Abstract
Main conclusion
A metabolic shift in green hairy root cultures of carrot from phenylpropanoid/benzenoid biosynthesis toward volatile isoprenoids was observed when compared with the metabolite profile of normal hairy root cultures.
Hairy roots cultures of Daucus carota turned green under continuous illumination, while the content of the major phenolic compound p-hydroxybenzoic acid (p-HBA) was reduced to half as compared to normal hairy roots cultured in darkness. p-Hydroxybenzaldehyde dehydrogenase (HBD) activity was suppressed in the green hairy roots. However, comparative volatile analysis of 14-day-old green hairy roots revealed higher monoterpene and sesquiterpene contents than found in normal hairy roots. Methyl salicylate content was higher in normal hairy roots than in green ones. Application of clomazone, an inhibitor of 1-deoxy-d-xylulose 5-phosphate synthase (DXS), reduced the amount of total monoterpenes and sesquiterpenes in green hairy roots compared to normal hairy roots. However, methyl salicylate content was enhanced in both green and normal hairy roots treated with clomazone as compared to their respective controls. Because methyl-erythritol 4-phosphate (MEP) and phenylpropanoid pathways, respectively, contribute to the formation of monoterpenes and phenolic acids biosynthesis, the activities of enzymes regulating those pathways were measured in terms of their in vitro activities, in both green and normal hairy root cultures. These key enzymes were 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR), an early regulatory enzyme of the MEP pathway, pyruvate kinase (PK), an enzyme of primary metabolism related to the MEP pathway, shikimate dehydrogenase (SKDH) which is involved in biosynthesis of aromatic amino acids, and phenylalanine ammonia-lyase (PAL) that catalyzes the first step of phenylpropanoid biosynthesis. Activities of DXR and PK were higher in green hairy roots as compared to normal ones, whereas the opposite trend was observed for SKDH and PAL activities. Gene expression analysis of DXR and PAL showed trends similar to those for the respective enzyme activities. Based on these observations, we suggest a possible redirection of metabolites from the primary metabolism toward isoprenoid biosynthesis, limiting the phenolic biosynthetic pathway in green hairy roots grown under continuous light.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The root of carrots (Daucus carota L.) is consumed worldwide because of its high levels of α-carotene and β-carotene (Fraser and Bramley 2004). Apart from carotenes, volatile terpenoids are present in D. carota roots, which are mainly responsible for the typical aroma and flavor of carrots. Mono- and sesquiterpenes represent approximately 98 % of the volatile compounds (Alasalvar et al. 2001; Kjeldsen et al. 2001). Interestingly, D. carota is one of the few dicot species where accumulation of simple phenolic compounds was noted even in absence of pathogen attack (Hartley and Harris 1981). Several studies have demonstrated that carrot cell walls contain significant quantities of p-hydroxybenzoic acid (p-HBA), which is presumably esterified with cell wall polymers (Schnitzler et al. 1992). p-HBA is associated predominantly with branched pectic polysaccharides, in contrast to p-hydroxybenzaldehyde (Kang et al. 2008). In addition, ferulic acid (mostly in dimer forms) and a range of other typical wall-bound phenolic aldehydes and cinnamic acid derivatives were also identified from D. carota roots (Sircar et al. 2007a; Kang et al. 2008). Thus, two major secondary metabolic pathways viz. isoprenoid/carotenoid and phenylpropanoid/benzenoid contribute to the formation of metabolites in D. carota roots.
Plant hydroxybenzoates are common mediators of plant responses of biotic and abiotic stress. Benzoate and hydroxybenzoate are simple small molecules. However, their evolving chemical and functional diversities and complexities are well reflected in their biosynthetic route (Wildermuth 2006). Although much progress has been made in elucidating the biosynthetic routes, still a part of the story remains elusive (Qually et al. 2012; Gaid et al. 2012).
Hairy root cultures are isolated root cultures where the absence of source–sink relationship with respect to metabolite synthesis and transport makes them attractive for studying secondary metabolic pathways (Mitra et al. 2002). Thus, to analyze the enzymatic route of p-HBA accumulation, hairy root cultures of D. carota were established with the rationale that hairy roots usually mirror the biosynthesis of metabolites in planta. Hairy root cultures of D. carota were shown to accumulate p-HBA as the principal wall-bound phenolic compound (Sircar et al. 2007a). Studies demonstrated that a CoA-independent C2 side-chain cleavage reaction contributed to p-HBA biosynthesis from p-hydroxycinnamic acid (Sircar and Mitra 2008). Further, synthesis of p-HBA was stimulated as a result of plant–environment interactions. Though a CoA-independent non-β-oxidative pathway for p-HBA biosynthesis was reported in recent years at the biochemical level (Sircar and Mitra 2009; Sircar et al. 2011), the regulation of this pathway under different environmental stimuli has yet to be examined. In recent past, involvement of alternative oxidase (AOX) in regulating phenylpropanoid metabolism was studied in hairy root cultures of D. carota. An increase in transcript accumulation of two AOX genes preceding the accumulation of phenylpropanoid derivatives and monolignols provided a new insight on the influence of AOX in phenylpropanoid biosynthesis (Sircar et al. 2012).
Hairy root culture of Daucus carota when cultivated under continuous illumination (250 μmol m−2 s−1) turned green (Fig. 1). Greening of hairy roots appears to be an unusual physiological phenomenon. We anticipated a possible light-dependent generation of active oxygen species in these green hairy roots of D. carota, which could induce a plausible metabolic shift to combat the photooxidative stress. Subsequent experiments elucidated the important role of different antioxidant and stress enzymes in combating photooxidative stress in green hairy roots upon continuous light exposure (Mukherjee et al. 2014).
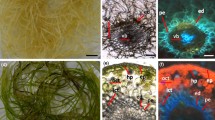
Images of dark-grown normal and light-grown green hairy roots of D. carota. a 21-day-old normal hairy roots culture of D. carota. b 21-day-old green hairy root cultures of D. carota. c Enlarged part of cross-sectioned normal hairy roots without chloroplasts in the cells. d Enlarged parts of green hairy roots with several chloroplasts in the cells. Bar 1.5 cm (a, b), 50 µm (c, d)
Abiotic stresses including light also affect secondary metabolism. Hairy root cultures cultivated under light were shown to accumulate increased levels of metabolites in different plant species (Flores et al. 1993; Jacob and Malpathak 2004). It was recently demonstrated that upon continuous illumination (24 h photoperiod), Epimedium pubescens plants suppressed the bioactive phenolics, epimeridin, by differentially regulating the expression of flavonoid pathway genes (Zeng et al. 2013). It was also confirmed that light levels lower than 10 μmol m−2 s−1 were sufficient to cause significant changes of root pigmentation and morphology by increasing root pigment content and root biomass (Vollsnes et al. 2012). As light is known to induce the phenylpropanoid metabolism in Arabidopsis thaliana roots (Hemm et al. 2004), we examined in detail the effect of light irradiation on the secondary metabolism in general, and on the content of volatile isoprenoid and non-volatile hydroxybenzoate compounds in particular, in green hairy roots of D. carota.
