Abstract
The interactions between plants and their herbivores are highly complex systems generating on one side an extraordinary diversity of plant protection mechanisms and on the other side sophisticated consumer feeding strategies. Herbivores have evolved complex, integrative sensory systems that allow them to distinguish between food sources having mere bad flavors from the actually toxic ones. These systems are based on the senses of taste, olfaction and somatosensation in the oral and nasal cavities, and on post-ingestive chemosensory mechanisms. The potential ability of plant defensive chemical traits to induce tissue damage in foragers is mainly encoded in the latter through chemesthetic sensations such as burning, pain, itch, irritation, tingling, and numbness, all of which induce innate aversive behavioral responses. Here, we discuss the involvement of transient receptor potential (TRP) channels in the chemosensory mechanisms that are at the core of complex and fascinating plant-herbivore ecological networks. We review how “sensory” TRPs are activated by a myriad of plant-derived compounds, leading to cation influx, membrane depolarization, and excitation of sensory nerve fibers of the oronasal cavities in mammals and bitter-sensing cells in insects. We also illustrate how TRP channel expression patterns and functionalities vary between species, leading to intriguing evolutionary adaptations to the specific habitats and life cycles of individual organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved multiple detection and defense mechanisms to withstand extensive tissue damage triggered by herbivores. Many species have developed tolerance to their consumers by increasing photosynthetic rate, growing compensatory tissues, and mobilizing resources from roots to shoots [201]. Another strategy involves production of specialized chemicals that induce repellant, toxic, and/or antinutritional effects in the herbivores. These compounds can be divided into several classes depending on their chemical properties and include alkaloids, terpenoids, phenolics, sulfur-containing compounds, proteinase, and growth inhibitors. On the other hand, as herbivores clearly cannot reject all food sources, they co-evolved complex protective mechanisms to avoid or resist the plants defensive traits. While plant-derived protective chemicals can cause serious negative effects such as liver damage, heart failure, or even death, herbivores may still consume them because of dietary superiority, palatability, or even addictive properties. For instance, locoweed (crazyweed) or larkspur plants are vastly consumed by grazing livestock due to their large protein content and pleasant taste [41]. However, overconsumption generates a variety of symptoms, including visual impairment, trembling, and neurological damage. Although intoxication of wild animals, such as deer, could be explained by a change of perception when starved, triggering consumption of more palatable species, the livestock behavior is an example of pure addiction to plant-induced psychoactive effect. Evidently, herbivores evolved complex sensory mechanisms to avoid intoxication. Essentially, taste, olfaction, and somatosensation in the oral and nasal cavities, as well as post-oral chemosensory mechanisms in the digestive tract serve to detect and avoid potentially toxic plants. In general, animals are attracted to sweet, floral fragrances, and flavors and repelled by bitter and pungent traits. Since many unpleasant, bitter-tasting plants are not harmful, it is essential to distinguish between mere bad taste and chemesthetic sensations such as heat, cold, pain, irritation, tingling, or numbness. The latter sensations are largely mediated by modulation of ion flow via voltage-gated Na+ and K+ channels, whose activation sustains the development and extinction of the action potential in somatosensory neurons, and via acid-sensing ion channels (ASIC) and transient receptor potential (TRP) channels, whose activation produces cationic influx, and an associated depolarization that initiates and/or modulates action potential firing. Because of their rising relevance for the understanding of the mechanisms of chemosensation, in this review, we will focus on the ecological role of TRP channels as mediators of multiple interactions between different classes of plant defensive chemicals and herbivores (Table 1).
Olfaction, gustation, and chemesthesis—detection frontiers
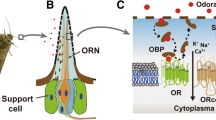
The sense of olfaction serves to the detection of airborne chemicals and in most vertebrates is mediated by two distinct systems. The main system perceives airborne chemicals and the accessory system detects fluid-phase chemicals. Olfaction arises once odorant molecules bind to the olfactory (odorant) receptors (OR) located on the cilia of olfactory neurons in the epithelium at the roof of the nasal cavity (Fig. 1; [177]).
