Abstract
Main conclusion
Expression of GmPSKγ1 , a novel PSK-encoding gene from soybean, increases seed size and yield in transgenic plants by promoting cell expansion.
Phytosulfokine-α (PSK-α), a sulfated pentapeptide hormone with the sequence YIYTQ, plays important roles in many aspects of plant growth and development. In this study, we identified a pair of putative precursor genes in soybean, GmPSKγ1 and -2, encoding a PSK-like peptide: PSK-γ. Similar to PSK-α in amino acid composition, the sequence of PSK-γ is YVYTQ, and the tyrosines undergo sulfonylation. Treatment of Arabidopsis seedlings with synthetic sulfated PSK-γ significantly enhanced root elongation, indicating that PSK-γ might be a functional analog of PSK-α. Expression pattern analysis revealed that the two GmPSKγ genes, especially GmPSKγ1, are primarily expressed in developing soybean seeds. Heterologous expression of GmPSKγ1 under the control of a seed-specific promoter markedly increased seed size and weight in Arabidopsis, and this promoting effect of PSK-γ on seed growth was further confirmed in transgenic tobacco constitutively expressing GmPSKγ1. Cytological analysis of transgenic Arabidopsis seeds revealed that PSK-γ promotes seed growth by inducing embryo cell expansion. In addition, expression analysis of downstream candidate genes suggested that PSK-γ signaling might regulate cell wall loosening to promote cell expansion in Arabidopsis seeds. Overall, our results shed light on the mechanism by which PSK-γ promotes seed growth, paving the way for the use of this new peptide for biotechnological improvement of crop seed/grain size and yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed growth is of great importance for agriculture productivity. In angiosperms, seed formation begins with a double fertilization event, generating a diploid embryo and a triploid endosperm (Chaudhury et al. 2001), and a protective seed coat originating from the maternal integument surrounds the embryo and endosperm (Figueiredo and Kohler 2014). Therefore, the coordinated growth and development of these three structures is required for the formation of normal-sized seed, which is one of the most important traits of seed yield (Li and Li 2016). Over the past decades, numerous signaling pathways and key regulatory factors have been identified that control seed size by regulating cell division and expansion in the endosperm as well as growth of the seed coat (reviewed by Orozco-Arroyo et al. 2015; Li and Li 2016). Conversely, knowledge of the mechanism underlying embryo growth regulation during seed development is rather scarce.
In the model species Arabidopsis thaliana, seed development has been divided into three stages: the early embryogenesis stage (0–6 days after pollination, DAP), the maturation stage (7–16 DAP), and the late maturation stage (17–20 DAP) (Baud et al. 2002). During the second stage, embryo cells undergo a period of expansion, a process that contributes significantly to the embryo’s volume and final seed size (Baud et al. 2002; Orozco-Arroyo et al. 2015). Nonetheless, the regulatory mechanism for embryonic cell growth is poorly understood and requires further investigation.
Phytosulfokine-α (PSK-α) is a disulfated pentapeptide of the sequence Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln that has been identified as a new peptide hormone functioning in many aspects of plant growth and development (Sauter 2015). A truncated disulfated tetrapeptide with the sequence Tyr(SO3H)-Ile-Tyr(SO3H)-Thr (named PSK-β) was also found in the cell culture medium from which PSK-α was initially isolated (Matsubayashi and Sakagami 1996). However, PSK-β was eventually classified as a degradation product of PSK-α (Yang et al. 1999), and only weak activity was detected (Matsubayashi et al. 1996). PSK-α is synthesized from prepropeptides of 80–120 amino acids that contain a putative signal peptide sequence at the N-terminus and the five-amino acid PSK-α motif near the C-terminus (Sauter 2015). PSK-α peptides mature through precursor processing by tyrosylprotein sulfotransferase (TPST) in the trans-Golgi (Komori et al. 2009) and subsequently by proteolytic enzyme activity in the apoplast (Srivastava et al. 2008). PSK-α precursors are encoded by small gene families widely distributed in the plant kingdom, including Asparagus officinalis (Matsubayashi and Sakagami 1996), A. thaliana (Matsubayashi et al. 2006), Oryza sativa (Yang et al. 1999; Lorbiecke and Sauter 2002), Zea mays (Lorbiecke et al. 2005), and Lotus japonicas (Wang et al. 2015a).
