temperature, it was necessary to grow A. thaliana at a PPFD of 800 µmol m−2 s−1 (data not shown).
Since the measurement of fluorescence excitation spectra is laborious and time consuming, the efficiency of fluorescence excitation was determined at only two narrow wavelength regions with a combination of UV-A-PAM (F470) and Mini-PAM (F660) portable fluorometers. The excitation ratio F 470/F 660 measured on the same leaves used for carotenoid extraction decreased linearly with increasing V-cycle pool size (Fig. 3). The regression coefficient of the relationship was not improved when instead of the V-cycle pool the sum of lutein and V-cycle pigment content was considered. A Run-test with p values of 0.1912 (V-cycle) and 0.1167 (V-cycle + Lut) confirmed the linear relationships.
Correlation between the V-cycle pool size (closed circles) or the sum of V-cycle and lutein (open circles), and the ratios of blue (F 470) and red (F 660) light-induced chlorophyll fluorescence. Individual data points are derived from pigment analysis and fluorescence measurements of leaves of the tt3 mutant acclimated to different irradiances
The influence of violaxanthin de-epoxidation on F 470/F 660 was tested in low and high light acclimated leaves of A. thaliana tt3 and F. sylvatica. Leaves were measured either after darkening or after 15 min illumination with strong light. Despite significant, and in the high light acclimated leaves strong, zeaxanthin formation, F 470/F 660 was not significantly affected by illumination (Fig. 4). In the high light acclimated leaves a small tendency to lower values was observed. However, this was not significant and its extent was small as compared to the difference of F 470/F 660 between the two light regimes for acclimation. In contrast, the epoxidation state of the V-cycle as shown by the numbers above the bars in Fig. 4 was significantly affected in all cases by the illumination of the leaves.
Ratios of blue (F 470) and red (F 660) light-induced chlorophyll fluorescence determined on leaves of the A. thaliana tt3 mutant and F. sylvatica, which were acclimated to low light (LL, 90–110 µmol m−2 s−1) or high light (HL, 420 µmol m−2 s−1) and shade or full sunlight, respectively. Before fluorescence measurements, leaf samples were either pre-darkened for 1 h (grey bars) or subsequently irradiated with white light (PPFD of 1200 µmol m−2 s−1) for 15 min (open bars). Above the bars, the epoxidation state of the leaf samples is indicated. Differences between F 470/F 660 were tested for each species separately with a Two-way ANOVA in combination with a Bonferroni post hoc test and were not significant for the light treatments. Means of n = 6, +SD
The fluorescence data show that in high light-acclimated plants blue light was less efficient to excite chlorophyll fluorescence than in low light-acclimated plants. Accordingly, blue light should also be less efficient in driving photosynthesis in high light-acclimated leaves. Therefore, the quantum yield of photosynthetic oxygen evolution was determined in the light limited range of photosynthesis. When photosynthesis was measured in red light, the quantum yields were high and remained largely constant at all growth irradiances (Fig. 5). Leaves from all light treatments showed the same F v/F m values of 0.77–0.78 except the leaves exposed to the highest PPFD of around 400 µmol m−2 s−1 where F v/F m was significantly lower at 0.75 (data not shown). The slope of the regression line for red light was not significantly different from zero (F test). Therefore, one can conclude that photoinhibition was, if at all, marginally affecting quantum yield only at the highest growth PPFD. In contrast, photosynthetic quantum yields declined under blue irradiation with increasing growth irradiance (Fig. 5). This suggests that an increased V-cycle pool size of high light acclimated leaves affected blue light-induced oxygen evolution.
