Abstract
Ginkgo biloba extract (GBE) helps lower cardiovascular disease risk. Diabetes mellitus (DM)-induced endothelial dysfunction is a critical and initiating factor in the beginning of diabetic vascular complications. It was reported that GBE causes an endothelial-dependent relaxation. This study was designed to figure out the molecular basis on which GBE protects from endothelial dysfunction in diabetes because the underlying mechanisms are unclear. Studies were performed in a normal control group and streptozotocin/nicotinamide-induced DM group. In aortas, notably diabetic aortas, GBE, and ginkgolide B (GB), a constituent of GBE, produced a dose-dependent relaxation. The relaxation by GB was abolished by prior incubation with L-NNA (an endothelial nitric oxide synthase (NOS) inhibitor), LY294002 (a phosphoinositide 3-kinase (PI3K) inhibitor), and Akt inhibitor, confirming the essential role of PI3K/Akt/eNOS signaling pathway. We also demonstrated that GB induced the phosphorylation of Akt and eNOS in aortas. The superoxide dismutase1 (SOD1) expression level decreased in DM aortas, but GB stimulation increased SOD activity and SOD1 expression in DM aortas. Our novel findings suggest that in DM aortas, endothelial-dependent relaxation induced by GB was mediated by activation of SOD1, resulting in activation of the Akt/eNOS signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are the leading cause of death in patients with diabetes. Endothelial dysfunction is also a pathophysiological distinctive feature characterized by diabetes [3]. Endothelial dysfunction is the situation where the endothelium loses its physiological properties but shows a tendency toward vasoconstriction and pro-thrombotic and pro-inflammatory states [3, 39]. Endothelial dysfunction is an important element in the development and progression of diabetic vascular complications [11].
Nitric oxide (NO) is pivotal for maintaining vascular homeostasis and function and is generated by NO synthase (NOS) [13]. The isoforms of NOS include endothelial constitutive NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). eNOS and nNOS are Ca2+-dependent constitutive NOS, whereas iNOS is Ca2+ independent. The NO produced by eNOS regulates physiological vasodilation [13]. Substantial evidence has shown that endothelial dysfunction characterized by the downregulated activity of eNOS is enormously involved in vascular dysfunction in diabetes [6, 22, 30, 36,37,38]. NO bioavailability depends on the balance of NO generation by eNOS and its elimination by oxidative stress [21]. Other scientists and we observed that the diabetes mellitus (DM) model presents a reduced endothelial-dependent relaxation response due to diminished NO bioavailability and increased oxidative stress consistent with high levels of reactive oxygen species [4, 19]. The increment of diminished-endothelial NO production and endothelial dysfunction by eNOS inactivation are of nice therapeutic target in diabetes. The activity of eNOS is positively controlled by serine/threonine protein kinase Akt by phosphorylation on Ser1177 [9]. The activity of eNOS can be excited by agonists such as insulin and flow-induced shear forces on the endothelial cell wall [12]. The role of endothelial function regulation and NO signaling in developing aortic complications in this disorder is still unclear.
Ginkgo biloba extract (GBE) is one of the most popular herbal supplements reported to have wide clinical therapeutic applications for peripheral vascular disease and age-dependent damages [8]. The mechanisms for the beneficial results of GBE are considered to be due to the scavenging of free radicals, redress in circulation, and antagonism of platelet-activating factors [7]. The pharmacological mechanism of GBE that causes endothelial-dependent relaxation in isolated rabbit aorta is reported via eNOS activation and NO releasing [5, 23]. However, the mechanisms underlying the GBE-induced relaxation response are exceedingly complex because of its different constituents. The mechanisms of these constituents in NO production are still unknown. Standardized GBE contains 24% flavonoids, 6% terpenes, and unknown compounds. Some GBE components have been identified. Flavonoids, such as isorhamnetin (Iso) and terpenes, including bilobalide (Bil), ginkgolide A (GA), and ginkgolide B (GB), are regarded as major active constituents of GBE [20]. These constituents also have vasorelaxant properties [10]. Ginkgolides, such as GA and GB and bilobalide, which are ginkgolide terpenoids, are two important bioactive constituents of GBE [43]. Previously, ginkgolides, including GB, were isolated from GBE. This research finding has led to the postulation that GBE and its constituents might have protective effects on diabetic vascular complications.
It has been reported that the ginkgolides, such as GA and GB, control the activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [25]. Because the activation of the PI3K/Akt signaling pathway is redox-sensitive, we made a hypothesis that the ginkgolides would cause an endothelial-dependent relaxation through activation of PI3K/Akt, potentially due to the antioxidant action. We also analyzed the acute effects of the ginkgolides on endothelial function in diabetic model mice and control mice to elucidate the beneficial effects of the constituents of GBE.
Materials and methods
Animal procurement and treatment
In this experimental study, male Institute of Cancer Research (ICR) mice (4 weeks old) were obtained from the Tokyo Animal Laboratories (Tokyo, Japan). All mice were housed under standard laboratory conditions and fed a standard laboratory diet and water ad libitum. All mice used in this study were treated in accordance with the principles and guidelines on animal care of Hoshi University Animal Care and Use Committee as reviewed by an ethics committee (approval No. P22-003) (accredited by the Ministry of Education, Culture, Sports, Science and Technology of Japan).