PSK-α was originally identified as a key effector inducing division in cell suspensions cultured at low density (Matsubayashi and Sakagami 1996; Matsubayashi et al. 1997). Recently, Heyman et al. (2013) reported that AtPSK5, transcriptionally activated by ERF115, regulates quiescent center (QC) cell division, therefore, maintaining stem cell niche longevity in root. Moreover, It has also been found that PSK-α plays important roles in cell expansion induction. Application of the PSK-α peptide or overexpression of the PSK-α precursor gene significantly enhanced root, hypocotyl, and leaf growth in Arabidopsis by promoting cell expansion (Kutschmar et al. 2009; Stührwohldt et al. 2011; Yu et al. 2016). It has also been reported that the cell growth induced by PSK-α might be attributed to enhanced cell wall loosening (Yu et al. 2016) and/or protoplast expansion (Stührwohldt et al. 2011). In addition, PSK-α signaling has promoting effects on multiple developmental events, including somatic embryogenesis (Hanai et al. 2000; Igasaki et al. 2003), tracheary element differentiation (Matsubayashi et al. 1999), cotton fiber elongation (Han et al. 2014), in vitro regeneration competence in recalcitrant legumes (Ochatt et al. 2018), and legume nodulation (Wang et al. 2015a), and regulates immune responses against biotrophic and necrotrophic pathogens in an antagonistic manner (Igarashi et al. 2012; Mosher et al. 2013).
PSK-α is perceived by PSKRs, members of a family of membrane-localized leucine-rich repeat (LRR) receptor kinases (Matsubayashi et al. 2002, 2006). The intracellular kinase domain of a PSKR, which is modified by phosphorylation (Kaufmann et al. 2017) and activated by Ca2+/calmodulin binding (Hartmann et al. 2014), possesses overlapping serine/threonine kinase and guanylate cyclase activity (Kwezi et al. 2011; Hartmann et al. 2014). Recent structural data reveal that PSK-α binding stabilizes PSKR heterodimerization with SERK proteins, resulting in allosteric activation of PSKR (Wang et al. 2015b). Ladwig et al. (2015) reported that a plasma membrane-localized module including CNGC17, H+-ATPase, and BAK1 interacts with PSKR to transduce PSK-α signaling. Despite increasing knowledge regarding PSKRs, the signaling cascade involved, especially key factors downstream of the receptor, remains largely unknown.
In this study, we identified a novel type of PSK, PSK-γ, from soybean. With a difference in only one amino acid, PSK-γ exhibits a function similar to that of PSK-α in promoting plant growth. Heterologous expression of one precursor gene, GmPSKγ1, which is primarily expressed in soybean seeds, remarkably increased seed size and weight in both transgenic Arabidopsis and tobacco through embryo cell expansion induction. Further investigation indicates that PSK-γ signaling might regulate cell wall loosening to promote cellular expansion in the seed. These results show that PSK-γ is a new positive regulator in seed growth and can be exploited to improve crop seed/grain size and yield.
Materials and methods
Plant materials and growth conditions
Arabidopsis [Arabidopsis thaliana (L.) Heynh.] ecotype Columbia (Col-0; ABRC, Columbus, OH, USA) and tobacco (Nicotiana tabacum L.) cultivar K326 (Biorun Biotechnology, Wuhan, Hubei, China) were used in this study. Seeds of Arabidopsis and tobacco were surface sterilized for 15–20 min in 3% (v/v) sodium hypochlorite solution containing 0.2% Tween-20 and then rinsed five times with sterile water. After stratification at 4 °C for 2 days, the seeds were germinated on Murashige and Skoog (MS) medium containing 1% (w/v) sucrose and 0.7% (w/v) agar. Seedlings were transplanted to a 3:1 (w/w) vermiculite:perlite mixture and cultured in environmental chambers under a light/dark cycle of 16/8 h. Temperatures were maintained at 23 °C and 26 °C for the growth of Arabidopsis and tobacco, respectively.
Peptide treatment
The peptides used in this study, including PSK-γ (YSO3VY SO3TQ), desulfated PSK-γ (YVYTQ), and randomly arranged pentapeptide (TYQYV), were synthesized by Chinese Peptide Company (Hangzhou, China). Each peptide was dissolved in sterilized ddH2O at 1 mM, which was used as a stock solution. Stratified Arabidopsis seeds were sown on MS agar plates containing 1 μM of each peptide. After 10 days of growth on the plates in the vertical position, seedlings were photographed to measure root length using Image J (National Institute of Health, Bethesda, MD, USA).
Gene cloning, vector construction and transformation
The full-length and truncated GmPSKγ1 CDS sequences were PCR amplified using cDNA reverse transcribed from RNA of soybean developing seeds (Glycine max Williams 82; seeds kindly provided by Professor Oliver Yu, Donald Danforth Plant Science Center, St. Louis, MO, USA); the primers used are listed in Suppl. Table S1. The obtained CDS fragments were cloned into the intermediate vector pRNAi downstream of the enhanced cauliflower mosaic virus (CaMV) 35S promoter using the restriction sites Nco I and BamH I. The resulting 35Sp:GmPSKγ1:OCSter and 35Sp:GmPSKγ1tc:OCSter cassettes were subsequently ligated into the binary vector pCambia 2300 using the restriction sites Kpn I and Sac I. For construction of the seed-specific expression plasmid, the promoter region (~ 2000 bp) of AtOLEO4 was PCR amplified from Arabidopsis (Col-0) genomic DNA using the primers listed in Suppl. Table S1. The plasmids were completed by replacing the 35S promoter with the AtOLEO4 promoter in 35Sp:GmPSKγ1 and 35Sp:GmPSKγ1tc (pCambia 2300) using Kpn I and Nco I. The seed-specific constructs were transformed into Arabidopsis by Agrobacterium-mediated transformation using the floral dip method that was performed by Pujie biotechnology company (Shanghai, China), and the 35Sp:GmPSKγ1 construct was transformed into tobacco by Agrobacterium-mediated transformation using the leaf disc method.