More specifically, we compared the contents of p-HBA and volatile compounds, especially the isoprenoid and benzenoid compounds from green and normal hairy roots of D. carota. The in vitro activities of p-hydroxybenzaldehyde dehydrogenase (HBD), the final enzyme of p-HBA formation, were measured in both green and normal hairy roots from the same subculture period to see the amount of active HBD enzymes present in the root tissues. We also measured the amount of active enzymes (in terms of their in vitro activities) of shikimate dehydrogenase (SKDH), phenylalanine ammonia-lyase (PAL) and 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR), the representative enzymes of shikimate/phenylpropanoid and isoprenoid/methyl-erythritol 4 phosphate (MEP) pathways, respectively, in green and normal hairy root tissues to draw any possible correlation. Expression analyses of PAL and DXR were also carried out to support the biochemical data. In addition, we compared the in vitro activities of pyruvate kinase (PK), a branching point enzyme between primary and secondary metabolism, in green and normal hairy roots to find clues for a possible metabolic shift. The green and normal hairy root cultures were treated with glyphosate, a competitive inhibitor of the 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS) enzyme in the shikimate pathway. The rationale behind was based on the assumption that blocking of EPSPS by glyphosate application would reduce the metabolic flow toward phenylpropanoid/hydroxybenzoate pathways which might enhance the levels of volatile terpenoids in hairy roots. Since clomazone is an inhibitor of 1-deoxy-d-xylulose 5-phosphate synthase (DXS), the first enzyme of the MEP pathway, the levels of terpenoid volatiles were also measured in clomazone-treated green and normal hairy root cultures. Finally, the possible redirection of metabolite biosynthesis from hydroxybenzoates to volatile terpenoids in green hairy roots of D. carota is discussed.
Materials and methods
Plant material
Seeds of Daucus carota L. (var. early nantes) were purchased from Sutton Seeds, Kolkata (India). Hairy root cultures of D. carota were established by infecting carrot discs with Agrobacterium rhizogenes as described by Sircar and Mitra (2008). Plant materials used in these studies were green hairy roots cultured under continuous light and normal hairy roots grown in darkness; culture and maintenance of hairy roots in the laboratory were described by Mukherjee et al. (2014).
Extraction and estimation of chlorophyll
Hairy root tissues (ca. 0.5 g) were homogenized in 80 % acetone in the dark. After centrifugation, the absorbance of the supernatant was measured at 664 nm and 647 nm. Total chlorophyll content was determined as described (Hall and Rao 1994).
Extraction and analysis of wall-bound phenolic acids
Cell wall-bound phenolic acids were extracted from both green and normal hairy roots as previously described (Sircar et al. 2007a). Separation of wall-bound phenolic acids was achieved by HPLC on RP-Hydro (Phenomenex™) C18 column (4 μm, 250 × 4.6 mm). Chromatograms were monitored simultaneously at 254 nm and 312 nm. p-HBA content was detected at 254 nm and identified by comparing its retention time with that one of an authentic standard obtained commercially (Sigma-Aldrich Chemicals, Bangalore, India). Further confirmation of the identity of the compound was achieved by matching of the UV spectra of the HPLC elute with that of the authentic standard as done earlier (Sircar et al. 2007b). For quantification, peak areas were determined using BREEZE™ software (version 3.20).
Inhibitor feeding experiment
Clomazone and glyphosate (both purchased from Fluka/Sigma-Aldrich Chemicals), were dissolved in water. The inhibitors were prepared as concentrated stock solutions and added to the culture media at appropriate concentrations after filter sterilization. Clomazone (10 μM) and glyphosate (50 μM) were added to the liquid medium at the time of subculture of hairy roots.
Estimation of hydrogen peroxide (H2O2)
Hydrogen peroxide content was determined following the method of Loreto and Velikova (2001). Hairy root tissues were homogenized in 2 mL of 0.1 % (w/v) trichloroacetic acid and centrifuged at 14,000g for 15 min at 4 °C. The supernatants were used to determine the H2O2 contents. An aliquot of 0.5 mL supernatant was added to 0.5 mL of 10 mM phosphate buffer (pH 7.0) containing 1 mL of 1 M potassium iodide solution (KI), and the absorbance was measured at 390 nm for quantification.
Extraction of headspace volatiles from hairy roots
Volatile compounds of hairy root tissues were extracted by dynamic headspace sampling techniques according to Bera et al. (2015). Hairy root tissues were kept in round-bottom flasks (250 mL capacity) with two open arms of 8 mm diameter each. One arm was fitted with a charcoal filter, through which ambient air could enter the flask and the other arm was connected with an 8-mm diameter glass column containing an adsorbent Porapak Q (80–100 mesh). Volatile-enriched air was dragged from the hairy root headspace through the adsorbent column using a vacuum pump at a flow rate of 4 L/min for 1 h at room temperature. Trapped volatiles were eluted from the adsorbing matrix into a glass vial with 200 μL of HPLC grade dichloromethane containing an appropriate concentration (1 µL) of ethyl hexanoate as an internal standard.
GC–MS analysis of volatile compounds
Gas chromatography–mass spectrometry (GC–MS) analysis was performed using a Shimadzu QP2010SE GC–MS system by following Maiti et al. (2014). Eluted compounds were separated on ZB-5 column (30 m × 0.25 mm i.d., film thickness 0.25 μm) with helium (He) as a carrier gas. The injection volume was 2 μL with split ratio 2:1. The injector temperature was set at 260 °C. The initial column oven temperature was set at 50 °C for 2 min, then increased at the rate of 2 °C min−1 to reach 60 °C (2 min hold), followed by an increment of temperature at the rate of 3 °C min−1 to 210 °C (2 min hold), and finally to 270 °C at a rate of 10 °C min−1, hold for 7 min. Column flow rate was 1 mL/min. The mass spectrometer conditions were as follows: ion source temperature, 200 °C; interface temperature, 280 °C; electron energy, 70 eV; scanning range of m/z, 40–600 a.m.u. The results were analyzed by Shimadzu GC-MS Solution software (ver. 2.6). The compounds were identified by comparing mass spectra of the components with those from the mass spectral library from NIST 05 (National Institute of Standards and Technology, Gaithersburg, MD, USA) and Wiley 8.0 (Wiley, New York, USA). Retention index values of these compounds were calculated and compared with data from literature (da Silva et al. 1999; Daferera et al. 2003; Asuming et al. 2005).
Assay of p-hydroxybenzaldehyde dehydrogenase (HBD)
Cell-free extracts were prepared from both green and normal hairy roots as described before (Sircar and Mitra 2008). Protein concentration was determined according to Bradford (1976). HBD activity was determined as essentially described by Sircar et al. (2011). Standard assay mixture consisted of 0.5 mM p-hydroxybenzaldehyde, 1 mM NAD+, 0.1 mM DTT and 100 μg protein. The final volume of the reaction mixture was 200 μL and was adjusted by 200 mM Tris–HCl buffer pH 7.5. The reaction mixture was incubated at 35 °C for 1 h and was stopped by adding 200 μL ice-cold acetic acid:methanol (1:9, v/v). The product formation was analyzed by HPLC following the method of Sircar et al. (2011).
Preparation of protein extracts for the PAL assay
Cell-free extracts were prepared by homogenizing hairy root tissue (ca. 0.5 g) in 100 mM Hepes buffer (2 mL buffer/g root tissue), pH 8.0, containing 20 % (w/w) polyvinylpyrrolidone (PVPP). Then the homogenate was centrifuged at 14,000g for 20 min and the supernatant was concentrated in an Amicon® ULTRA-4 CFU membrane concentrator (Millipore, Billerica, MA, USA). All steps were carried out at 4 °C. The soluble protein content was determined according to Bradford (1976).
Assay of PAL
PAL activity was determined according to Sircar and Mitra (2008) with suitable modification. The reaction mixture contained 450 μL of 100 mM Tris–HCl (pH-8.3) buffer, 50 μL of 100 mM l-phenylalanine and 100 μg protein extract. The reaction mixture was incubated 37 °C for 60 min and terminated by 500 μL chilled acetic acid and methanol (1:9, v/v) mixture. This mixture was then used for the detection of product formation by HPLC as described by Sircar and Mitra (2008).