Experimental design
After 1 week of animal acclimatization, a single dose of 200 mg/kg of streptozotocin (STZ; dissolved in a citrate acid buffer; Wako, Osaka, Japan) was administered by a tail vein injection 15 min after an intraperitoneal administration of 1.5 g/kg of nicotinamide (NA; dissolved in normal saline; Sigma-Aldrich, St. Louis, MO, USA) for the induction of type 2 diabetes [22, 36, 37]. The presence of DM at 12–16 weeks after NA-STZ injection was confirmed by assaying plasma glucose levels. In subsequent experiments, mice with a plasma glucose level of more than 400 mg/dL were used.
The mice were anesthetized with isoflurane for surgical procedures and subsequently euthanized via thoracotomy and exsanguination, and plasma samples were collected and centrifuged at 3500 rpm for 10 min and kept at − 20℃ until glucose levels were measured. Plasma glucose levels were analyzed using a commercial assay kit (Wako). The thoracic aorta of all animals was removed immediately and placed in a petri dish filled with cold modified Krebs–Henseleit solution (KHS; 118.0 mM NaCl, 4.7 mM KCl, 25.0 mM NaHCO3, 1.8 mM CaCl2, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 11.0 mM glucose).
Experimental protocol for vascular reactivity assessment
The aortas were cleaned of excess connective tissue and fat and cut into rings of 2 mm in length. In all experiments, great care was taken to avoid damaging the luminal surface of the endothelium. Aortic rings were suspended in the organ bath system filled with KHS and continuously aerated with a mixture of 5% CO2 and 95% O2 at 37℃. The vascular isometric force was monitored using an isometric transducer (TB-611 T; Nihon Kohden, Tokyo, Japan) linked to a PowerLab recording system (AD Instruments, Australia).
The rings were equilibrated for 45 min under a resting tension of 1.5 g before the experiment. During the equilibration period, the rings were washed every 15 min. At the end of the equilibration, all segments were stimulated with potassium chloride (80 mM) to determine smooth muscle contractility. After rinsing with KHS to base-line tension, rings were equilibrated for 30 min by observing various agonist-induced responses. Rings were then contracted with a submaximal concentration of prostaglandin F2α (PGF2α; 10−6 to 3 × 10−6 M) (Fuji Pharma, Tokyo, Japan), which produced approximately 1 g force. After reaching a plateau of contraction, cumulative concentration–response curves to acetylcholine (ACh; 10−9 to 10−5 M) (Daiichi-Sankyo Pharmaceuticals, Tokyo, Japan), GBE (10−8 to 10−5 g/mL) (Tokiwa, Chiba, Japan), Iso (10−9 to 10−5 M) (Tokiwa), Bil (10−9 to 10−5 M) (Tokiwa), GA (10−9 to 10−5 M) (Tokiwa), and GB (10−9 to 10−5 M) (Tokiwa) were obtained for relaxations. Rings were incubated 30 min before the experiment with GB (10−6 M) to determine the participation of GB in response to ACh. Rings were incubated 30 min before the experiment with NG-nitro-L-arginine (L-NNA; 10−4 M, an eNOS inhibitor) (Sigma-Aldrich, St. Louis, MO, USA) to determine the participation of NO in response to GB. Rings were incubated with 1L-6-hydroxymethyl-chiro-inositol 2[(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate] (Akt inh; 10−6 M, an Akt inhibitor) (Calbiochem, San Diego, CA, USA) and LY294002 (LY; 10–6 M, a PI3K inhibitor) (Calbiochem) 30 min before the experiment with GB, to determine the mechanisms in response to GB.
Nitrite and nitrate in the aorta
The nitrite and nitrate concentrations were measured by ENO20 NOx Analyzer (Eicom, Kyoto, Japan), based on the liquid chromatography method with post-column derivatization with Griess reagent, as described previously [19, 22, 36,37,38]. Briefly, the isolated aortas were cleared from surrounding tissue and cut into pieces of 4 mm length, added to tubes containing KHS with or without LY294002 or Akt inh (both at 10−6 M), and incubated for 30 min at 37℃. GB (10−6 M) was added to the tube for 20 min. Finally, the semi-dried aorta was weighed and frozen in liquid nitrogen. The frozen aortas were used for Western blotting to determine the SOD activity. The quantitative results for NO production (NOx; nitrate + nitrite level) were expressed in mol/g (weight of the aorta)/min.