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was employed to determine the expression patterns of GmPSK genes and transcript levels of GmPSKγ1 in transgenic plants. Samples were homogenated in liquid nitrogen to extract total RNA using an RNAprep pure Plant kit (Tiangen, Beijing, China), and 1 μg RNA of each sample was reverse transcribed using a PrimeScript RT reagent kit (Perfect RealTime) (TaKaRa, Dalian, China). Quantitative RT-PCR was carried out with cDNA and gene-specific primers (Suppl. Table S1); the Hieff qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) and a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) were used. The PCR procedure and data analysis were performed in accordance with methods established in a previous study (Yu et al. 2014).
Paraffin-embedded sections
Arabidopsis seeds at 10 DAP were dissected from siliques using needles and fixed in FAA (50% ethanol, 5% formaldehyde, 10% acetic acid, by vol.) solution overnight. The fixed seeds were dehydrated in a graded ethanol series and embedded in paraffin. Sections were prepared at 10 μm thickness using a Leica RM2235 rotary microtome, stained with 0.05% toluidine blue, and examined and photographed under bright-field microscopy (Zeiss Axio Scope A1).
Results
Sequence, phylogeny, and expression pattern of GmPSK precursors
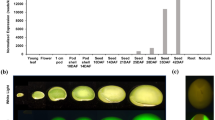
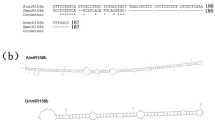
In a search of the genome database of Glycine max Williams 82 (www.phytozome.net/search.php) using AtPSK sequences as queries, we identified 16 putative GmPSK genes encoding PSK precursors. Among them, 14 harbor a single PSK-α pentapeptide motif near the C-terminus; these were designated GmPSKα1-14 (Fig. 1a). With the exception of GmPSKα4 (170 aa in length), these GmPSKα precursor proteins have a typical length of ~ 80 aa and contain the conserved PSK signature motif C(x)6–12CxxRR(x)3–4AHxDYIYTQ (Sauter 2015) (Fig. 1a). The other two putative GmPSK precursors consist of 94 amino acids, a typical length of PSK precursors, but exhibit less conservation in the PSK signature motif. Obvious variations include substitution of the isoleucine in YIYTQ by valine and replacement of the double arginine by lysine and threonine (Fig. 1a). These two atypical PSK precursors are highly conserved in protein sequence, sharing 91% amino acid identity, and might have originated from a gene duplication event during evolution. Because the name PSK-β has been assigned to the tetrapeptide YIYT (Matsubayashi and Sakagami 1996), we named the predicted YVYTQ pentapeptide with tyrosine-sulfonylation PSK-γ and accordingly, its precursor proteins in soybean GmPSKγ1 and -2 (Fig. 1a).
GmPSKγ1 and -2 are predicted to encode a novel type of phytosulfokine precursor protein. a Protein sequence alignment of AtPSKs and predicted GmPSKs. The full-length amino acid sequences of these proteins, with the exception of GmPSKα4 (indicated by #), were aligned using BioEdit software. GmPSKα4 is much longer than is a typical PSK precursor protein, and only the relatively conserved COOH-terminal portion was aligned. Stars indicate consensus amino acids; circles and triangle indicate the di-basic amino acids and isoleucine in the PSK signature motif, respectively. b Phylogenetic tree of AtPSKs and predicted GmPSKs. GmPSKγs are close to AtPSK1 in phylogeny. The tree was constructed using MEGA6 with the neighbor-joining method, and bootstrap values from 1000 replications were included. c Quantitative RT-PCR analyses of GmPSKγ1 and -2 expression in various soybean tissues. Values are the mean ± SD of three independent biological replicates normalized against the reference gene GmEF1β
Phylogenetic relationship analysis revealed that although there are additional members, the GmPSK family comprises orthologs of the reported Arabidopsis PSK family. Indeed, both GmPSKγ proteins are likely orthologs of AtPSK1 (Fig. 1b). Quantitative RT-PCR was employed to examine the expression patterns of GmPSK genes in various tissues of G. max. The results showed that the 14 GmPSKα genes were expressed in a tissue-specific or ubiquitous pattern (Suppl. Fig. S1); in contrast, GmPSKγ1 was dominantly expressed in developing seeds, and GmPSKγ2 was expressed in seeds, pods (without seeds), and roots, with the highest expression level detected in seeds (Fig. 1c). These results indicate that GmPSKγ genes might encode a novel type of phytosulfokine that mainly functions in seed development.