Extraction and spectrophotometric assay of shikimate dehydrogenase (SKDH)
The enzyme was assayed following the method of Diaz et al. (1997) with suitable modifications. A cell-free extract was prepared by homogenizing 0.32 g of both, normal and green hairy roots, in the presence of 0.1 M K-phosphate buffer (pH 7.4), containing 0.5 mM dithiothreitol (DTT), 2 mM l-cysteine, 2 mM EDTA, 8 mM 2-mercaptoethanol (β-Mesh) and 0.5 g of polyvinylpyrrolidone (PVPP). The homogenate was centrifuged at 14,000g for 15 min at 4 °C, and the supernatant was used for further analyses. The enzyme activity was determined spectrophotometrically at 25 °C in 1 mL reaction mixture containing 4 mM shikimic acid, 2 mM NADP+ and 50 μg crude protein in 0.1 (M) Tris–HCl buffers (pH-9.2). The reaction was initiated by adding the crude extract and the activity was determined by measuring the reduction of NADP+ at 340 nm for 15 min.
In-gel assay of SKDH
In-gel assay of SKDH was performed in 10 % polyacrylamide gel slab using 0.25 M Tris base containing 0.5 M glycine as running buffer (pH 8.3) (Laemmli 1970). Staining of SKDH was carried out for 30 min according to Díaz et al. (1997) with suitable modification. The staining solution (20 mL) contained 4 mg of shikimic acid, 2 mg of NADP, 2 mg of MTT [3-(4,5-dimethyl-2-il)-2,5-diphenyltetrazolium bromide)], and 1 mg PMS (phenazine methosulphate) in 0.1 M Tris–HCl buffer (pH 9.0).
Extraction and assay of pyruvate kinase (PK)
Fresh green and normal hairy roots (0.5 g) were homogenized with 2 mL of 100 mM Hepes buffer (pH 8.0). The homogenate was centrifuged at 14,000g for 30 min at 4 °C and the enzyme activity was determined by measuring the decrease in absorbance at 340 nm (Bergmeyer et al. 1974). The assay was performed for 15 min at 37 °C in a total volume of 1 mL containing 100 mM potassium phosphate buffer (pH 7.6), 17 mM phosphoenol pyruvate, 1.3 mM NADH, 100 mM MgSO4, 44 mM ADP, lactic acid dehydrogenase (1 unit) and 50 μg crude enzyme extract.
Preparation of protein extract from chloroplast/leucoplast and assay of 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR)
DXR enzyme activity was determined after isolation of proteins from chloroplasts and leucoplasts from green and normal hairy roots, respectively. Fresh hairy roots tissues (2 g) were crushed in 3.5 mL of 0.5 M sucrose solution (thus maintaining the normal volume of chloroplasts through osmoregulation) in a pre-chilled mortar and pestle as essentially described earlier (Nishida 1963). Crushed materials were filtered through cheese cloth and the collected filtrates were centrifuged at 2500g for 10 min at 4 °C. Upon transfer to fresh microcentrifuge tubes, the supernatants were centrifuged at 30,000g for 5 min at 4 °C. After discarding the supernatants, the pellets were used as source of isolated chloroplasts and leucoplasts. Subsequently, the pellets were suspended in 600 μL of extraction buffer consisting of 150 mM Tris–HCl (pH 7.2), 5 mM MgCl2, 5 mM 2-mercaptoethanol and 1 mM thiamine diphosphate as described (Ramak et al. 2013). After centrifugation at 30,000g for 5 min, the supernatants were concentrated in an Amicon® Ultra membrane concentrator (Millipore) following the manufacturer’s instruction. The concentrated extracts were used for the determination of the total protein content (Bradford 1976).
DXR activity was determined spectrophotometrically as described recently by Ramak et al. (2013). To 500 μL reaction mixture, 1 mM 1-deoxy-D-xylulose 5-phosphate (Sigma-Aldrich Chemicals), 1 mM MnCl2, 0.125 mM NADPH and 100 μg of chloroplast/leucoplast-isolated concentrated crude protein were added. The oxidation of NADPH was monitored at 340 nm for 15 min at 25 °C.
Isolation of core cDNA fragment of DXR
Total RNA pools from D. carota hairy roots were isolated using the RNeasy Mini Plant Kit (Qiagen). Oligo (dT)-primed reverse transcription was carried out at 42 °C with RevertAid™ M-MuLV reverse transcriptase (Fermentas). Core cDNA fragment was amplified by PCR using Taq DNA polymerase (Fermentas) and degenerate primer 1 and primer 2 (Table 1). These degenerate primers were designed from conserved regions of plant DXR Souret et al. (2002). The PCR cycle with degenerate primer was conducted at 94 °C for 3 min, followed by 10 cycles of touchdown PCR comprising denaturation, 94 °C for 45 s: for annealing starting at 65 °C and ending at 45 °C for 45 s, and extension at 72 °C for 1 min. After touchdown, 30 cycles of PCR were used with an annealing temperature of 53.3 °C and with final extension of 72 °C for 10 min. The PCR products were separated by electrophoresis in a 1 % agarose gel. The bands were stained by ethidium bromide; the bands of expected size were extracted from the gel and purified using QIAquick gel extraction kit (Qiagen). The purified DNA was cloned in pDrive vector using Qiagen PCR cloning kit (Qiagen) following the manufacturer’s instructions. The cloned product was sequenced at Eurofins Genomic India Pvt Ltd (Bangalore, India).
Expression studies of DXR and PAL genes
The 14-day-old normal and green hairy roots were used for isolation of total RNA using RNeasy Plant mini kit (Qiagen) following the manufacturer’s instruction. An aliquot of 2 μg total RNA was used for reverse transcription reactions, carried out as described above under “Isolation of core cDNA fragment of DXR”. Gene-specific primer 3 and primer 4 (Table 1) were designed from the core DXR cDNA sequence isolated earlier and used for amplifying a 265-bp fragment of the coding sequence to find out the relative density of transcript accumulation. To analyze PAL transcript accumulation, forward and reverse primers (5 and 6) were designed based on sequence information from NCBI database (Table 1). Actin served to normalize the RT-PCR results and was amplified with forward primer 7 and reverse primer 8 (Table 1). PCR reactions for DXR were performed under the following conditions: 94 °C for 3 min followed by 27 cycles of amplification (94 °C for 45 s, 51.7 °C for 45 s, 72 °C for 1 min) and final extension at 72 °C for 10 min. Similar PCR conditions were used for amplification PAL and actin, except that the annealing temperatures were 52.3 °C and 53.2 °C, respectively. PCR amplified products were separated on 1 % (w/v) agarose gel, stained with ethidium bromide and photographed using MicroBis (DNR Bio-Imaging System) gel-imaging system. ImageJ software, which analyzes the pixel intensity of PCR bands, was used to compare the DXR and PAL expression levels relative to that of actin.
Statistical analysis
All the experiments were done in triplicate and results obtained were analyzed using ANOVA through Microsoft Excel software.
Results
Phenolic acid and total chlorophyll contents in green and normal hairy roots
The cell wall-bound p-HBA content was found to be reduced in green hairy roots. In 14-day-old green and normal hairy root cultures, the p-HBA contents were 1.1 ± 0.18 mg/g dry mass and 2.1 ± 0.2 mg/g dry mass, respectively. The p-HBA content in green hairy roots was 48 % lower (P ≤ 0.002) compared to normal hairy roots (Fig. 2). Green hairy roots of D. carota also accumulated less amount of other soluble phenolics including p-HBA. Total chlorophyll content found in green hairy roots was 1.2 mg/g fresh mass whereas in normal hairy roots the amount was negligible.