Human olfaction and gustation. a Localization of olfactory epithelium covering the superior nasal concha and directions of air entering nasal cavity. Modified from P.J. Lynch and C. Jaffe. b Schematic representation of laminar and circuit organization of the olfactory bulb. Olfactory bipolar neurons, located in the olfactory epithelium, contain 5 to 20 hair-like cilia trapping odorant molecules. Neurons are surrounded by columnar supporting cells, containing yellow-brown pigment, giving them yellow tint. Together with olfactory glands, supporting cells produce mucus that helps to dissolve and detect airborne chemicals. Olfactory stimulation is the only sensory information that directly reaches the cerebral cortex, while other sensations are conveyed through the thalamus. c Structure of human taste buds. Taste buds are located along the edges of upper surface of the tongue and palate in different types of papillae. They are flask-shaped consisting of 50 to 150 cells (gustatory or basal cells). Gustatory hairs, which extend through the taste pore from the gustatory cells, are sensitive to chemicals present in the saliva. Sensory dendrites present around the gustatory cells receive signals from several receptor cells and conduct a signal to brain. Modified from Marieb and Hoehn [137]
The OR gene superfamily is one of the largest in the human genome comprising 390 putatively functional genes and 465 pseudogenes organized into 18 gene families and 300 subfamilies [167]. It covers up to 3% of the human genome, illustrating their critical function in mammalian physiology [179]. The ratios of gene to pseudogene are highest in rodents and lowest in human, indicative of a greater evolutionary pressure for olfaction in the rodent survival. Allelic exclusion of OR genes causes expression of typically one odorant receptor allele in each sensory neuron, although the mechanism underlying this process is still not fully understood [167]. ORs are activated in different combinations, allowing multitude odorants discrimination [96].
Taste is the sensory impression of the food or other substances on the tongue and in the soft tissues of the mouth. The five basic tastes modalities, recognized by humans as well as most animals are bitter, salty, sour, sweet, and umami, all giving information on the nutrient composition of food and helping to prevent ingestion of toxic and harmful products. The taste buds are the basic anatomical structures detecting tastes, located in the stratified epithelium of the tongue, palate, pharynx, larynx, and epiglottis [177]. In humans, most of the taste buds are situated at the back of the tongue with the number that varies significantly between different people (Fig. 1c) [98, 177]. Taste receptor cells (TRCs) can be divided into three types, each responding to diverse gustatory stimulation. In contrast, the so-called “chemesthesis” (also known as trigeminality or general chemical sense) is related to the chemical activation of sensory nerves resulting in touch and thermal sensations, as well as a wide variety of other sensations such as tingling, itch, numbness, and pain [116]. Chemesthesis (Fig. 2) is associated with nerve fibers originating from the trigeminal (TG) and dorsal root ganglia (DRG) [193, 194] and their stimulation by different chemicals induces pungent sensation as burning, tingling, or cooling also in absence of an olfactory stimulation [116].
Involvement of the mammalian TRP channels in chemosthesis and taste. Chemesthesis comprises a broad range of sensations, associated with diverse mechanisms present in a wide array of sensory structures, including nociceptors, other free nerve endings, and keratinocytes. Oronasal chemesthetic signals are conducted by somatosensory fibers in the trigeminal (V), glossopharyngeal (IX), and vagus (X) nerves [186]. Sensory neurons and oronasal keratinocytes express multiple TRP channels implied in chemesthesis and gustation. Another member of the TRP superfamily, TRPM5, has been identified in the subset of taste bud cells (type II) that to function in signal transduction for sweet, bitter, and umami taste [181, 182]. The molecular mechanism of sensing these tastes involves activation of G protein-coupled receptors (GPCRs), followed by IP3-mediated release of Ca2+ from intracellular stores resulting in Ca2+-induced activation of TRPM5 [126, 180, 182]. Reproduced with permission from Roper [186]
Most odorants at sufficient concentrations stimulate TG nerve endings in addition to the olfactory nerve [86, 199]. Trigeminal chemosensory nerve endings in the nasal and oral cavity are a first recognition mechanism protecting from noxious stimuli, and these present in the mouth also enhance the flavor. Nociceptive TG neurons can be stimulated by a large group of chemicals classified as irritants, including natural plant compounds, air pollutants, and endogenous substances. It has been clearly established that several members of the TRP protein superfamily are essential for olfaction, taste, thermo-, osmo-, mechano-, and chemosensation (Fig. 3) [24, 39, 94, 154, 156, 176, 186].