The PSK-γ peptide promotes Arabidopsis root growth
To test the physiological activities of PSK-γ, we treated wild-type Arabidopsis seedlings with 1 μM sulfated PSK-γ peptide and then observed root growth, which is reported as a sensitive system for evaluating the activity of PSK-α (Kutschmar et al. 2009). At 10 days of age, PSK-γ treatment caused an approx. 31% increase in primary root length compared to seedlings treated with mock or 1 μM randomly arranged pentapeptide having the same amino acid composition as PSK-γ (Fig. 2a, b, d, e). To assess the effect of the sulfonylation modification on the activity of PSK-γ, we also treated Arabidopsis seedlings with 1 μM desulfated PSK-γ (dsPSK-γ), and the results showed that dsPSK-γ treatment caused an 11% increase in primary root length compared to controls (Fig. 2a, c, d, e), indicating that dsPSK-γ retains part of PSK-γ activity. These findings demonstrate that the PSK-γ peptide has a promoting effect on root growth, which has also been reported for PSK-α (Kutschmar et al. 2009).
Treatment with the PSK-γ peptide promotes root growth in Arabidopsis. Ten-day-old Arabidopsis (Col-0) seedlings grown on vertical MS agar plates containing mock (a), 1 μM PSK-γ (b), 1 μM desulfated PSK-γ (dsPSK-γ, c), and 1 μM randomly arranged pentapeptide with the same amino acid composition as dsPSK-γ (random, d). Bars = 1 cm. e Lengths of the Arabidopsis primary roots shown in a–d. Values are the mean ± SD from three independent biological replicates with a total of ~ 40 seedlings per treatment. Different letters indicate significantly different values, P < 0.01, ANOVA test
Expression of GmPSKγ1 promotes seed growth in Arabidopsis
Based on the findings that GmPSKγ1 and -2, especially GmPSKγ1, are primarily expressed in soybean seeds, it is conceivable that PSK-γ plays a role in seed growth and development. To test this assumption, a seed-specific expression plasmid was constructed and transformed into Arabidopsis due to its high transformation efficiency compared to soybean; in this case, GmPSKγ1 was expressed under control of the promoter of AtOLEO4 (At3g27670) (Op:γ1, Fig. 3a), a seed/embryo-specific gene reported by Baud et al. (2009) and verified by qRT-PCR expression data and microarray information from PlaNet (Suppl. Fig. S2a and b). A seed-specific expression plasmid of a truncated form of GmPSKγ1 devoid of the peptide-encoding sequence was also constructed (Op:γ1tc, Fig. 3a) and transformed as a negative control. More than 20 independent transgenic lines were generated for each construct. Op:γ1 lines 2 and 11, and Op:γ1tc line 10 showed relatively high transcript abundance in developing seeds (Fig. 3b) and were chosen for further analyses. Phenotypical observation of dry mature seeds revealed that GmPSKγ1 expression in Arabidopsis notably increased seed size (Fig. 3c). Specifically, the seed length of the GmPSKγ1-expressing lines increased by 15–20% compared to that of wild type and the truncated control, and the seed width increased by 15–17% (Table 1). Accordingly, the weight of the dry seeds increased by 20–31% in GmPSKγ1-expressing lines compared to controls (Table 1), while seed number per silique, another key factor determining yield, was not influenced by GmPSKγ1 expression (Table 1). As expected, seed-specific expression of GmPSKγ1 did not influence the growth of vegetative organs, such as leaves and roots (Suppl. Fig. S3), demonstrating that GmPSKγ1 expression was the direct factor enhancing seed growth.
Seed-specific expression of GmPSKγ1 promotes seed growth in Arabidopsis. a Schematic representation of GmPSKγ1 expression constructs. The GmPSKγ1 CDS, in both full-length and truncated forms, was constructed under control of the seed-specific AtOLEO4 promoter. b Semi-quantitative RT-PCR analyses of GmPSKγ1 transcript abundances in developing seeds of the indicated transgenic Arabidopsis lines. Expression of AtActin2 was used as an internal control. c Comparison of dry mature seeds of wild-type and the indicated transgenic Arabidopsis lines. Bars = 1 mm
Expression of GmPSKγ1 promotes embryo cell growth in Arabidopsis seeds
In a mature Arabidopsis seed, the embryo occupies the entire space between the integuments and is thus one of the major determinants of seed size. Embryos of seeds at 10 DAP were dissected and observed under a stereomicroscope, and the embryos of GmPSKγ1-expressing seeds appeared to be markedly larger than were those of wild-type and truncated control seeds (Fig. 4a, b). To reveal the cellular basis underlying the enlarged embryo, seeds were cross sectioned at 10 DAP, a time during which embryo cells undergo expansion and differentiation (Baud et al. 2002). The results showed that expression of GmPSKγ1 significantly promoted cell expansion in embryos (Fig. 4c). In contrast to controls, the transverse areas of radical cells, cotyledonal spongy cells, and palisade cells in GmPSKγ1-expressing seeds increased by approximately 42, 52, and 29%, respectively (Fig. 4d–f). Therefore, seed-specific expression of GmPSKγ1 promotes embryo cell growth, leading to seed size and weight enhancement.