HBD activities in green and normal hairy roots
HBD acts as the final enzyme of the hydroxybenzoate pathway by catalyzing the formation p-HBA from p-hydroxybenzaldehyde. The HPLC-based stopped assay method was used to determine the activities of HBD in both, green and normal hairy roots, of 14-day-old cultures (Fig. 3a). Normal hairy roots showed an activity of 6.0 pkat/mg protein, whereas in green hairy roots the HBD activity was found to be 0.4 pkat/mg protein. Thus, as compared to normal hairy roots, the amount of active HBD enzyme was found to be 15-fold less (P ≤ 0.00001) in green hairy roots (Fig. 3b).
Assay of HBD in normal and green hairy roots. a HPLC chromatograms in green and normal hairy roots of D. carota. Top chromatogram of the HBD-catalyzed reaction from normal hairy roots. Bottom chromatogram of the HBD-catalyzed reaction from green hairy roots. Formation of p-HBA is marked by (arrow). Chromatograms were monitored at 254 nm. b Activities of HBD from crude protein extract of 14-day-old green and normal hairy roots. Activities were determined by measuring the p-HBA formation through HPLC. Each value is the mean ± SD of a triplicate analysis from at least three independent extractions. Mean values are significant different at the P ≤ 0.05 level
PAL activities in green and normal hairy roots
PAL, an enzyme of the phenylpropanoid pathway, was assayed by the HPLC-based stopped assay measuring the amount of trans-cinnamic acid formation. In vitro enzyme activities were monitored from 14-day-old normal and green hairy roots. The activity of PAL in normal hairy roots was 80 pkat/mg protein, whereas in green hairy roots the value was 54 pkat/mg protein. Therefore, as compared to the normal ones, a 1.5-fold less (P ≤ 0.001) amount of active PAL enzyme was formed in green hairy roots (Fig. 4a).
Comparison of PAL, SKDH, PK, and DXR activities in 14-day-old green and normal hairy roots of D. carota. a PAL activity was determined by measuring the production of trans-cinnamic acid per 60 min by HPLC. Enzyme activity is expressed in pkat/mg protein. The activities of SKDH (b), PK (c) and DXR (d) were determined spectrophotometrically by measuring reduction of NADP, oxidation of NADH, and oxidation of NADPH, respectively, at 340 nm. For SKDH and PK, enzyme activities were expressed in nkat/mg protein and mU/mg protein, respectively. DXR activity was expressed in pkat/mg protein. Each value for all enzyme assays represents the mean ± SD from at least three independent protein extraction preparations. Mean values are significant different at the P ≤ 0.05 level
SKDH activities in green and normal hairy roots
SKDH is responsible for the synthesis of aromatic amino acid and a large number of phenolic compounds in plants (Herrmann 1995). To correlate the activity of this enzyme with the phenolic acid content in both types of hairy roots, SKDH activities were determined spectrophotometrically in 14-day-old green and normal hairy roots. The specific activities of SKDH in normal hairy roots and green hairy roots were found to be 2 nkat/mg protein and 1.11 nkat/mg protein, respectively (Fig. 4b). Hence, in 14-day-old normal hairy roots, the level of the active SKDH enzyme was 1.8-fold (P ≤ 0.001) higher than in green hairy roots. During in-gel SKDH assay, only one isozyme band appeared in both, green and normal hairy root cultures. Image analysis demonstrated an enhanced SKDH activity (increased by 80 %) in normal hairy roots in comparison to green hairy roots. The more intense band in normal hairy roots indicated the generation of more active SKDH protein in normal hairy roots as compared to green hairy roots at a particular time point (Suppl. Fig. S1).
Pyruvate kinase activities in green and normal hairy roots
Pyruvate and glyceraldehyde-3-phosphate, the products of the primary metabolism, are the starting points of the MEP pathway, primarily to synthesize the phytol chain of chlorophyll in green hairy roots. However, in normal hairy roots, this pathway may not be as active as in green ones. The activity of pyruvate kinase (PK) converting phosphoenol pyruvic acid into pyruvic acid, amounted to 45 mU/mg protein in green hairy roots, whereas in normal hairy roots the activity amounted to 29.3 mU/mg protein. Thus, at a particular time point, the amount of active PK present in green hairy roots was 1.5-fold (P ≤ 0.001) higher as compared to normal hairy roots (Fig. 4c).
DXR activities in green and normal hairy roots
DXR as a key enzyme of the MEP pathway catalyzes the production of MEP from 1-deoxy-d-xylulose 5-phosphate. DXR activity was determined spectrophotometrically after extracting the proteins from chloroplasts or leucoplasts of both green and normal hairy roots from 14-day-old cultures. Activities of the DXR enzyme were found to be 0.2 pkat/mg protein and 0.1 pkat/mg protein, for green and normal hairy roots, respectively. Thus, green hairy roots exhibited a twofold (P ≤ 0.002) higher activity of the DXR enzyme as compared to normal hairy roots (Fig. 4d).
DXR and PAL expression in green and normal hairy roots
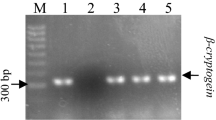
Core cDNA fragment of DXR gene was isolated using degenerate primers designed by Souret et al. (2002) (Table 1). The resulting sequence of 287 bp was cloned and sequenced. Amplified sequence showed homology with the amino acid sequence of different plant DXR (Suppl. Fig. S2) available in the public database with a percentage identity of 92 %. Therefore, the resulting sequence was defined as core cDNA of DXR, and appeared to be suitable for expression studies. Accumulation of DXR and PAL transcripts in both green and normal hairy roots was studied by RT-PCR after normalization of PCR cycle numbers (Suppl. Fig. S3). Gene-specific primers (Table 1) resulted in amplification of 265 bp and 765 bp fragments of DXR and PAL genes, respectively. The level of actin which was detected as a 70-bp PCR product, served as control for normalization of other transcript levels. In green hairy roots, DXR transcript accumulation was found to be 5-fold higher than that of normal hairy roots whereas PAL transcript accumulation was 3.9-fold higher in normal hairy roots (Fig. 5).
Expression analysis of DXR and PAL genes in 14-day-old green (G) and normal (N) hairy roots of D. carota. a Total RNA (2 μg) was reverse transcribed and used as template for semi-quantitative RT-PCR analysis. b, c DXR and PAL transcript levels were normalized on the basis of actin transcript amounts. SD values are indicated (n = 3)
Comparative analysis of volatile compounds from green and normal hairy roots
Plant roots emit a wide range of volatile organic compounds including terpenoids, fatty acid derivatives and benzenoids in its surroundings (Loreto and Schnitzler 2010). However, no information on the volatile profile was available for hairy root cultures of D. carota. Through GC–MS analysis, we have identified 31 major volatile compounds, including monoterpenes, sesquiterpenes and one benzenoid compound (Suppl. Table 1; Suppl. Fig. S4). Several monoterpenes and sesquiterpenes were present in volatiles of hairy roots. However, for clarity of the presentation, the contents of individual monoterpenes and sesquiterpenes were added together in two groups and presented as total monoterpene and sesquiterpenes, respectively. Comparative volatile analysis revealed 2.3-fold (P ≤ 0.0004) and 1.3-fold (P ≤ 0.024) increments in total monoterpene and total sesquiterpene contents, respectively, in 14-day-old green hairy roots compared to normal hairy roots of the same age (Fig. 6a, b). The only detectable volatile benzenoid compound was methyl salicylate, the content of which was 1.9-fold (P ≤ 0.001) higher in normal hairy roots as compared to the green ones (Fig. 6c). Hence in green hairy roots, isoprenoid contents were higher than in normal hairy roots but the benzenoid content was lower. This observation raised the question as to whether blocking of isoprenoid biosynthesis could enhance the levels of benzenoid compounds.