Western blot analysis
Western blot analysis was performed as described in previous studies [19, 22, 36,37,38]. Briefly, frozen aortas (explained in the “Nitrite and nitrate in the aorta” section) were lysed with RIPA buffer supplemented with protease inhibitors. Total protein lysates were centrifuged at 13,000 rpm for 10 min at 4℃, and protein concentrations were analyzed by the BCA method (Thermo Scientific, Rockford, IL, USA). An equal amount of protein was separated using SDS/PAGE and transferred to a PVDF membrane by electroblotting. The membranes were blocked with ImmunoBlock (Sumitomo Pharma, Osaka, Japan) for 90 min at room temperature and then incubated overnight at 4℃ with primary antibodies. Phosphorylated Akt (p-Akt), Akt, phosphorylated eNOS (p-eNOS), eNOS, SOD1, SOD2, SOD3, and β-actin protein levels were analyzed using specific primary antibodies as below: anti-p-Akt (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA), anti-Akt (1:1000 dilution; Cell Signaling Technology), anti-p-eNOS (1:1000 dilution; Cell Signaling Technology), anti-eNOS (1:1000 dilution; BD Biosciences, NJ, USA), anti-SOD1 (1:1000 dilution; Enzo Life Sciences, Inc., Farmingdale, NY, USA), anti-SOD2 (1:1000 dilution; BD Biosciences), anti-SOD3 (1:1000 dilution; Enzo Life Sciences, Inc.), and anti- β -actin (1:5000 dilution; Sigma Chemical, St. Louis, MO, USA). After washing, membranes were probed with peroxidase-conjugated secondary antibodies at 37℃ for 20 min. The protein signal was detected using the Tridento femto (Gene Tex, LA, IN, USA) and visualized by Light-capture (ATTO, NY, USA) and analyzed using CS Analyzer (version 3.0, ATTO). Protein activity following treatments was quantified as the levels of the phosphor-protein divided by total protein expression, and β -actin was used for normalization of the total protein expression.
SOD activity
The frozen aortas were homogenized in RIPA buffer supplemented with protease inhibitors, and protein concentrations were analyzed by the BCA method (explained in the “Western blot analysis” section). Then the activity of SOD was determined according to the manufacturer’s recommendations (Dojin Science Inc., Kumamoto, Japan).
Statistical analysis
Results are expressed as means ± standard error (SE), in which n represents the number of mice. Statistical analysis was performed by 2-way repeated measurements or 1-way analysis of variance (ANOVA) followed by post hoc Tukey’s multiple comparison test, which compares each dose-mean with the other dose-mean. A difference was considered significant when P ≤ 0.05. Data analysis was performed in GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA).
Results
Basic characteristics of mice with type 2 diabetes
The NA/STZ-induced type 2 DM model is a metabolic disorder that does not cause obesity and causes hyperglycemia [22, 29, 36, 37]. This study confirmed our previous study results [22, 36, 37] and the results of a study by Masiello et al. [29] in the NA/STZ-induced DM model. Mice body weight in control (47.3 ± 4.8 g) and DM (43.8 ± 5.5 g) groups were not different. The DM group (936.6 ± 29.0 mg/dL) showed dramatically increased levels of non-fasting plasma glucose compared with the control (221.1 ± 31.8 mg/dL; P < 0.001). These data are consistent with features of type 2 DM and our previous reports [22, 36, 37].
Effect of GBE on vasorelaxation
GBE (10−8 to 10−5 g/mL) strongly relaxed the contraction-induced PGF2α in a concentration-dependent manner, as shown in Fig. 1. The significant relaxation was produced by 3 × 10−6 g/mL of GBE in DM aortas.
Relaxation effects of Ginkgo biloba extract (GBE) in aortas of control and DM. The ordinate shows the relaxation response normalized by prostaglandin F2α (PGF2α)-induced precontraction, and the abscissa shows the concentration of GBE. Each point represents the mean ± SE (n = 5). ***P < 0.001, compared with control
Effect of constituents of GBE on vasorelaxation
GBE mainly consists of flavonoids such as isorhamnetin and terpenoids such as bilobalide, GA, and GB. The relaxation by each constituent (isorhamnetin, bilobalide, or GA) was much weaker in the aortas of both control and DM groups (Fig. 2a–c). Isorhamnetin and GA at high concentrations caused vasorelaxation but were not markedly different between control and DM. GB provoked a concentration-dependent relaxation in all groups (Fig. 2d). DM caused an enhancement in GB-induced relaxation response.
Relaxation effects of the constituent of GBE in aortas of control and DM. Isorhamnetin (a), bilobalide (b), ginkgolide (GA) (c), and ginkgolide (GB) (d)-induced relaxation in aortas. The ordinate shows the relaxation response normalized by PGF2α -induced precontraction, and the abscissa shows the concentration of various constituents of GBE. Each point represents the mean ± SE (n = 5). ***P < 0.001, compared with control
Figure 3 shows the effect of GB on relaxation induced by ACh in control and DM aortic rings. GB significantly intensified the maximum relaxation induced by ACh in a dose-related manner.
Effect of GB on relaxation induced by acetylcholine (ACh) in aortic rings precontracted with PGF2α isolated from control (a) and DM (b). The ordinate indicates the relaxation response normalized by PGF2α-induced precontraction, and the abscissa shows the concentration of ACh. Each point represents the mean ± SE (n = 5). *P < 0.05, compared with control. ## P < 0.01, ### P < 0.001, compared with DM
Pretreatment for 30 min with L-NNA was performed to observe the involvement with GB-induced relaxation via NO activation. In the presence of L-NNA (10−4 M), the GB-induced relaxation responses completely disappeared (Fig. 4a), suggesting that GB-induced vascular relaxation response depends on NO via eNOS activation. The difference between the control and DM groups was not significant.