The relationship between the relative quantum yields of oxygen evolution in blue and red light to fluorescence excitation efficiencies is shown in Fig. 6a. Leaves acclimated to low light and showing a high ratio of blue to red light-induced chlorophyll fluorescence had quantum yields of light limited photosynthesis under blue and red irradiation in a ratio of about 0.8. With decreasing fluorescence excitation efficiency by blue light, the relative quantum yield in blue light declined further. The relationship of the relative quantum yield in blue light to V-cycle contents is tested in Fig. 6b. As expected, low V-cycle pool sizes correlated with high quantum yields in blue light and the efficiency of blue light to induce oxygen evolution strongly declined when the V-cycle pool size was enlarged.
a Relationship between the chlorophyll fluorescence ratio of blue (F 470) and red (F 660) excitation light and the ratio of photosynthetic quantum yields of oxygen evolution under blue and red irradiation. b Relationship between the quantum yield ratio and the V+A+Z leaf content of tt3 plants. Data points in both panels are obtained from single leaves acclimated to varying light conditions
The acclimation of V-cycle pool size to growth irradiance is a widespread phenomenon. To test if the F 470/F 660 excitation ratio varies with light acclimation also in field grown plants, leaves of the herbaceous plant V. hirundinaria and the woody plants F. sylvatica and C. sanguinea were collected along a natural light gradient. As a measure for growth irradiance experienced by the leaves their leaf mass per area (LMA) was used. In all three species, a clear dependence of F 470/F 660 on LMA could be observed (Fig. 7). However, the slopes differed, which may be related to different leaf anatomies between the species. Especially F. sylvatica has more sclerenchymatic tissue than the other two species. For F. sylvatica and C. sanguinea also linear correlations between V-cycle pool size and F 470/F 660 were obtained (data not shown).
Effect of light acclimation determined as leaf mass per area (LMA) of leaves from Cornus sanguinea, Fagus sylvatica and Vincetoxicum hirundinaria on the chlorophyll fluorescence ratio of blue (F 470) and red (F 660) excitation light. Individual data from leaves collected at the Botanical Garden of Kiel University (C. sanguinea and F. sylvatica) or during a field trip in Southern Germany (V. hirundinaria). The regression coefficients are R 2 = 0.86, R 2 = 0.67 and R 2 = 0.72, respectively
Discussion
The major issue of the experiments was to explore, if the fluorescence excitation ratio F 470/F 660 has a potential to serve as an indicator of carotenoid content. This was clearly successful and the changes in F 470/F 660 induced by varying light acclimation seem to be largely caused by changes in the pool size of the V-cycle carotenoids. A strong correlation was obtained between relative fluorescence excitation and V-cycle pigment contents (Fig. 3) and this group of pigments may be mostly, but potentially not entirely, responsible for the observed changes in F 470/F 660. Among the carotenoids, the V-cycle pigments showed the strongest change over the irradiance range. Furthermore, it is known that they do not transfer energy to chlorophyll (Caffari et al. 2001). Also β-carotene displays a rather inefficient energy transfer of only 35 % in PSII (de Weerd et al. 2003), which contributes the largest part to Fo in C3 plants (Pfündel 1998), but we detected only minor changes in β-carotene contents (Fig. 2). Lutein showed a 20 % increase, which was much less than the increase of V-cycle pool size (Fig. 2). Although they displayed only minor changes, one cannot exclude that both β-carotene and lutein may have to some extent contributed to the observed changes in F 470/F 660. However, the carotenoid species which is most closely related to the observed changes in excitation efficiency is that of the V-cycle pigments.
The relative proportion of violaxanthin and zeaxanthin, i.e. the epoxidation state of the V-cycle, did not have a significant influence on F 470/F 660 (Fig. 4). This has first a practical advantage since it allows to detect only the V-cycle pool size irrespective of the recent irradiation regime and second it indicates that at least the de-epoxidizable violaxanthin is in vivo equally ineffective as a light harvesting pigment as zeaxanthin.