Seed-specific expression of GmPSKγ1 promotes embryonic cell growth in Arabidopsis seeds. a Comparison of embryos dissected from the developing seeds (10 days after pollination, DAP) of wild-type and the indicated transgenic Arabidopsis lines. Bars = 0.5 mm. b Measurement of the length of embryonic cotyledons at 10 DAP. c Transverse sections of developing seeds at 10 DAP. The paraffin-embedded sections were stained with toluidine blue. Bars = 100 μm. Measurement of transverse areas of radical cells (d) and spongy (e) and palisade (f) cells in cotyledons at 10 DAP. Values are the mean ± SD from three independent biological replicates, with a total of 212–244 cells measured for each cell type. Different letters indicate significantly different values, P < 0.01, ANOVA test
Constitutive expression of GmPSKγ1 promotes growth of seeds and vegetative organs in tobacco
To investigate whether the effect of GmPSKγ1 on seed growth is conserved in the plant kingdom, we constructed a constitutive expression plasmid comprising GmPSKγ1 driven by the enhanced CaMV 35S promoter (Fig. 5a) and transformed it into Nicotiana tabacum. Twenty-three independent transgenic lines were generated, among which lines 7, 11, and 22 exhibited high transcript levels of GmPSKγ1 in developing seeds (Fig. 5b). Phenotypic observation of dry mature seeds revealed that GmPSKγ1 expression in tobacco remarkably increased seed size (Fig. 5c): the projected areas of GmPSKγ1-expressing seeds were 12–30% larger than those of wild-type seeds (Fig. 5d). Moreover, the weight of the dry seeds increased by 7–29% (Fig. 5e). Therefore, the effect of PSK-γ on seed growth is conserved between Arabidopsis and tobacco, indicating that PSK-γ function might be conserved in plant species, at least in dicots.
Constitutive expression of GmPSKγ1 promotes growth of seeds and vegetative organs in tobacco. a Schematic representation of the GmPSKγ1 constitutive expression construct. Full-length GmPSKγ1 CDS was constructed under control of the enhanced CaMV 35S promoter. b Quantitative RT-PCR analysis of GmPSKγ1 transcript levels in developing seeds of GmPSKγ1-overexpressing tobacco lines. Values are the mean ± SD of three independent biological replicates normalized against the reference gene NtUBC2. c Comparison of dry mature seeds of wild-type and the indicated transgenic tobacco lines. Bar: 1 mm. d Measurement of the projected areas of dry mature seeds shown in (c). n = 144–152. e 100 seed weight of the mature dry seeds shown in (c). n = 3 biological replicates. f One-month-old wild-type and GmPSKγ1-overexpressing tobacco plants. Bar = 2 cm. Leaf (the largest one) areas (g) and fresh weight of above-ground parts (h) of the 1-month-old tobacco plants shown in (f). n = 3 biological replicates. i Seventeen-day-old wild-type and GmPSKγ1-overexpressing tobacco seedlings grown on vertical MS-agar plates. Bars = 1 cm. j Primary root length of the tobacco seedlings shown in (i), n = 3 biological replicates. Different letters indicate significantly different values, P < 0.01, ANOVA test
Due to the constitutive 35S promoter, GmPSKγ1 was also highly expressed in vegetative organs such as leaves (Suppl. Fig. S4), significantly promoting tobacco leaf growth (Fig. 5f and g) and increasing plant weight (Fig. 5h). Additionally, GmPSKγ1-expressing tobacco seedlings developed longer roots than did wild-type seedlings (Fig. 5i and j). These data demonstrate that similar to PSK-α, PSK-γ can also promote plant vegetative growth.
GmPSKγ1 expression in Arabidopsis seeds regulates expression of genes associated with cell growth
To investigate the mechanism by which GmPSKγ1 promotes seed growth, we performed RNA sequencing (RNA-Seq) to identify differentially expressed genes (DEGs) in 10 DAP Arabidopsis seeds between Op:γ1 and Op:γ1tc transgenic lines. The RNA-Seq raw data were submitted to the National Center for Biotechnology Information Gene Expression Omnibus under the series entry GSE121567. However, due to lack of biological replicates, these data can only serve as a guidance to understand the regulation mechanism of PSKγ1; therefore, qRT-PCR with biological replicates was performed to verify the expression of the candidate DEGs. The results showed that a number of genes with putative functions in plant cell growth were differentially expressed in GmPSKγ1-expressing seeds (10 DAP) relative to controls. Particularly, two cell expansion-promoting genes, Cel2 (At1G02800, encoding an endo-1,4-β-glucanase) and SAUR74 (At3G12955, encoding a SAUR-like auxin-responsive protein), were significantly upregulated (Fig. 6a). In contrast, several cell expansion-inhibiting genes were downregulated, including peroxidase-encoding genes POD11 (At1G68850), -49 (At4G36430), and -64 (At5G42180) and ethylene-responsive factor family genes ERF13 (At1G77640), -53 (At2G20880), and -71 (At2G47520) (Fig. 6b).