Effect of L-NNA, LY294002, or Akt inhibitor on GB-induced relaxation response and GB-stimulated nitric oxide (NO) production. a–c The aortic rings from control and DM were incubated with cumulative doses of GB in the presence of L-NNA (10−4 M) (a), LY294002 (LY; 10−6 M) (b), or Akt inhibitor (Akt inh; 10−6 M) (c) for 30 min. Each point represents the mean ± SE (n = 6). d Effects of GB on NO production in control and DM. The aortic rings of control or DM were determined the levels of GB (10−6 M, 20 min)-stimulated NO production in the absence or the presence of LY and Akt inh (relatively, 10−6 M, 30 min). Each point represents the mean ± SE (n = 8). **P < 0.01, compared with non-stimulated control aorta. ### P < 0.001, compared with non-stimulated DM aorta. $ P < 0.05, compared with GB-stimulated control aorta. & P < 0.05, && P < 0.01, compared with GB-stimulated DM aorta
Polyphenolic compounds induce endothelial-dependent relaxation through PI3K/Akt/eNOS signaling pathway [33, 37]. Therefore, we tested the effect of LY294002 and Akt inhibitor on GB-induced relaxation response. LY294002 (10−6 M) and Akt inhibitor (10−6 M) completely cleared up the GB-induced relaxation response but were not strongly different between the control and DM groups (Fig. 4b and c). NO production evoked by the GB (10−6 M) is summarized in Fig. 4d. GB produced NO in the aortas of both control and DM groups. Incubation with LY294002 and Akt inhibitor also abolished NO production by GB. Therefore, it was apparent that pretreatment to a PI3K inhibitor and Akt inhibitor attenuated the NO production and endothelial-dependent relaxation evoked by GB, suggesting that the GB-induced endothelial NO production and relaxation response are mediated by activation of the PI3K/Akt signaling pathway.
Activation of eNOS via the Akt pathway
We investigated the GB-induced phosphorylation of Akt (at Ser473) and eNOS (at Ser1177) in aortas to correctly understand the mechanism of GB-induced endothelial-dependent relaxation. GB-induced phosphorylation of Akt and eNOS was demonstrated by Western blotting. The phosphorylation of Akt was found to be increased after GB stimulation in DM and controls, though there was no significant difference between DM and controls under non-stimulation and GB stimulation (Fig. 5a and b). GB induced eNOS phosphorylation in the DM aortas and tended to increase eNOS phosphorylation in the controls, though there was no significant difference between DM and controls under non-stimulation and GB stimulation (Fig. 5a and c). These data also suggested that the GB-induced phosphorylation of eNOS at Ser1177 is probably Akt-dependent.
Mechanisms of GB-induced NO production in the aorta: Western blotting showing the protein expression of p-Akt (at Ser473), Akt, p-eNOS (at Ser1177), and eNOS in the aortas of control and DM. Aortas were treated with 10−6 M of GB for 20 min. a Representative data of Western blot was utilized to measure expression. b, c The quantitative analysis of the expression of p-Akt (b) and p-eNOS (c), respectively. Phosphorylation of Akt and eNOS protein levels was expressed relative to the amount of total protein. The results are expressed as a mean ± SE (n = 5). **P < 0.01, compared with non-stimulated control aorta. # P < 0.05, compared with non-stimulated DM aorta
Effect of GB on the activity of the antioxidant enzyme in the aorta
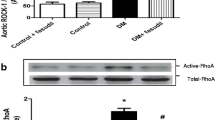
The activity and expression of antioxidant enzymes are the major response to relieving oxidative stress damage in aortas. As shown in Fig. 6a, the activity of total SOD was reduced in the DM group compared to the control in the basal condition, whereas GB significantly elevated the activity of total SOD in DM aortas but not in control aortas. The aortic expression level of the SOD isoform was measured by Western blot analysis (Fig. 6b–e). Western blot analysis revealed remarkably reduced SOD1 protein in DM aortas compared with controls, whereas SOD2 and SOD3 proteins were not observed between DM and control aortas. However, the GB stimulation significantly increased SOD1 in DM aortas compared with non-stimulated DM aortas, although SOD1 levels did not differ significantly between DM and control under GB stimulation. SOD2 and SOD3 protein changes induced by GB were not observed in DM or control aortas. These results suggested that GB was capable of ameliorating oxidative stress via SOD1 activity in the DM aorta.
Effects of GB on activity and expression of superoxide dismutase (SOD) in aortas of control and DM. a The total SOD activity in the aorta of control and DM was determined in the absence or presence of GB (10−6 M, 20 min). b Representative data of Western blot was utilized to measure expression. c–e The quantitative analysis of the expression of SOD1 (c), SOD2 (d), and SOD3 (e), respectively. The results are expressed as a mean ± SE (n = 5–6). *P < 0.05, compared with non-stimulated control aorta. # P < 0.05, ## P < 0.01, compared with non-stimulated DM aorta
Discussion
The present findings indicate that GB included in GBE is potent inducer of the endothelial formation of NO as assessed in isolated aortas. This response depends on Akt/eNOS signal activation and is partly a redox-sensitive mechanism (Fig. 7) in DM aortas. These findings support a key role of the SOD/Akt/eNOS/NO pathway in the protective effects of GB in DM.