Comparison of major volatiles emission in green and normal hairy roots. Volatile compounds were trapped from 14-day-old green and normal hairy roots, using dynamic headspace and were subsequently analyzed by GC–MS. Major headspace volatile compounds were analyzed from both green and normal of hairy roots. a Total monoterpene. b Total sesquiterpene. c Methyl salicylate. Aqueous clomazone (10 μM) and glyphosate (50 μM) solutions were used to inhibit isoprenoid biosynthesis and phenolic biosynthesis in green and normal hairy roots. Studies of volatile emission in clomazone- and glyphosate-treated hairy roots were performed following the same procedure as done in previous analyses. d–g Comparison of major volatiles in inhibitor-treated green and normal hairy roots: d Total monoterpene. e Total sesquiterpene. f Methyl salicylate. g Total monoterpenes in glyphosate-treated green and normal hairy roots. The values are the mean ± SD of at least three independent extractions. Mean values are significant different at the P ≤ 0.05 level
Glyphosate competitively inhibits EPSP synthase enzyme of the shikimate pathway. In this study, glyphosate was used to examine the effect on volatile terpenoid emission upon blocking the shikimate pathway. Feeding of glyphosate at a concentration of 0.05 mM was carried out according to a protocol for tobacco (Haderlie et al. 1977). An increased emission of volatile monoterpenes was observed in glyphosate-treated hairy roots; a 2.4-fold enhanced amount of volatile monoterpenes was noticed in glyphosate-treated green hairy roots as compared to treated normal hairy roots (Fig. 6g). However, the volatile sesquiterpene contents remained more or less unaffected in both types of hairy roots. Surprisingly, a huge increment of volatile methyl salicylate emission was observed in both green and normal hairy roots (Suppl. Fig. S5).
Clomazone, a potent inhibitor of 1-deoxy-d-xylulose 5-phosphate synthase (DXS) was fed to both, green and normal hairy roots of D. carota, to study the role of the MEP pathway on volatile emission. Upon clomazone treatment, no monoterpene and sesquiterpene volatiles were detected in treated green hairy roots as compared to untreated green ones (Fig. 6d, e). On the other hand, in clomazone-treated normal hairy roots, monoterpene and sesquiterpene contents were reduced by 2.5-fold and 3-fold, respectively, as compared to controls (untreated normal hairy roots) (Fig. 6d, e).
The methyl salicylate level increased in clomazone-treated green and normal hairy roots by 1.3-fold and 1.4-fold, respectively, as compared to untreated hairy roots (Fig. 6c–f). However, in clomazone-treated normal hairy roots, methyl salicylate content was twofold (P ≤ 0.001) higher than in treated green hairy roots (Fig. 6f).
Discussion
Hairy roots developed through A. rhizogenes-mediated transformation were exploited as a system for the study of secondary metabolism (Rhodes et al. 1997). Secondary metabolism of hairy roots generally mirrors that of the species from which they were established. The hairy root cultures reflect to some extent similar operations of secondary metabolic pathways both in their route and enzymology of those in intact plant roots, but there are a few concerns. As the hairy root system itself acts as source and sink of metabolites, metabolites which are normally exported to the rest of the plant may accumulate in hairy roots, a situation not usually occurring in normal roots (Hamill et al. 1987).
Hairy roots of several plant species turned green when cultured under continuous light, and maintained their branched root morphology with the formation of chlorophyll in the plastids. These green hairy roots had an altered (usually enhanced) metabolite production as established in normal hairy roots grown under laboratory photoperiodic conditions (Taya et al. 1994). The amount of total chlorophyll in D. carota green hairy roots was comparable to the levels found in hairy roots of Ipomoea aquatica (1.7 mg/g fresh mass) cultivated under continuous light (Tone et al. 1997). These D. carota green hairy roots exhibited a reduced amount of p-HBA accumulation in the cell wall compared to normal hairy roots. This appeared to be in sharp contrast with earlier findings that in general light played a positive role in up-regulating phenylpropanoid metabolism in intact roots of Arabidopsis thaliana (Hemm et al. 2004).
Continuous light exposure generated photooxidative stress in green hairy roots (Mukherjee et al. 2014) leading to the generation of excess H2O2 in root tissues. Behnke et al. (2010), during their investigation on isoprene non-emitting poplar, also observed H2O2 accumulation when exposed to high light and temperature. It was suggested that the generated H2O2 triggered a signaling cascade that ultimately down-regulated the phenylpropanoid pathway (Behnke et al. 2010). Green hairy root accumulated 1.2 μM H2O2 per gram fresh tissue whereas in normal hairy roots the level was 0.7 μM H2O2 per gram fresh tissue (Suppl. Fig. S6). It is plausible that this uplifted level of H2O2 in green hairy roots down-regulates the phenolic biosynthesis by some unknown mechanism. These findings raised questions as to a possible shift of the carbon flow toward other metabolic directions in green hairy roots.
HBD converts p-hydroxybenzaldehyde to p-HBA in a CoA-independent non-β-oxidative pathway of p-HBA formation in hairy roots of D. carota (Sircar and Mitra 2008, 2009). Though the activity of benzaldehyde dehydrogenase was reported in a few plant species (Gaid et al. 2009), HBD has only been studied extensively at the biochemical level in hairy roots of D. carota recently (Sircar et al. 2011). In methyl jasmonate-treated D. carota hairy roots, it was found that enhanced accumulation of p-HBA was preceded by a substantial increment in HBD activity (Sircar et al. 2011). We found it important to check the activity of HBD in green hairy roots because the level of p-HBA was reduced in green hairy roots as compared to normal hairy roots. In green hairy roots, HBD activity was found to be 15-fold lower compared to that one found in normal hairy roots. This result suggests that the pathway to p-HBA formation might have been suppressed in hairy roots when they turned green. The result raised the further question whether a general decrease in the enzyme activities of shikimate/phenylpropanoid pathway might occur in green hairy roots.
PAL catalyzes the first step of phenylpropanoid biosynthesis by converting l-phenylalanine into trans-cinnamic acid. The enzyme plays an important regulatory role in the formation of different phenolic compounds, including hydroxybenzaldehydes and fragrant methoxybenzaldehydes (Chakraborty et al. 2008; Kundu et al. 2012). The regulation of all isoforms of this enzyme in different developmental stages and under different environmental stimuli was extensively studied in the past (Liang et al. 1989). Hairy green roots of D. carota exhibited a reduced enzyme activity and reduced transcript accumulation which is in contrast to a previous study in Phaseolus vulgaris, where significant increment in PAL transcript accumulation was evident under light irradiation (Liang et al. 1989). In contrast, reduced PAL and tyrosine ammonia lyase (TAL) activities were found along with reduced secondary metabolite formation in wheat seedlings grown under low pressure sodium lamps (Guerra et al. 1985). Thus, the suppressed PAL activity in green hairy carrot roots could be due to continuous light irradiation which is indispensible for maintaining the green roots. Hence the question arises whether a similar suppression in the enzyme activities of the shikimate pathway could be observed in green hairy roots.
The shikimate pathway is a metabolic route which produces aromatic amino acids and a myriad of phenolic compounds in bacteria and plants (Herrmann 1995). SKDH catalyzes the third and fourth steps of the shikimate pathway by converting 3-dehydroquinate to shikimate. In a previous study, it was reported that the SKDH activity was developmentally regulated, and a correlation between phenolic content and SKDH activity was evident (Díaz et al. 1997). Recently, a modulatory role of SKDH in fragrant 2-hydroxy-4-methoxybenzaldehyde (MBALD) biosynthesis has been demonstrated in Hemidesmus indicus (Kundu et al. 2012). In elicitor-treated roots, an increased accumulation of MBALD was preceded by an increase in the activity of SKDH as compared to controls. In our study, compared to normal hairy roots, D. carota green hairy roots accumulated a lower amount of phenolics along with a reduced activity of SKDH. Hence, as compared to normal hairy roots, suppression of enzyme activities of shikimate/phenylpropanoid/hydroxybenzoate pathways in green hairy roots of D. carota was observed.