The phylogenetic tree and main structural features of mammalian TRP channels. TRPs can be classified into six subgroups containing TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPV (vanilloid), TRPP (polycystin), and TRPML (mucolipin) [155, 156]. Seventh subgroup contains non-mammalian, TRPN (NOMPC-like), expressed in fish [192] and invertebrates [218]. All TRP channels have an ancient origin related to TRPY subfamily of fungi [23, 52, 171]. Reproduced with permission from Nilius et al. [159]
Transient receptor potential cation channels
TRPs are cation channels, with variable permeability to Ca2+ and Mg2+, ranging from Ca2+ impermeable (TRPM4 and TRPM5 are only permeable to monovalent cations), over non-selective Ca2+-permeable (most TRP channels) to highly Ca2+ selective (TRPV5 and TRPV6) [125, 157, 158]. Many TRPs are able to conduct other divalent cations such as Ba2+, Cd2+, Co2+, Mn2+, or Zn2+ [27]. The cation permeability of TRP channels highly depends on the pore structure. The structure-function analysis of TRP pores revealed essential residues of the selectivity filter necessary to distinguish between different ions. For instance, neutralization of D546 of TRPV1 and the corresponding 682 of TRPV4 reduces their Ca2+ and Mg2+ conductance. A further mutation of D672 amino acid of TRPV4 promotes additional selectivity decrease for divalent cations and changes the monovalent permeability [170].
TRP channels are expressed in numerous tissues and cell types, and exhibit great diversity in activation mechanisms [40, 145, 176, 215]. They are formed by homo- or hetero-multimerization of four subunits consisting of six transmembrane (TM) segments with the cationic pore loop located between fifth and sixth TM segments (Fig. 3). The carboxyl-(C)- and amino-(N)-termini are located intracellularly and are involved in the mechanisms of channel opening and closing (gating), enzymatic activity, and interactions with many proteins through various residues or domains, as for instance ankyrin (ANK) repeats [64, 156].
Plant secondary metabolites—elaborate chemical defense system
Alkaloids—psychoactive modulators
Alkaloids such as caffeine, nicotine, strychnine, cocaine, and quinine are widely produced by many plants. They function as insect repellents and vertebrate deterrents by inducing neurotoxic effects due to their functional similarity with endogenous neurotransmitters. For that reason, they often induce drug addiction in animals, as for instance, wallabies search for opioid containing poppy plants [43], dogs learn to harass certain toads releasing hallucinogen bufotenin [42], and dolphins eat toxic pufferfish [161]. Drug dependence is mediated by cortico-mesolimbic dopaminergic system and reward/pleasure pathways and modifies neuronal signaling pathways in the brain, which in turn alters behaviors. It has been shown that members of the canonical (TRPC) and vanilloid (TRPV) subfamilies of TRP channels are involved in drug abuse due to their function in psychostimulant-induced behavioral and neuronal plasticity [222].
Plants from the Nicotiana genus produce nicotine (3-[(2S)-1-methylpyrrolidin-2-yl]pyridine) in the roots as a response to leaf wounding (Fig. 4). This alkaloid activates nicotinic acetylcholine receptors (nAChRs) in plant consumers, influencing sensory and motor functions controlled by the cholinergic pathway [45, 57, 120].
Nicotine synthesis in plants. a Tissue damage caused by herbivory attack enhances the production of the plant phythormone jasmonate (JA), which is transported into the roots, activating nicotine synthesis by condensation of the methylpyrrolinium cation derived from putrescine and nicotinic acid [100, 101]. Nicotine is then transported to leaves, where is deposited into vacuoles. b Simplified biosynthesis diagram of the four main tobacco alkaloids. Diverse amino acids are used as building blocks in the synthesis of the pyridine, pyrrolidine, and piperidine rings, required to produce nicotine, nornicotine, anabasine, and anatabine [29, 100]
One of the best examples is a role of TRPCs in nicotine-induced behaviors of Caenorhabditis elegans [63]. This nematode expresses TRP-1 and TRP-2 (TRPC homologs) in multiple types of neurons [226, 229], acting downstream of nAChRs signaling pathway involved in a variety of responses to nicotine, such as stimulated crawl-speed followed by adaptation to this chemical. When nicotine addicted C. elegans were placed in nicotine-free environment they presented withdrawal symptoms, where the worms became dependent on nicotine for their normal locomotion patterns [63]. Moreover, C. elegans worms having null mutations in trp-1 and trp-2 display reduced behavioral responses to nicotine. Although the precise mechanism is not clear it was proposed that TRP-2 acts as a receptor-operated cation channel coupled to the Gq-PLCβ pathway. This signaling is a common pathway in mammalian responses to nicotine, suggesting evolutionarily conserved role of TRPC channels and nAChRs in nicotine sensing.