Despite the increasing number of studies indicating the instrumental role of GBE in the treatment of cardiovascular diseases [31, 32], its clinical merit is still underrepresented with the lack of knowledge about its cellular and molecular mechanisms of action [43]. Another previous study demonstrated that GBE induced dose-dependent vascular relaxation through the NO pathway in the isolated rat aorta, suggesting that GBE may modulate NO production [23]. The present experiments declared that GBE-induced relaxation response was increased in the aortas of mice with DM. However, there is little information regarding the mechanisms of vasorelaxation induced by GBE. GBE is a distinct plant extract. Its major constituents are terpenoids (such as bilobalide, GA, and GB) and 30 types of flavonoids [27]. In the present study, we examined the relative contribution of each constituent in GBE-induced relaxation responses and compared the relaxation responses induced by major constituents such as terpenoids (bilobalide, GA, and GB) and flavonoids (isorhamnetin). Isorhamnetin, bilobalide, and GA showed very weaker vasorelaxation than GB. The maximum relaxation induced by GB in DM was significantly stronger than in control. Therefore, it can be suggested that GB may contribute to GBE’s effects sufficiently on vasorelaxation response in DM.
Recent studies have clarified that GB protects endothelial cells [24, 27]. Similarly, in the present study, we showed GB causes vasorelaxation responses. Our study clarified that GB enhanced vasorelaxation to ACh in both control and DM mice, which may be related to increased eNOS activity and NO production. NO is a critical factor for regulating aortic responses, and endothelial dysfunction may be associated with decreased NO production in mice with DM [19, 22, 36,37,38]. These results suggested that the mechanisms underlying GB’s effect in preventing endothelial dysfunction could involve an enhanced NO production, leading to improved endothelial-dependent responses.
We researched whether the activation of GB-induced relaxation response was mediated by the PI3K/Akt/eNOS/NO pathway. PI3K/Akt signaling is an important pathway in controlling endothelial function [2, 40]. PI3K can stimulate Akt and phosphorylate eNOS, leading to NO production and vascular relaxation [2]. Aortic rings were preincubated with LY294002 to research whether the PI3K is related to the changes in vascular relaxation response induced by GB. The results in the control and DM groups showed that the preincubation completely suppressed the vasorelaxation effects of GB in LY294002. The results suggest that PI3K is involved in the vasorelaxation induced by GB in the aorta from both control and DM.
The PI3K/Akt signaling pathway is linked to vascular health [35]. Akt is a serine/threonine protein kinase recruited to the endothelial cell membrane by way of its binding to PI3K-produced phosphoinositides. PI3K can phosphorylate the Ser473 site of downstream Akt, which leads to kinase activation, and is involved in endothelial-dependent relaxation [34, 40]. In the present study, we observed that preincubation with Akt inhibitor significantly attenuated the GB-induced relaxation responses in the aortas of control and DM groups. We revealed that GB induced concurrent phosphorylation of Akt. Additionally, eNOS is a downstream regulator of PI3K and Akt [1]. As a rate-limiting enzyme for NO synthesis, eNOS plays a critical role in maintaining vascular functions [14, 16, 22, 36,37,38]. Moreover, eNOS leads to NO production by activating PI3K/Akt-mediated phosphorylation [14, 42]. Depression to eNOS activity can reduce NO synthesis or activity, induce oxidative stress, and increase vascular resistance and endothelial dysfunction [9, 15]. To research whether the eNOS is related to the changes in GB-induced relaxation response, the aortic rings of mice from control and DM groups were preincubated with L-NNA. The results showed that preincubating the DM and control with L-NNA, an NOS inhibitor, completely suppressed the GB-induced relaxation responses. GB also induced eNOS phosphorylation in the DM and control aortas. These results suggest that eNOS is involved in the vasorelaxation caused by GB in the aorta of mice from the control and DM groups.
Furthermore, the GB high concentration elicited a stronger reaction in the DM than the control. The data in Fig. 4a–c show that there is no significant difference in GB-induced relaxation between DM and controls and that GB-induced relaxation disappears in the presence of an NOS inhibitor, a PI3K inhibitor, and an Akt inhibitor, implying that GB-induced relaxation at high concentrations activates the PI3K/Akt/eNOS signaling pathway in the DM aortas.