Plastidial isoprenoid biosynthesis initiates from pyruvate and glyceraldehyde-3-phosphate through an enzymatic reaction catalyzed by DXS enzyme (Rohmer 1999). PK, an enzyme of the primary metabolism, produces pyruvic acid from phosphoenol pyruvate. Generally, the ratio of phosphoenol pyruvate to pyruvate is essential to maintain the flow of several biochemical pathways including isoprenoid biosynthetic pathways (Andre and Benning 2007). We anticipated that green roots might produce an enhanced amount of isoprenoid volatiles in addition to chlorophyll. Indeed, in green roots, the activity of PK was 1.5-fold higher than in normal hairy roots. This is plausible as the requirement of pyruvate as a precursor is needed in green hairy roots for uplifting isoprenoid biosynthesis.
According to the contemporary metabolic control theory, metabolic pathways are regulated by partial rate limitation occurring at each of a number of enzymatic steps (Ap Rees and Hill 1994). The enzymes DXS and DXR catalyze the first two steps of the MEP pathway, respectively. DXR plays a crucial role in controlling the isoprenoid biosynthesis in plants (Mahmoud and Croteau 2001). Recent work, however, showed that DXS also has a high ability to control the flux into the MEP pathway (Wright et al. 2014). Although measuring DXS activity would have been useful for better clarification of the MEP pathway regulation, current experimental limitation for the tricky assay of DXS activity at the authors’ disposal compelled them to choose DXR assay as a marker enzyme for the MEP pathway. Further, it is well established that the control over a metabolic pathway is shared between all the enzymes of that pathway, and that generally an increase in all enzymes of a pathway is necessary for an increased flux (Fell 1992). It is well agreed that the control in the MEP pathway is shared between its different enzymes (Banerjee and Sharkey 2014), as was also hypothesized earlier by Carretero-Paulet et al. (2002). In our study, DXR enzyme activity and expression were found to be increased in green hairy roots compared to normal hairy roots. These results are in agreement with the previous report where light-induced transcript accumulation of DXR gene was found in Arabidopsis (Carretero-Paulet et al. 2002). In Artemisia annua transformed roots, transcript accumulation of DXR under light irradiation was not markedly different from that one in dark-grown transformed roots; but when 14-day-old dark-grown transformed roots were shifted to light irradiation for 3 days, transcript accumulation of DXR increased drastically (Souret et al. 2002). The enhanced DXR activity in green hairy carrot roots raised the question on the content of isoprenoid volatiles in these hairy roots.
The aroma of D. carota roots mainly depends on the volatile isoprenoid composition. A major role of these root volatiles comprises ecological interactions with belowground microorganisms, other plants and herbivores (Holopainen and Gershenzon 2010). Constituents of root volatiles have been determined in some plant species including carrot roots (Kjeldsen et al. 2001). In the past, in carrot roots, 36 different volatile compounds were found of which monoterpenes and sesquiterpenes were the major constituents (Kjeldsen et al. 2001). By solid-phase extraction-based trapping of volatiles and subsequent GC–MS analysis of elutes, we have identified 31 major volatile compounds, including monoterpenes, sesquiterpenes and one benzenoid compound in green hairy roots of D. carota. It was demonstrated earlier through feeding experiments with labeled precursor that monoterpenes are exclusively synthesized by the MEP pathway, whereas sesquiterpenes are formed by both, the mevalonic acid (MVA) pathway and the MEP pathway, in carrot leaves and roots (Hampel et al. 2005). Green chloroplasts are formed in hairy roots of D. carota when cultivated under continuous illumination. Because of chloroplast formation, it can be assumed that the chloroplast-specific MEP pathway were more effectively operated in green hairy roots than in normal hairy roots. Comparative volatile analyses showed increased monoterpenes and sesquiterpene contents in green hairy roots compared to normal hairy roots, which suggested that in green hairy roots the MEP pathway was more active than in normal hairy roots.
Clomazone inhibits MEP pathway by inhibiting the DXS, the first enzyme of MEP pathway (Flores-Pérez et al. 2010). Aqueous solution of clomazone was used to block the DXS activity in green and normal hairy roots. In green hairy roots, the content of monoterpenes and sesquiterpenes was almost nil as compared to normal hairy roots. Further an altered morphology in green hairy roots was also noticed. Surprisingly, clomazone-treated normal hairy roots showed an unaltered morphology, but monoterpenes and sesquiterpene contents were reduced as compared to normal untreated hairy roots. It was earlier established that MVA and MEP pathways share common intermediates and a metabolic cross-talking between the two pathways exists (Hampel et al. 2005). Although sesquiterpenes are normally synthesized by the MVA pathway, the apparent reason of suppression of sesquiterpene contents in normal hairy roots was perhaps due to cross-talking between MEP and MVA pathways (Rodríguez-Concepción and Boronat 2015).
Methyl-d-erythritol-2,4-cyclopyrophosphate (MEcPP), an intermediate of the MEP pathway was reported to accumulate in higher amounts in spinach leaves under intense light and excess temperature (Rivasseau et al. 2009; Mongelard et al. 2011). Higher accumulation of MEcPP was also observed in glyphosate-treated horseweed and ryegrass under intense light and temperature (Ge et al. 2012). However, without glyphosate treatment, these plants failed to accumulate MEcPP even under intense light and temperature. Glyphosate was known to inhibit the shikimate pathway by blocking EPSPS, but the reason for accumulation of a MEP derivative (MEcPP) upon glyphosate treatment is biochemically elusive till today. In our study, glyphosate treatment also increased the monoterpene content significantly in both, green and normal hairy roots (Fig. 6g). Therefore, by some unknown mechanism, the terpenoid biosynthesis is modulated in hairy roots after blocking of the shikimate pathway by glyphosate treatment.
Methyl salicylate is a benzenoid ester (commonly found in scents of moth pollinated flowers) and is an important component of plant defense (Ross et al. 1999). Interestingly, the content of methyl salicylate was suppressed in green hairy roots as compared to normal hairy roots. This observation correlates well with our previous data from D. carota, where suppressed levels of phenolic acids accumulation were observed in green hairy roots (Mukherjee et al. 2014). In clomazone-treated green hairy roots, methyl salicylate content was increased as compared to untreated green hairy roots. It is plausible that blocking of DXS by clomazone in green hairy roots limited the metabolic flow in the MEP pathway and channelized the carbon flow toward the benzenoid pathway. Increment in methyl salicylate content in clomazone-treated normal hairy roots was rather puzzling. It is conceivable that in normal hairy roots, clomazone treatment reduced the synthesis of volatile terpenoids by inhibiting the interconnected MEP and MVA pathways and thereby increased the methyl salicylate content.
It is clear that volatile terpenoids play an important role in protection against a variety of abiotic stresses including high light irradiation (Vickers et al. 2009). The presence of terpenoids improves the ability of plants to deal with internal oxidative changes resulting from abiotic stresses. Activation of carbon sources alternative to fixed photosynthates possibly stimulates the formation of volatile terpenoids in plants upon exposition to abiotic stresses Lorato et al. (1996); Vickers et al. 2009). These isoprenoid volatiles play important physiological and ecological roles in protecting plants from environmental constraints; in particular, the role of monoterpenes in stabilizing chloroplast membranes during light stress is well documented (Loreto and Schnitzler 2010).