Qantitative RT-PCR analysis of expression of upregulated (a) and downregulated (b) genes associated with cell growth in 10 DAP Arabidopsis seeds. AtActin2 was used as an internal control. Values represent the mean ± SD from three lines of each genotype with two biological replicates for each line. *P < 0.05, **P < 0.01, Student’s t test
Discussion
PSK-γ is a novel type of phytosulfokine from soybean
Over the past 2 decades, PSK-α precursors have been extensively identified and studied in multiple plant species. Although weak sequence conservation is observed among these precursor proteins, the PSK-α pentapeptide motif is highly conserved (Lorbiecke and Sauter 2002). However, it is still unknown whether any novel PSK peptide or PSK-α isoform exists in the plant kingdom. In this study, we identified a pair of soybean genes that encode a new type of PSK: PSK-γ. Both GmPSKγ precursors have a typical amino acid length and a PSK signature motif, which is, however, less conserved compared to PSK-α. The isoleucine in PSK-α is substituted by a valine in PSK-γ. Regardless, given that isoleucine and valine are similar in chemical characteristics, it is conceivable that the two types of PSK peptides have similar biological functions. As expected, treatment of Arabidopsis seedlings with synthetic sulfated PSK-γ markedly promoted root elongation, a conserved function also reported for PSK-α (Kutschmar et al. 2009). Moreover, similar to PSK-α, sulfonylation of the tyrosines in PSK-γ is important for its full activity. Another sequence variation between PSK-γ and PSK-α is that the di-basic amino acids upstream from the PSK-α pentapeptide are replaced by one basic amino acid (lysine) and a threonine in PSK-γ. Although di-basic residues are speculated to be required for proteolytic processing of precursors (Srivastava et al. 2008), a single basic residue in the case of OsPSK, a well-characterized rice PSK-α, is sufficient for maturation (Yang et al. 1999), indicating that maturation of PSK-γ from its precursors might occur with a less-conserved PSK signature motif. Based on their phylogenetic relationship, GmPSKγs are orthologs of AtPSK1. These data led us to hypothesize that PSK-γ evolved from a PSK-α ancestor present in the soybean genome and retained conserved activities.
PSK-γ promotes seed growth by enhancing embryo cell expansion
Although the seed development process has been extensively studied in Arabidopsis, little is known about the embryo enlargement process during seed maturation. In this study, we found that GmPSKγ genes, especially GmPSKγ1, are primarily expressed in developing soybean seeds, indicating potential roles in seed development. By expressing GmPSKγ1 under the control of a seed/embryo-specific promoter, we found that both the seed size and weight of transgenic Arabidopsis lines to be notably increased. This promoting effect of PSK-γ on seed size and weight was further confirmed by constitutive expression of GmPSKγ1 in tobacco. Moreover, detailed analysis of cytological alterations in transgenic Arabidopsis seeds at 10 DAP (middle maturation stage) revealed that PSK-γ significantly promoted cell expansion in embryo tissues, including cotyledons and radicles, resulting in an enlarged embryo. This observation is reminiscent of the function of PSK-α in promoting cell elongation and expansion in various tissues (Kutschmar et al. 2009; Stührwohldt et al. 2011; Yu et al. 2016). On the basis of these findings, it is reasonable to presume that although slightly different in amino acid composition, PSK-γ and PSK-α share similar biochemical features and conserved functions in promoting plant cell growth.
PSK-γ promotes embryo cell expansion possibly by loosening the cell wall
The growth of plant cells requires two basic cellular actions: cell wall loosening mediated by cell wall-remodeling enzymes and protoplast expansion driven by intracellular turgor pressure. Our previous data showed that overexpression of AtPSK4 in Arabidopsis promotes cell expansion by affecting cell wall development (Yu et al. 2016), and Stührwohldt et al. (2011) also reported that PSK-α peptide application induced protoplast expansion. These findings indicate that PSK-α regulates cell growth with dual functions. To investigate the molecular mechanism of PSK-γ regulation of embryo cell expansion, qRT-PCR analysis was performed to identify DEGs in developing seeds between GmPSKγ1-expressing and control Arabidopsis lines. It was shown that a number of genes associated with cell expansion to be differentially regulated, among which, the endo-1,4-β-glucanase-encoding gene Cel2 was significantly upregulated. Endo-1,4-β-glucanases are important factors responsible for promoting cell growth by loosening the cell wall structure (Cosgrove 2005; Yu et al. 2013); therefore, increase in Cel2’s expression potentially promotes cell expansion. Moreover, two peroxidase family genes, POD49 and -64, were transcriptionally repressed in GmPSKγ1-expressing seeds. It is known that peroxidases act as inhibitors of cell expansion by enhancing cross-linking and deposition of cell wall materials (Brownleader et al. 2000; Raggi et al. 2015). Thus, repression of these genes by PSK-γ signaling facilitates cell expansion. Although more evidence is required, our data indicate that PSK-γ might regulate embryo cell expansion by loosening cell wall.