Cholinergic neurons and nAChRs also play a role in learning and memory pathways, mood and processing of painful stimuli [143]. Because of its toxicity, nicotine has been used historically in agriculture to control pests. However, its high toxicity in mammals, short life span, and shallow insecticide efficiency (acting only on soft bodied insects with piercing mouth apparatus and mites) nicotine use has been limited. Nicotine poisoning produces various symptoms related to malfunction of the central nervous system. For example, nicotine intoxication of honeybees induces ascending motor paralysis of the nerve cord resulting in complete body paralysis with occasional twitching of tarsus, antenna, or abdomen [139]. The pungency of nicotine was originally related to a specific activation of nAChRs present in nociceptive fibers [57, 120, 195, 196]. However, nicotine was later found to activate mouse and human TRPA1 channels, to activate mouse sensory neurons, and to trigger ventilatory responses in mice in a TRPA1-dependent manner [57, 206]. Interestingly, nicotine was shown to have a relatively slow but sustained stimulatory effect on TRPA1 (Fig. 5a), which contrast with the rapidly-installing and quickly desensitizing action on nAChRs. Furthermore, nicotine induced two types of intracellular Ca2+ responses in mouse trigeminal ganglion neurons: rapid and quickly desensitizing ones mediated by nAChRs and slower, more sustained ones mediated by TRPA1. It could be therefore concluded that nAChRs and TRPA1 confer sensory neurons the ability to respond to nicotine in a wide range of concentrations over prolonged periods. Of note, nicotine has a bimodal effect on mouse TRPA1, activating when applied in low micromolar concentrations and inhibiting in the milimolar range (Fig. 5b). However, the physiological relevance of the inhibitory effect is not yet clear. Taken together, these findings indicate that the irritating effects of nicotine in mammals may be attributed to nAChRs and TRPA1 activation. This further supported the already existing notion that TRPA1 mediates the detection of chemicals such as isothiocyanates, caffeine, and phenols, whose production in plants is enhanced upon herbivore attack.
Activation of mTRPA1 by nicotine. a Time course of the effects of nicotine on the amplitude of mTRPA1 currents measured at − 75 and + 50 mV in stably transfected CHO cells. The horizontal lines indicate the periods of extracellular application of nicotine at the indicated concentrations. The colored data points correspond to the current traces shown to the inset. b Concentration-dependent modulation of mTRPA1 currents by nicotine. Reproduced with permission from Talavera et al. [206]
The tobacco specialist Manduca sexta (tobacco hornworm) tolerates nicotine at doses (LD50 1.5 g/kg BW) that are fatal to other herbivores (LD50 for mice 0.0003 g/kg BW, birds 0.0178 g/kg BW, and humans 0.002 g/kg BW) [223] and is less prone to parasitoids when fed with a nicotine-rich diet [19]. The larvae cope with high doses of nicotine via at least three different strategies. First, after absorption from food, the alkaloid is transported via hemolymph and excreted with feces by Malpighian tubes containing specific ABC transporters [11, 114, 133]. Second, nicotine is metabolized in the neural sheath. Third, it has been proposed that M. sexta larval neurons are less sensitive to nicotine when compared to those of other animals. This change in sensitivity could be attributed to the insect nervous tissue, which is protected by a very efficient ion-impermeable sheath, or to a distinct composition of nAChR subunits and/or different TRPA1 activation properties. It has been shown that the genome of M. sexta encodes a single TrpA1 gene and that the protein is expressed in the lateral and medial styloconic sensilla [2]. It was proposed that MsexTrpA1 functions as a molecular integrator of chemical and thermal input in gustatory receptor neurons (GRNs) stimulated by aristolochic acid, a bitter compound produced by the plant family Aristolochiaceae (birthworts).