Conclusion
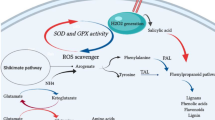
Our results indicate a shift in metabolite biosynthesis from phenolics to volatile terpenoids occurred in green hairy roots of carrot (Fig. 7). Carbon is generally redirected to volatile production under abiotic stress conditions. These volatile terpenoids perhaps protect the green hairy roots, thus justifying the metabolic expenses of production. It is plausible that, during greening, the primary metabolites that are normally channelized toward shikimate/phenylpropanoid pathways are used by the MEP pathway, thus limiting the carbon flow toward shikimic/phenylpropanoid pathways. Future work will investigate details of the expression and identify the regulatory enzymes of the MEP pathway in green hairy roots.
Possible connection of MEP pathway and p-HBA biosynthetic pathway. Both pathways share a common metabolite as their starting materials. Due to chlorophyll biosynthesis in green hairy roots, the major carbon pool was channelized toward the MEP pathway resulted in increased volatile terpenoids content. A concomitant reduction of the levels of phenylpropanoid/benzenoid derivatives in green roots was noticed. The compounds and selected enzymes are as follows: CDP-ME 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-MEP 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, DMAPP dimethylallyl diphosphate, DXP 1-deoxy-d-xylulose 5-phosphate, DXR 1-deoxy-d-xylulose 5-phosphate reductoisomerase, DXS 1-deoxy-d-xylulose 5-phosphate synthase, EPSP 5-enolpyruvyl-shikimate-3-phosphate, FPP farnesyl diphosphate, FPPS FPP synthase, GPP geranyl diphosphate, HBD p-hydroxybenzaldehyde dehydrogenase, HDR 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, IPP isopentenyl diphosphate, MEP methyl-erythritol 4-phosphate, MTS monoterpene synthase, PAL phenylalanine ammonia lyase, PK pyruvate kinase, SKDH shikimate dehydrogenase
Author contribution statement
CM and AM conceived and designed research. CM conducted experiments. TS did GC–MS analysis. CM, TS and AM analyzed data. AM wrote the final manuscript. All authors read and approved the manuscript.
Abbreviations
- DXR:
-
1-Deoxy-d-xylulose 5-phosphate reductoisomerase
- DXS:
-
1-Deoxy-d-xylulose 5-phosphate synthase
- EPSPS:
-
5-Enolpyruvyl-shikimate-3-phosphate synthase
- HBD:
-
p-Hydroxybenzaldehyde dehydrogenase
- MEP:
-
Methyl-erythritol 4-phosphate
- MVA:
-
Mevalonic acid
- PAL:
-
Phenylalanine ammonia-lyase
- p-HBA:
-
p-Hydroxybenzoic acid
- PK:
-
Pyruvate kinase
- SKDH:
-
Shikimate dehydrogenase
References
Alasalvar C, Grigor JM, Zhang D, Quantick PC, Shahidi F (2001) Composition of volatiles, phenolics, sugars, antioxidant vitamins and sensory quality of different coloured carrot varieties. J Agric Food Chem 49:1410–1416
Andre C, Benning C (2007) Arabidopsis seedlings deficient in a plastidic pyruvate kinase are unable to utilize seed storage compounds for germination and establishment. Plant Physiol 145:1670–1680
Ap Rees T, Hill SA (1994) Metabolic control analysis of plant metabolism. Plant Cell Environ 17:587–599
Asuming WA, Beauchamp PS, Descalzo JT, Dev BC, Dev V, Frost S, Ma CW (2005) Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem Syst Ecol 33:17–26
Banerjee A, Sharkey T (2014) Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat Prod Rep 31:1043–1055
Behnke K, Kaiser A, Zimmer I, Brüggemann N, Janz D, Polle A, Hampp R, Hänsch R, Popko J, Schmitt-Kopplin P, Ehlting B, Rennenberg H, Barta C, Loreto F, Schnitzler J (2010) RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: a transcriptomic and metabolomic analysis. Plant Mol Biol 74:61–75
Bera P, Samanta T, Kotamreddy JNR, Mitra A (2015) Inter-specific variation in headspace scent volatiles composition of four commercially-cultivated jasmine flowers. Nat Prod Res 29:1328–1335
Bergmeyer HU, Gawehn K, Grassl M (1974) Enzymatic assay of pyruvate kinase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York, pp 509–510
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:1151–1154
Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepción M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-d-erythritol 4-phosphate pathway. Plant Physiol 129:1581–1591
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase-mediated biosynthesis of 2-hydroxy-4-methoxybenzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165:1033–1040
da Silva MHL, Andrade EHA, Zoghbi MGB, Luz AIR, da Silva JD, Maia JGS (1999) The essential oils of Lantana camara L. occurring in North Brazil. Flavour Frag J 14:208–210
Daferera DL, Ziogas BN, Polissiou MG (2003) The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot 22:39–44
Díaz J, Barceló R, de Caceres FMA (1997) Changes in shikimate dehydrogenase and the end products of the shikimate pathway, chlorogenic acid and lignins, during the early development of seedlings of Capsicum annum. New Phytol 136:183–188
Fell DA (1992) Metabolic control analysis: a survey of its theoretical and experimental development. Biochem J 286:313–330
Flores HE, Doi Y, Cuello JL, Maldonado-Mendoza IE, Loyola-Vargas VM (1993) Green roots: photosynthesis and photoautotrophy in an underground plant organ. Plant Physiol 101:363–371
Flores-Pérez Û, Pérez-Gil J, Closa M, Wright LP, Botella-Pavía P, Phillips MA, Ferrer A, Gershenzon J, Rodríguez-Concepción M (2010) PLEIOTROPIC REGULATORY LOCUS 1 (PRL1) integrates the regulation of sugar responses with isoprenoid metabolism in Arabidopsis. Mol Plant 3:101–112
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Gaid MM, Sircar D, Beuerle T, Mitra A, Beerhues L (2009) Benzaldehyde dehydrogenase from chitosan-treated Sorbus aucuparia cell cultures. J Plant Physiol 166:1343–1349
Gaid MM, Sircar D, Müller A, Beuerle T, Liu B, Ernst L, Hänsch R, Beerhues L (2012) Cinnamate: CoA ligase initiates the biosynthesis of a benzoate-derived xanthone phytoalexin in Hypericum calycinum cell cultures. Plant Physiol 160:1267–1280
Ge X, d’Avignon DA, Ackerman JH, Sammons RD (2012) Observation and identification of 2-C-methyl-d-erythritol-2,4-cyclopyrophosphate in horseweed and ryegrass treated with glyphosate. Pestic Biochem Physiol 104:187–191
Guerra D, Anderson AJ, Salisbury FB (1985) Reduced phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities and lignin synthesis in wheat grown under low pressure sodium lamps. Plant Physiol 78:126–130
Haderlie HC, Widholm JM, Slife FW (1977) Effect of glyphosate on carrot and tobacco cells. Plant Physiol 60:40–43
Hall DO, Rao KK (1994) Photosynthesis, 5th edn. Cambridge University Press, Cambridge, pp 39–51
Hamill JD, Parr AJ, Rhodes MJC, Robins RJ, Walton NJ (1987) New routes to plant secondary products. Biotechnology 5:800–804
Hampel D, Mosandl A, Wüst M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66:305–311
Hartley RD, Harris PJ (1981) Phenolic constituents of cell walls of dicotyledonous. Biochem Syst Ecol 9:198–203
Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C (2004) Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J 38:765–778
Herrmann KM (1995) The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell 7:907–919
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15:176–184