In conclusion, our study demonstrates that PSK-γ, a novel PSK from soybean, can promote seed growth and weight by inducing embryo cell expansion. The findings highlight the potential use of PSK-γ signaling in molecular breeding to generate crops with larger seed/grain size and higher yield.
Author contribution statement
LY and LL conceived and designed the research. LY and YL performed the experiments. LY, YL, SZ, JY and EW analyzed the data. LY and LL wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- DAP:
-
Days after pollination
- PSK:
-
Phytosulfokine
References
Baud S, Boutin J, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40:151–160
Baud S, Wuilleme S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60:933–947
Brownleader MD, Hopkins J, Mobasheri A, Dey PM, Jackson P, Trevan M (2000) Role of extensin peroxidase in tomato (Lycopersicon esculentum Mill.) seedling growth. Planta 210:668–676
Chaudhury AM, Koltunow A, Payne T, Luo M, Tucker MR, Dennis ES, Peacock WJ (2001) Control of early seed development. Annu Rev Cell Dev Biol 17:677–699
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Figueiredo DD, Kohler C (2014) Signalling events regulating seed coat development. Biochem Soc Trans 42:358–363
Han J, Tan J, Tu L, Zhang X (2014) A peptide hormone gene, GhPSK promotes fibre elongation and contributes to longer and finer cotton fibre. Plant Biotechnol J 12:861–871
Hanai H, Matsuno T, Yamamoto M, Matsubayashi Y, Kobayashi T, Kamada H, Sakagami Y (2000) A secreted peptide growth factor, phytosulfokine, acting as a stimulatory factor of carrot somatic embryo formation. Plant Cell Physiol 41:27–32
Hartmann J, Fischer C, Dietrich P, Sauter M (2014) Kinase activity and calmodulin binding are essential for growth signaling by the phytosulfokine receptor PSKR1. Plant J 78:192–202
Heyman J, Cools T, Vandenbussche F, Heyndrickx KS, Van Leene J, Vercauteren I, Vanderauwera S, Vandepoele K, De Jaeger G, Van Der Straeten D, De Veylder L (2013) ERF115 controls root quiescent center cell division and stem cell replenishment. Science 342:860–863
Igarashi D, Tsuda K, Katagiri F (2012) The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J 71:194–204
Igasaki T, Akashi N, Ujino-Ihara T, Matsubayashi Y, Sakagami Y, Shinohara K (2003) Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol 44:1412–1416
Kaufmann C, Motzkus M, Sauter M (2017) Phosphorylation of the phytosulfokine peptide receptor PSKR1 controls receptor activity. J Exp Bot 68:1411–1423
Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y (2009) Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci USA 106:15067–15072
Kutschmar A, Rzewuski G, Stührwohldt N, Beemster GT, Inzé D, Sauter M (2009) PSK-alpha promotes root growth in Arabidopsis. New Phytol 181:820–831
Kwezi L, Ruzvidzo O, Wheeler JI, Govender K, Iacuone S, Thompson PE, Gehring C, Irving HR (2011) The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependent signaling in plants. J Biol Chem 286:22580–22588
Ladwig F, Dahlke RI, Stührwohldt N, Hartmann J, Harter K, Sauter M (2015) Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 27:1718–1729
Li N, Li Y (2016) Signaling pathways of seed size control in plants. Curr Opin Plant Biol 33:23–32
Lorbiecke R, Sauter M (2002) Comparative analysis of PSK peptide growth factor precursor homologs. Plant Sci 163:321–332
Lorbiecke R, Steffens M, Tomm JM, Scholten S, von Wiegen P, Kranz E, Wienand U, Sauter M (2005) Phytosulphokine gene regulation during maize (Zea mays L.) reproduction. J Exp Bot 56:1805–1819
Matsubayashi Y, Sakagami Y (1996) Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA 93:7623–7627
Matsubayashi Y, Hanai H, Hara O, Sakagami Y (1996) Active fragments and analogs of the plant growth factor, phytosulfokine: structure-activity relationships. Biochem Biophys Res Commun 225:209–214
Matsubayashi Y, Takagi L, Sakagami Y (1997) Phytosulfokine-alpha, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high- and low-affinity binding sites. Proc Natl Acad Sci USA 94:13357–13362
Matsubayashi Y, Takagi L, Omura N, Morita A, Sakagami Y (1999) The endogenous sulfated pentapeptide phytosulfokine-alpha stimulates tracheary element differentiation of isolated mesophyll cells of zinnia. Plant Physiol 120:1043–1048
Matsubayashi Y, Ogawa M, Morita A, Sakagami Y (2002) An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 296:1470–1472
Matsubayashi Y, Ogawa M, Kihara H, Niwa M, Sakagami Y (2006) Disruption and overexpression of Arabidopsis phytosulfokine receptor gene affects cellular longevity and potential for growth. Plant Physiol 142:45–53