The strong correlation between the fluorescence index F 470/F 660 and the V-cycle pool size could be used to detect dynamic changes in the latter non-invasively by using the fluorescence excitation ratio. Although in this study, chlorophyll fluorescence was determined on detached leaves, the employed apparatus generally allows measurements with attached leaves non-destructively even under field conditions. This has a range of potential applications. The data in Fig. 7 suggest that F 470/F 660 might be a good indicator in other species besides A. thaliana as well, although species-specific calibration may be necessary. It is not yet clear, how light is sensed and transformed in the acclimation of the V-cycle, and a means to follow the light-induced pigment changes in attached leaves during the process of light acclimation could help in the elucidation of the underlying mechanisms. Non-destructive methods allow large phenotyping experiments for detection of mutants (Niyogi et al. 1997). The close correlation between irradiance and V-cycle pool size (Björkman and Demmig-Adams 1994; Niinemets et al. 2003) gives also the opportunity to deduce from leaves information about the light climate to which they were exposed during the preceding days. Simultaneous measurement of UV-A screening using the same technique provides additional information about the light climate during which the same leaves developed. Such measurements could be used for the quality control of green leafy vegetables, since high light grown leaves contain generally more antioxidants.
The photochemical reflectance index has originally been developed to follow the formation of zeaxanthin, thereby indicating reductions in light-use efficiency of photosynthesis (Gamon et al. 1990, 1992; Garbulsky et al. 2011). In the meantime it was shown that PRI also reflects V-cycle pool size (Gamon and Berry 2012; Wong and Gamon 2015a). Simultaneous measurement of PRI and F 470/F 660 may help extract the component responding to zeaxanthin formation from PRI.
Besides the methodical progress, our data have also some importance concerning the function of carotenoids as light harvesting pigments. In the reported experiments, more than 30 % reduction of the relative efficiency of fluorescence excitation at 470 nm upon acclimation of A. thaliana plants to high light at 9 °C (Fig. 3) was accompanied by a decline of similar magnitude in the relative quantum efficiency of photosynthetic oxygen evolution when measured with light in a broad blue range (ca. 400–480 nm) (Fig. 5).
Apparently, decreased photosynthetic quantum efficiency and decreased fluorescence excitation efficiency are related phenomena (Fig. 6a). A reduced quantum efficiency of leaf photosynthesis in the blue spectral region as compared to the red region is a phenomenon that is well known (McCree 1972a; Inada 1976; Terashima et al. 2009; Hogewoning et al. 2012). Based on photosynthetic action spectra in green algae, where light scattering effects have only a minor effect on absorption spectra, Haxo (1960) concluded that light absorbed by carotenoids is not entirely transferred to chlorophyll. Hogewoning et al. (2012) did a thorough analysis of the shape of the wavelength dependence of photosynthesis. They concluded that the low level in the blue region was not caused by a biased excitation of the two photosystems but rather by the presence of non-photosynthetic pigments, either carotenoids or flavonoids. We provide strong evidence that the responsible non-photosynthetic pigments are rather carotenoids than flavonoids. It is known that flavonoid accumulation is induced by high light and low temperature (Bilger et al. 2007; Agati et al. 2009; Løvdal et al. 2010), which occurred in our plants as well (Fig. II, Supplemental). However, the high light-induced spectral changes in fluorescence excitation in the wavelength range between 440 and 520 nm were independent of the presence of flavonoids as the excitation spectra were identical in the tt3 and tt5 genotypes (Fig. 1). Accordingly, above 440 nm all changes in the excitation spectra must have resulted from pigments different from polyphenolics and, hence, carotenoids are the only remaining candidates. The identity of carotenoids as the pigments leading to the observed changes in fluorescence excitation efficiency above 440 nm is further supported by the fluorescence excitation ratio spectra using tt5 LL leaves as reference and transformed into absorbance units (inset Fig. 1). These spectra show, identically for both mutants, a peak at 490 nm and resemble at wavelengths above 440 nm carotenoid spectra. Below 440 nm, apparently polyphenolics dominate the difference spectrum. The difference between this spectrum and the average V-cycle absorbance spectrum may be caused by spectral flattening (reduction of the central peak to a shoulder) and by the influence of the direct environment of the pigments in the thylakoid membrane (bathochromic shift of about 14 nm). A shift of 17 nm in difference spectra between V and Z determined in 100 % acetone or in isolated chloroplasts was observed by Yamamoto et al. (1972) and explained by a difference in the absorbance spectra of the pigments in acetone and in vivo. Croce et al. (1999) reported absorbance maxima for LHC-bound V and Z at 492 and 501 nm, which were shifted by 18-20 nm as compared to those obtained in 80 % acetone. Therefore, the spectral data provide evidence that carotenoids were responsible for the observed changes in fluorescence excitation efficiency. One might also conclude that these carotenoids are bound to proteins.