Jacob A, Malpathak N (2004) Green hairy cultures of Solanum khasianum Clarke—a new route to in vitro solasodine production. Curr Sci 87:1442–1447
Kang YH, Parker CC, Smith AC, Waldron KW (2008) Characterization and distribution of phenolics in carrot cell wall. J Agric Food Chem 56:8558–8564
Kjeldsen F, Christensen LP, Edelenbos M (2001) Quantitative analysis of aroma compounds in carrot (Daucus carota L.) cultivars by capillary gas chromatography using large-volume injection technique. J Agric Food Chem 49:4342–4348
Kundu A, Jawali N, Mitra A (2012) Shikimate pathway modulates the elicitor-stimulated accumulation of fragment 2-hydroxy-4-methoxybenzaldehyde in Hemidesmus indicus root. Plant Physiol Biochem 56:104–108
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liang X, Dron M, Cramer CL, Dixon RA, Lamb CJ (1989) Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem 264:14486–14492
Lorato F, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M, Sharkey TD (1996) Different sources of reduced carbon contribute to form three classes of terpenoid emitted by Quercus ilex L. leaves. Proc Natl Acad Sci USA 93:9966–9969
Loreto F, Schnitzler J (2010) Abiotic stress and induced BVOCs. Trends Plant Sci 15:156–166
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98:8915–8920
Maiti S, Moon UR, Bera P, Samanta T, Mitra A (2014) The in vitro antioxidant capacities of Polianthes tuberosa L. flower extracts. Acta Physiol Plant 36:2597–2605
Mitra A, Mayer MJ, Mellon FA, Michael AJ, Narbad A, Parr AJ, Waldron K, Walton NJ (2002) 4-Hydroxycinnamoyl-CoA-hydratase/lyase, an enzyme of phenylpropanoid cleavage from Pseudomonas, causes formation of C6–C1 glucose conjugates when expressed in hairy roots of Datura stramonium. Planta 15:79–89
Mongelard G, Seemann M, Boisson AM, Rohmer M, Bligny R, Rivasseau C (2011) Measurement of carbon flux through the MEP pathway for isoprenoid synthesis by 31P-NMR spectroscopy after specific inhibition of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate reductase. Effect of light and temperature. Plant, Cell Environ 34:1241–1247
Mukherjee C, Sircar D, Chatterjee M, Das S, Mitra A (2014) Combating photooxidative stress in green hairy roots of Daucus carota cultivated under light irradiation. J Plant Physiol 171:179–187
Nishida K (1963) Osmotic swelling of isolated chloroplast. Plant Cell Physiol 4:247–256
Qually AV, Widhalm JR, Adebesin F, Kish CM, Dudareva N (2012) Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc Natl Acad Sci USA 109:16383–16388
Ramak P, Osaloo SK, Ebrahimzadeh H, Sharifi M, Behmanesh M (2013) Inhibition of the mevalonate pathway enhances carvacrol biosynthesis and DXR gene expression in shoot cultures of Satureja khuzistanica Jamzad. J Plant Physiol 170:1187–1193
Rhodes MJC, Parr AJ, Walton NJ (1997) Studies of secondary metabolic pathways in transformed roots. In: Doran PM (ed) Hairy roots: culture and applications. Harwood Academic Publishers, Amsterdam, pp 31–41
Rivasseau C, Seemann M, Boisson AM, Streb P, Gout E, Douce R, Rohmer M, Bligny R (2009) Accumulation of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate in illuminated plant leaves at supraoptimal temperatures reveals a bottleneck of the prokaryotic methylerythritol 4-phosphate pathway of isoprenoid biosynthesis. Plant Cell Environ 32:82–92
Rodríguez-Concepción M, Boronat A (2015) Breaking new grounds in the regulation of early steps of plant isoprenoid biosynthesis. Curr Opin Plant Biol 25:17–22
Rohmer M (1999) The discovery of a mevalonate independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Ross JR, Nam KH, D’Auria JC, Pichersky E (1999) S-Adenosyl-l-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys 367:9–16
Schnitzler JP, Madlung J, Rose A, Seitz HU (1992) Biosynthesis of p-hydroxybenzoic acid in elicitor treated carrot cell cultures. Planta 188:594–600
Sircar D, Mitra A (2008) Evidence for p-hydroxybenzoate formation involving phenylpropanoid chain-cleavage in hairy roots of Daucus carota. J Plant Physiol 165:407–414
Sircar D, Mitra A (2009) Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota 2. Confirming biosynthetic steps through feeding of inhibitors and precursors. J Plant Physiol 166:1370–1380
Sircar D, Roychowdhury A, Mitra A (2007a) Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota. J Plant Physiol 164:1358–1366
Sircar D, Dey G, Mitra A (2007b) A validated HPLC method for simultaneous determination of 2-hydroxy-4-methoxybenzaldehyde and 2-hydroxy-4-methoxybenzoic acid in root organs of Hemidesmus indicus. Chromatographia 65:349–353
Sircar D, Mukherjee C, Beuerle T, Beerhues L, Mitra A (2011) Characterization of p-hydroxybenzaldehyde dehydrogenase, the final enzyme of p-hydroxybenzoic acid biosynthesis in hairy roots of Daucus carota. Acta Physiol Plant 33:2019–3024
Sircar A, Cardoso HG, Mukherjee C, Mitra A, Arnholdt-Schmitt B (2012) Alternative oxidase (AOX) and phenolic metabolism in methyl-jasmonate treated hairy root cultures of Daucus carota L. J Plant Physiol 169:657–663
Souret FF, Weathers PJ, Wobbe KK (2002) The mevalonate-independent pathway is expressed in transformed roots of Artemisia annua and regulated by light and culture age. In Vitro Cell Dev Biol Plant 38:581–588
Taya M, Sato H, Kino-Oka M, Tone S (1994) Characterization of Pak-Bung green hairy roots cultivated under light irradiation. J Ferment Bioeng 78:42–48
Tone S, Taya M, Kino-Oka M (1997) Alteration of metabolite formation and morphological properties of hairy roots by environmental stimuli. In: Doran PM (ed) Hairy roots: culture and applications. Harwood Academic Publishers, Amsterdam, pp 65–72
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Vollsnes AV, Melø TB, Futsaether CM (2012) Photomorphogenesis and pigment induction in lentil seedling roots exposed to low light conditions. Plant Biol 14:467–474
Wildermuth MC (2006) Variation on a theme: synthesis and modification of plant benzoic acids. Curr Opin Plant Biol 9:288–296
Wright LP, Rohwer JM, Ghirardo A, Hammerbacher A, Ortiz-Alcaide M, Raguschke B, Schnitzler J-P, Gershenzon J, Phillips MA (2014) Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiol 165:1488–1504
Zeng S, Liu Y, Wang Y (2013) Light stress suppresses the accumulation of epimedins A, B, C, and icariin in Epimedium, a traditional medicinal plant. Acta Physiol Plant 35:3271–3275
Acknowledgments
This work was supported by a research grant [No. 38(1201)/08/EMR-II to A. Mitra] from the Council of Scientific and Industrial Research (CSIR), India. Chiranjit Mukherjee was supported from this grant in the form of a senior research fellow. Facilities created from a research grant (4-25/2013/TS-I) of Ministry of Human Resource Development (MHRD) India were utilized in this work. Finally, we thank Ishita Paul, a member of our research group, for checking grammatical errors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mukherjee, C., Samanta, T. & Mitra, A. Redirection of metabolite biosynthesis from hydroxybenzoates to volatile terpenoids in green hairy roots of Daucus carota . Planta 243, 305–320 (2016). https://doi.org/10.1007/s00425-015-2403-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2403-4