In this study, the ACh-induced relaxation tended to be smaller in rings from DM but not significantly. These results are consistent with previous studies [22, 36]. When aortic rings from DM and controls were stimulated with GB, the ACh-induced relaxation responses were significantly higher in the stimulated groups than in the non-stimulated groups. Previous studies have shown that eNOS is activated via two distinct pathways: an increase in intracellular Ca2+ levels and eNOS phosphorylation via the Akt signaling pathway [22, 36, 37]. ACh-induced eNOS activation/NO production through increased intracellular Ca2+ levels is a well-known endothelial function. However, Akt plays no role in the ACh-induced relaxation response. Furthermore, our previous study reported that ACh induced a relaxation response, which did not change under the stimulation of an Akt inhibitor, suggesting that ACh-induced NO production is independent of the Akt pathway [22]. In the present study, we found that GB signaling activates eNOS via Akt activity. The results of the present study suggest that GB benefits the vascular endothelium by activating the Akt/eNOS signaling pathway, independent of ACh pathway regulation in the aorta. However, Fig. 3 indicates that the ACh-dependent and GB-induced NO production act synergistically but not additively. The results of the present study indicate that the total NO production in the endothelium is limited.
Oxidative stress contributes to DM-induced endothelial dysfunction [17, 28]. The most important cellular mechanism to provide a shield against endothelial dysfunction involves the activity of SODs, which consists of the cytoplasmic Cu (copper)/Zn SOD (SOD1), the mitochondrial Mn-SOD (SOD2), and the extracellular SOD (SOD3) [18]. In DM-induced endothelial dysfunction, oxidative stress-generating systems can generate intracellular and extracellular superoxide [17, 28]. The antioxidants such as SOD are the primary defense against superoxide. In the current study, the SOD activity tended to decrease in DM, whereas GB stimulation increased the SOD activity, suggesting that the GB-treated DM aortas were being protected from superoxide.
Moreover, the levels of SOD1 in DM aortas significantly decreased, whereas GB treatment increased SOD1 levels against the reduction in DM aortas. We assumed that there is no change in expression of SOD2 or SOD3 proteins in both control and DM aortas, and there is no increase in SOD2 or SOD3 proteins after GB stimulation. These results suggest the inability of SOD1 to restore by GB stimulation in DM aortas completely. On the other hand, these results indicate that the GB stimulation has no effect on the levels and activity of SODs in control aortas.
Endothelial cells can produce reactive oxygen species (ROS). The vascular production of ROS can significantly increase in diabetes. Increased ROS production can reduce NO bioavailability and endothelial function. ROS acts as a chemical messenger for cell signaling to mediate Phosphatase and Tensin Homolog Deleted from Chromosome 10 (PTEN)/PI3K/Akt pathway. PTEN is located upstream of the PI3K/Akt pathway and clearly plays a role in inhibiting PI3K/Akt expression. When PTEN expression is upregulated, its negative regulation significantly reduces PI3K/Akt expression. We performed testing at the protein level by using Western blot analysis. We found that any changing trend of SOD1 and Akt expression levels was consistent with the changing trend of SOD activity in the GB-treated aortas from DM. This finding suggests that the PI3K/Akt signal pathway is closely related to SOD1 activity change in DM aortas. However, it is still unclear how the increased SOD1 activity activated the PI3K/Akt signal pathway. This notion is consistent with studies showing ischemic reperfusion injury in a rabbit model [41]. On the other hand, the present study found that GB induced SOD1 activation in DM aortas. At the same time, we showed that GB induced Akt phosphorylation regardless of the levels and activity of SODs in control aortas. Thus, in both DM and control aortas GB induced Akt phosphorylation. However, this relationship between the SOD activity and Akt phosphorylation under GB stimulation was observed only in DM aortas. We intended to examine the GB-induced endothelial relaxation under SOD1 inhibition. However, there was a limitation in the GB powder and DM mice, and we were not able to conduct these experiments. Liu et al. reported that GB upregulated the SOD1 levels in cerebral ischemia [26]. We believe that GB improves SOD1 expression, which controls the PTEN/PI3K/Akt/eNOS signaling pathway and induces NO-mediated endothelial function. However, further investigations are needed to discover the mechanisms.
In summary, this study has shown that GBE causes an endothelial-dependent relaxation mediated by NO produced by GB stimulation. It is suggested that this effect of GB is mediated by activation of the PI3K/Akt signaling pathway, causing the phosphorylation of eNOS. The major new finding of the present study is that GB prevents endothelial dysfunction in the DM aorta. The mechanism underlying the protective effect of GB might involve increased aortic SOD1 activity and increased NO bioavailability in endothelial cells. GB-induced Akt and eNOS phosphorylation may also contribute to the protective effect of GB against diabetic endothelial dysfunction. However, our experimental system also has certain limitations. Results indicate that GB-induced relaxation was related to the PI3K/Akt/eNOS/NO signaling pathway. However, we could not clarify how SOD1 makes PI3K signaling work in DM. The relationship between the SOD1 activity and PI3K/Akt signaling pathway in DM is unclear and needs further research. Regardless, our work would add GB in GBE to the growing list of remedies for diabetic vascular complications because we could at least partially reveal the mechanism on a molecular level.
Data availability
Supporting data will be made available to readers upon request to the corresponding authors.