While a limited efficiency of carotenoids as light harvesting pigments has been proposed several times, we show here for the first time that the extent of this phenomenon can be affected by acclimation to irradiance. McCree (1972a) reported photosynthetic action spectra of a range of crop species grown in a growth chamber or in the field in Texas during spring and summer. Taking the average of his data one can calculate a 7 % reduction of quantum yield in field grown leaves as compared to growth chamber leaves at wavelengths between 450 and 500 nm. This difference is much smaller than that shown here, which reached almost 50 %. However, irradiance in McCree’s growth chamber was 100 ± 20 W m−2, which corresponds to about 470 ± 90 µmol m−2 s−1 (McCree 1972b). This irradiance is much higher than that used by us for low light grown plants. Hogewoning et al. (2012) used different growth light spectra to affect acclimation of the photosynthetic apparatus to shade light or to blue light. This may not have caused large variations in carotenoid contents.
To conclude, we are providing the first evidence that chlorophyll excitation and, hence, photosynthesis by blue light are affected by the dynamic light acclimation of the V-cycle pool size. We can unambiguously exclude a contribution of polyphenolics to the observed variations in fluorescence excitation in the blue spectral region. Since the excitation ratio F 470/F 660 can be measured on attached leaves even under field conditions it provides a simple means to detect V-cycle pigment contents and their dynamics non-intrusively in leaves.
References
Adams WW, Demmig-Adams B (1992) Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident light. Planta 186:390–398
Agati G, Stefano G, Biricolti S, Tattini M (2009) Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot 104:853–861
Bilger W, Björkman O, Thayer SS (1989) Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol 91:542–551
Bilger W, Fisahn J, Brummet W, Kossmann J, Willmitzer L (1995) Violaxanthin cycle pigment contents in potato and tobacco plants with genetically reduced photosynthetic capacity. Plant Physiol 108:1479–1486
Bilger W, Veit M, Schreiber L, Schreiber U (1997) Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant 101:754–763
Bilger W, Rolland M, Nybakken L (2007) UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem Photobiol Sci 6:190–195
Björkman O, Demmig-Adams B (1994) Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis, ecological studies, vol 100. Springer, Berlin, pp 17–70
Caffari S, Croce R, Breton J, Bassi R (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J Biol Chem 276:35924–35933
Caffari S, Tibiletti T, Jennings RC, Santabarbara S (2014) A comparison between plant photosystem I and photosystem II architecture and functioning. Curr Protein Pept Sci 15:296–331
Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I (2002) The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ 25:1663–1676
Croce R, Weiss S, Bassi R (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J Biol Chem 274:29613–29623
Davies BH (1976) Carotenoids. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments. Academic Press, London, pp 38–165
De Weerd FL, Dekker JP, van Grondelle P (2003) Dynamics of β-carotene-to-chlorophyll singlet energy transfer in the core of photosystem II. J Phys Chem B 107:6214–6220
Demmig B, Winter K, Krüger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves. Plant Physiol 84:218–224
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Phys 43:599–626
Demmig-Adams B, Adams WW, Hebr U, Neimanis S, Winter K, Krüger A, Czygan F-C, Bilger W, Björkman O (1990) Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol 92:293–301
Demmig-Adams B, Garab G, Adams W, Govindjee (2014) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Springer, Dordrecht
Gamon JA, Berry JA (2012) Facultative and constitutive pigment effects on the photochemical reflectance index (PRI) in sun and shade conifer needles. Isr J Plant Sci 60:85–95
Gamon JA, Field CB, Bilger W, Björkman O, Fredeen AL, Peñuelas J (1990) Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sun flower leaves and canopies. Oecologia 85:1–7
Gamon JA, Peñuelas J, Field CB (1992) A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens Environ 41:35–44
Garbulsky MF, Penuelas J, Gamon JA, Inoue Y, Filella I (2011) The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens Environ 115:281–297