Mosher S, Seybold H, Rodriguez P, Stahl M, Davies KA, Dayaratne S, Morillo SA, Wierzba M, Favery B, Keller H, Tax FE, Kemmerling B (2013) The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J 73:469–482
Ochatt S, Conreux C, Mcolo RM, Despierre G, Magnin-Robert J, Raffiot B (2018) Phytosulfokine-alpha, an enhancer of in vitro regeneration competence in recalcitrant legumes. Plant Cell Tiss Org 135:189–201
Orozco-Arroyo G, Paolo D, Ezquer I, Colombo L (2015) Networks controlling seed size in Arabidopsis. Plant Reprod 28:17–32
Raggi S, Ferrarini A, Delledonne M, Dunand C, Ranocha P, De Lorenzo G, Cervone F, Ferrari S (2015) The Arabidopsis class III peroxidase AtPRX71 negatively regulates growth under physiological conditions and in response to cell wall damage. Plant Physiol 169:2513–2525
Sauter M (2015) Phytosulfokine peptide signalling. J Exp Bot 66:5161–5169
Srivastava R, Liu JX, Howell SH (2008) Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis. Plant J 56:219–227
Stührwohldt N, Dahlke RI, Steffens B, Johnson A, Sauter M (2011) Phytosulfokine-alpha controls hypocotyl length and cell expansion in Arabidopsis thaliana through phytosulfokine receptor 1. PLoS One 6:e21054
Wang C, Yu H, Zhang Z, Yu L, Xu X, Hong Z, Luo L (2015a) Phytosulfokine is involved in positive regulation of Lotus japonicus nodulation. Mol Plant Microbe Interact 28:847–855
Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J (2015b) Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525:265–268
Yang H, Matsubayashi Y, Nakamura K, Sakagami Y (1999) Oryza sativa PSK gene encodes a precursor of phytosulfokine-alpha, a sulfated peptide growth factor found in plants. Proc Natl Acad Sci USA 96:13560–13565
Yu L, Sun J, Li L (2013) PtrCel9A6, an endo-1,4-beta-glucanase, is required for cell wall formation during xylem differentiation in populus. Mol Plant 6:1904–1917
Yu L, Chen H, Sun J, Li L (2014) PtrKOR1 is required for secondary cell wall cellulose biosynthesis in Populus. Tree Physiol 34:1289–1300
Yu L, Liu Y, Li Q, Tang G, Luo L (2016) Overexpression of phytosulfokine-alpha induces male sterility and cell growth by regulating cell wall development in Arabidopsis. Plant Cell Rep 35:2503–2512
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31500197 and 31570241), the 973 National Key Basic Research Program in China (2015CB158300), and the Shanghai Key Program of Supporting Program (15230500100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2019_3101_MOESM1_ESM.jpg
Fig. S1 Expression patterns of GmPSKα genes. Quantitative RT-PCR analyses of the expression patterns of GmPSKα genes in various soybean tissues. Values are the mean ± SD of three independent biological replicates normalized against the reference gene GmEF1β
425_2019_3101_MOESM2_ESM.jpg
Fig. S2 AtOLEO4 is specifically expressed in Arabidopsis seeds. a Quantitative RT-PCR analysis of the AtOLEO4 expression profile. AtOLEO4 is specifically and highly expressed in developing seeds. Values are the mean ± SD of three independent biological replicates normalized against the reference gene AtActin2. b Microarray data from PlaNet (http://aranet.mpimp-golm.mpg.de/, probeset ID: 258240_at) show that AtOLEO4 is primarily expressed in seed embryos at various stages, including triangle, heart, torpedo, and mature stages
425_2019_3101_MOESM3_ESM.jpg
Fig. S3 Seed-specific expression of GmPSKγ1 does not influence Arabidopsis vegetative growth. a Four-week-old wild-type and OLEOp:GmPSKγ1 transgenic Arabidopsis plants. Bar = 2 cm. b, c Measurement of the leaf length (the 7th and 8th leaves, b) and fresh weight (c) of the 4-week-old Arabidopsis plants shown in a. n =34–44. d Ten-day-old seedlings of the indicated genotypes grown on vertical MS-agar plates. Bars = 2 cm. e Primary root length of the Arabidopsis seedlings shown in d. n =26–34
425_2019_3101_MOESM4_ESM.jpg
Fig. S4 Quantitative RT-PCR analysis of GmPSKγ1 transcript levels in leaves of 35S:GmPSKγ1 transgenic tobacco lines. Values are the mean ± SD of three independent biological replicates normalized against the reference gene NtUBC2
Rights and permissions
About this article
Cite this article
Yu, L., Liu, Y., Zeng, S. et al. Expression of a novel PSK-encoding gene from soybean improves seed growth and yield in transgenic plants. Planta 249, 1239–1250 (2019). https://doi.org/10.1007/s00425-019-03101-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03101-w