References
Abeyrathna P, Su Y (2015) The critical role of Akt in cardiovascular function. Vascul Pharmacol 74:38–48. https://doi.org/10.1016/j.vph.2015.05.008
Anwar MA, Samaha AA, Ballan S, Saleh AI, Iratni R, Eid AH (2017) Salvia fruticosa induces vasorelaxation in rat isolated thoracic aorta: role of the PI3K/Akt/eNOS/NO/cGMP signaling pathway. Sci Rep 7:686. https://doi.org/10.1038/s41598-017-00790-9
Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP (2011) Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34:S285–S290. https://doi.org/10.2337/dc11-s239
Azul L, Leandro A, Boroumand P, Klip A, Seiça R, Sena CM (2020) Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic Biol Med 146:264–274. https://doi.org/10.1016/j.freeradbiomed.2019.11.002.
Chen X, Salwinski S, Lee TJ (1997) Extracts of Ginkgo biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin Exp Pharmacol Physiol 24:958–959. https://doi.org/10.1111/j.1440-1681.1997.tb02727.x
Davel AP, Wenceslau CF, Akamine EH, Xavier FE, Couto GK, Oliveira HT, Rossoni LV (2011) Endothelial dysfunction in cardiovascular and endocrine-metabolic diseases: an update. Braz J Med Biol Res 44:920–932. https://doi.org/10.1590/s0100-879x2011007500104
De Smet PA (2002) Herbal remedies. N Engl J Med 347:2046–2056. https://doi.org/10.1056/NEJMra020398
Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE (2000) Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil 81:668–678. https://doi.org/10.1016/s00039993(00)90052-2
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605. https://doi.org/10.1038/21224
Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J (2001) Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol 133:117–124. https://doi.org/10.1038/sj.bjp.0704064
Fetterman JL, Holbrook M, Westbrook DG, Brown JA, Feeley KP, Breton-Romero R, Linder EA, Berk BD, Weisbrod RM, Widlansky ME, Gokce N, Ballinger SW, Hamburg NM (2016) Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol 15:53. https://doi.org/10.1186/s12933-016-0372-y
Fleming I, Fisslthaler B, Dixit M, Busse R (2005) Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 118:4103–4111. https://doi.org/10.1242/jcs.02541
Förstermann U, Münzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714. https://doi.org/10.1161/CIRCULATIONAHA.105.602532
Fraccarollo D, Widder JD, Galuppo P (2008) Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation 118:818–827. https://doi.org/10.1161/CIRCULATIONAHA.107.717702
Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601. https://doi.org/10.1038/21218
Gadkari TV, Cortes N, Madrasi K (2013) Agmatine induced NO dependent rat mesenteric artery relaxation and its impairment in salt-sensitive hypertension. Nitric Oxide 35:65–71. https://doi.org/10.1016/j.niox.2013.08.005
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
Heistad DD (2006) Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol 26:689–695. https://doi.org/10.1161/01.ATV.0000203525.62147.28
Ishida K, Taguchi K, Matsumoto T, Kobayashi T (2014) Activated platelets from diabetic rats cause endothelial dysfunction by decreasing Akt/endothelial NO synthase signaling pathway. PLoS One 9:e102310. https://doi.org/10.1371/journal.pone.0102310.
Kleijnen J, Knipschild P (1992) Ginkgo biloba. Lancet 340:1136–1139. https://doi.org/10.1016/0140-6736(92)93158-j
Kim JH, Auger C, Kurita I, Anselm E, Rivoarilala LO, Lee HJ, Lee KW, Schini-Kerth VB (2013) Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide 35:54–64. https://doi.org/10.1016/j.niox.2013.08.002
Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K (2004) Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension 44:956–962. https://doi.org/10.1161/01.HYP.0000147559.10261.a7
Kubota Y, Tanaka N, Umegaki K, Takenaka H, Mizuno H, Nakamura K, Shinozuka K, Kunitomo M (2001) Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci 69:2327–2336. https://doi.org/10.1016/s0024-3205(01)01303-0
Li R, Chen B, Wu W, Li J, Qi R (2009) Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor κB activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. J Pharmacol Sci 110:362–369. https://doi.org/10.1254/jphs.08275fp
Liu J, Wu P, Xu Z, Zhang J, Liu J, Yang Z (2020) Ginkgolide B inhibits hydrogen peroxide-induced apoptosis and attenuates cytotoxicity via activating the PI3K/Akt/mTOR signaling pathway in H9c2 cells. Mol Med Rep 22:310–316. https://doi.org/10.3892/mmr.2020.11099
Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, Li F, Gu S, Zong S, Zhou J, Cao L, Wang Z, Xiao W (2019) Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 24:441–452. https://doi.org/10.1007/s12192-019-00977-1
Liu X, Zhao G, Yan Y, Bao L, Chen B, Qi R (2012) Ginkgolide B reduces atherogenesis and vascular inflammation in ApoE(-/-) mice. PLoS One 7:e36237. https://doi.org/10.1371/journal.pone.0036237.