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145:1506–1520
Haxo FT (1960) The wavelength dependence of photosynthesis and the role of accessory pigments. In: Allen MB (ed) Comparative Biochemistry of Photoreactive Systems. Symposia on comparative biology of the Kaiser Foundation Research Institute, vol 1. Academic Press, New York, pp 339–360
Hogewoning SW, Wientjes E, Douwstra P, Trouwborst G, van Ieperen W, Croce R, Harbinson J (2012) Photosynthetic quantum yield dynamics: from photosystems to leaves. Plant Cell 24:1921–1935
Huner NPA, Maxwell DP, Gray GR, Savitch LV, Krol M, Ivanov AG, Falk S (1996) Sensing environmental temperature change through imbalances between energy supply and energy consumption: redox state of photosystem II. Physiol Plant 98:358–364
Inada K (1976) Action spectra for photosynthesis in higher plants. Plant Cell Physiol 17:355–365
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1872:182–193
Kirakosyan A, Kaufman P, Warber S, Zick S, Aaronson K, Bolling S, Chang SC (2004) Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol Plant 121:182–186
Li J, Ou-Lee T-M, Raba R, Amundson R, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5:171–179
Lichtenthaler H, Pfister K (1978) Praktikum der Photosynthese. Quelle & Meyer, Heidelberg
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light harvesting complex at 2.72 Å resolution. Nature 428:287–292
Logan BA, Barker DH, Demmig-Adams B, Adams WW (1996) Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant Cell Environ 19:1083–1090
Løvdal T, Olsen KM, Slimestad R, Verheul M, Lillo C (2010) Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 71:605–613
Markstädter C, Queck I, Baumeister J, Riederer M, Schreiber U, Bilger W (2001) Epidermal transmittance of leaves of Vicia faba for UV radiation as determined by two different methods. Photosynth Res 67:17–25
Martiskainen J, Kananavičius R, Linnanto J, Lehtivuori H, Keränen M, Aumanen V, Tkachenko N, Korppi-Tommola J (2011) Excitation energy transfer in the LHC-II trimer: from carotenoids to chlorophylls in space and time. Photosynth Res 107:195–207
McCree KJ (1972a) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9:191–216
McCree KJ (1972b) Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric Meteorol 10:443–453
Niinemets Ü, Kollist H, Garcia-Plazaola JI, Hernandez A, Becerill JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ 26:1787–1801
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Phys Plant Mol Biol 50:333–359
Niyogi KK, Bjorkman O, Grossman AR (1997) Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell 9:1369–1380
Olsen KM, Slimestad R, Lea US, Brede C, Løvdal T, Ruoff P, Verheul M, Lillo C (2009) Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ 32:286–299
Pfündel E (1998) Estimating the contribution of photosystem I to total leaf chlorophyll fluorescence. Photosynth Res 56:185–195
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Sheahan JJ (1996) Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae). Am J Bot 83:679–686
Siefermann-Harms D (1987) The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant 69:561–568
Sims DA, Gamon JA (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Rem Sens Environ 81:337–354
Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R (2009) Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol 50:684–697
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Wong CYS, Gamon JA (2015a) Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytol 206:187–195
Wong CYS, Gamon JA (2015b) The photochemical reflectance index provides an optical indicator of spring photosynthetic activation in evergreen conifers. New Phytol 206:196–208
Yamamoto HY, Wang YY, Kamite L (1972) An ascorbate-induced absorbance change in chloroplasts from violaxanthin de-epoxidation. Plant Physiol 49:224–228
Acknowledgments
Funding by the German Federal Ministry of Education and Research as part of the WeGa research network (Project-No: 0315542B) is gratefully acknowledged. We thank Andrea Behrens, Kathrin Fischer, Lili Beckmann and Christian Pawlitzki for the measurements with the field grown plants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nichelmann, L., Schulze, M., Herppich, W.B. et al. A simple indicator for non-destructive estimation of the violaxanthin cycle pigment content in leaves. Photosynth Res 128, 183–193 (2016). https://doi.org/10.1007/s11120-016-0218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0218-1