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25:29–38. https://doi.org/10.1161/01.ATV.0000150649.39934.13
Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G (1998) Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 47:224–229. https://doi.org/10.2337/diab.47.2.224
Meza CA, La Favor JD, Kim D, Hickner RC (2019) Endothelial dysfunction: is there a hyperglycemia-induced imbalance of NOX and NOS? Int J Mol Sci 20:3775. https://doi.org/10.3390/ijms20153775
Morgenstern C, Biermann E (2002) The efficacy of Ginkgo special extract EGb 761 in patients with tinnitus. Int J Clin Pharmacol Ther 40:188–197. https://doi.org/10.5414/cpp40188
Muir AH, Robb R, McLaren M, Daly F, Belch JJ (2002) The use of Ginkgo biloba in Raynaud’s disease: a double-blind placebo-controlled trial. Vasc Med 7:265–267. https://doi.org/10.1191/1358863x02vm455oa
Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB (2004) Red wine polyphenols-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J 19:455–457. https://doi.org/10.1096/fj.04-2146fje
Ou HC, Lee WJ, Lee SD, Huang CY, Chiu TH, Tsai KL, Hsu WC, Sheu WH (2010) Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharm 248:134–143. https://doi.org/10.1016/j.taap.2010.07.025
Roviezzo F, Cuzzocrea S, Lorenzo AD, Brancaleone V, Mazzon E, Paola RD, Bucci M, Cirino G (2007) Protective role of PI3-kinase-Akt-eNOS signaling pathway in intestinal injury associated with splanchnic artery occlusion shock. Br J Pharmacol 151:377–383. https://doi.org/10.1038/sj.bjp.0707233
Taguchi K, Matsumoto T, Kamata K, Kobayashi T (2012) Inhibitor of G protein-coupled receptor kinase 2 normalizes vascular endothelial function in type 2 diabetic mice by improving β-arrestin 2 translocation and ameliorating Akt/eNOS signal dysfunction. Endocrinology 153:2985–2996. https://doi.org/10.1210/en.2012-1101
Taguchi K, Hida M, Hasegawa M, Matsumoto T, Kobayashi T (2016) Dietary polyphenol morin rescues endothelial dysfunction in a diabetic mouse model by activating the Akt/eNOS pathway. Mol Nutr Food Res 60:580–588. https://doi.org/10.1002/mnfr.201500618
Taguchi K, Hida M, Narimatsu H, Matsumoto T, Kobayashi T (2017) Glucose and angiotensin II-derived endothelial extracellular vesicles regulate endothelial dysfunction via ERK1/2 activation. Pflugers Arch 469:293–302. https://doi.org/10.1007/s00424-016-1926-2
Widlansky ME, Gokce N, Keaney JF Jr, Vita JA (2003) The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42:1149–1160. https://doi.org/10.1016/S0735-1097(03)00994-X
Xia T, Guan W, Fu J (2016) Tirofiban induces vasorelaxation of the coronary artery via an endothelium-dependent NO-cGMP signaling by activating the PI3K/Akt/eNOS pathway. Biochem Biophy Res Commun 474:599–605. https://doi.org/10.1016/j.bbrc.2016.03.110
Yu QJ, Yang Y (2016) Function of SOD1, SOD2, and PI3K/AKT signaling pathways in the protection of propofol on spinal cord ischemic reperfusion injury in a rabbit model. Life Sci 148:86–92. https://doi.org/10.1016/j.lfs.2016.02.005
Zhang L, Liu Q, Lu L (2011) Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1a accumulation via phosphatidylinositol 3-kinase/Akt pathway. J Pharmacol Exp Ther 338:485–491. https://doi.org/10.1124/jpet.111.180992
Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C (2004) Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev 22:309–319. https://doi.org/10.1111/j.1527-3466.2004.tb00148.x
Acknowledgements
We thank I. Tano and N. Bessho for technical assistance. We thank Enago (www.Enago.jp) for the English language review.
Funding
This work was partly supported by the JSPS KAKENHI Grant Numbers JP21K06811 (to Kumiko Taguchi) and JP21K06878 (to Tsuneo Kobayashi).
Japan Society for the Promotion of Science,JP21K06811,JP21K06878
Author information
Authors and Affiliations
Contributions
Kumiko Taguchi conceived and designed the study, performed the statistical analysis, and wrote the manuscript. Kumiko Taguchi and Kanami Okudaira conducted the experiments. Takayuki Matsumoto and Tsuneo Kobayashi helped develop the project, perform some experiments, and write the manuscript. Tsuneo Kobayashi is the guarantor of this work and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The animal study was reviewed and approved by the Hoshi University Animal Care and Use Committee. The application of approval number is P22-003. Consent to participate is “not applicable” as human study is not included in this work.
Human and animal ethics
Human ethics is “not applicable” as human study is not included in this work. This study was performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals from the Committee for the Care and Use of Laboratory Animals of Hoshi University (Tokyo, Japan).
Consent for publication
All authors have read the text of the article. All authors agreed with the content; all gave explicit consent to submit and are completely satisfied with its publication. The obtained consent was from the responsible authorities at the institute/organization where the work has been carried out.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taguchi, K., Okudaira, K., Matsumoto, T. et al. Ginkgolide B caused the activation of the Akt/eNOS pathway through the antioxidant effect of SOD1 in the diabetic aorta. Pflugers Arch - Eur J Physiol 475, 453–463 (2023). https://doi.org/10.1007/s00424-023-02790-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02790-3