Caffeine (1,3,7-trimethylxanthine), a drug of choice for many, is another alkaloid produced by many plants with an associated protective function against herbivores (Fig. 6a). Caffeine is biosynthesized from the plant nucleotide xanthosine in a four-step sequence involving three methylations (Fig. 6b) [14, 53]. Few ecological theories exist to explain caffeine function in plants. The autotoxic theory claims that caffeine, released from leaves and seeds falling into the ground, inhibits germination and growth of other plants around the coffee plants. Exogenously applied caffeine affects metabolic pathways as well as can inhibit cell division. Secondly, the chemical defense theory postulates a protective effect of caffeine against the fungus Gibberella xylarioides [79] and against herbivores. Caffeine sprays prevent feeding of tobacco horn-worms or snails [83] and inhibit enzymes in the insect nervous system causing paralysis and death. Caffeine can produce more intriguing behavioral effects in insects, such as construction of spider webs with no symmetry [221] and drowning of mosquitoes larvae. Interestingly, some Citrus flowers contain caffeine to protect them from its main pathogens, e.g., Hemileia vastatrix, Colletotrichum kahawae, and low, non-repellent caffeine concentrations (below 0.058 mg/ml) in their nectars, attracting pollinating honeybees [225]. Bees detect caffeine with neurons located in sensilla of the mouthparts, which express HsTRPA [108], a member of the TRPA channel subfamily, which may serve as sensor for this bitter chemical [95]. Caffeinated honeybees show altered pollinating behavior, with increased recognition of caffeine containing nectars, suggesting a chemical-induced enhancement of the insect’s memory [225].
Caffeine-producing plants and its biosynthesis. a Phylogeny tree of the evolutionary position of caffeine-producing plants with respect to other eudicots. Caffeine is commonly produced by plants belonging to Coffea and Rubia genus and many other plants such as Theobroma (cacao), Ilex (yerba mate), Paullinia (guaraná), Cola (cola nut), and Camellia tea (tea) species, although they evolved distinct pathways to produce this chemical [47, 53]. Coffea plants are hard wood trees or shrubs producing a fruit, containing usually two seeds called coffee beans [47]. Caffeine is present in the highest concentrations in the young shoots and leaves. Depending on species green coffee beans contain variable amounts of caffeine (up to 3.3% of dry mass) [14, 15]. b Simplified schema of the caffeine biosynthetic pathway involving three methylations of nucleotide xanthosine. Three methyltransferase enzymes are essential in the synthesis: xanthosine methyltransferase (XMT), theobromine synthase [7-methylxanthine methyltransferase (MXMT)], and caffeine synthase [3,7-dimethylxanthine methyltransferase (DXMT)]. Reproduced with permission from Denoeud et al. [53]
This is beneficial for both species, as plants ensure pollinations and bees may locate and revisit their food sources more easily. In birds, exposure to caffeine doses below 30 mg/kg causes several cardiovascular and respiratory effects, inducing more awakeness and alertness and enhancement of their productivity [70]. Higher doses are highly toxic, causing seizures and death. In larger animals, caffeine can induce symptoms as hyperactivity, restlessness, vomiting, an elevated heart rate and hypertension, seizures, and finally death. For humans, the lethal caffeine dose is approximately 10 g if taken at once, which corresponds to roughly 105 cups of coffee. Several mechanisms are responsible for caffeine’s pharmacological and toxic effects. For instance, activation of ryanodine receptors (RyRs) leading to the emptying of intracellular Ca2+ stores represents a well-known and widely studied effect [214, 230]. Caffeine also inhibits phosphodiesterases increasing cAMP, thereby causing cardiovascular modulation [59], and blocks adenosine receptors playing key roles in behavioral and cognitive functions [34]. Mouse TRPA1 is rapidly activated by caffeine in transfected cells as well as in capsaicin-insensitive DRG neurons. Neurons from animals lacking TRPA1 are deficient in rapid responses to caffeine and only exhibit a slower intracellular Ca2+ increase that may be attributed to RyR receptors activity [150]. Wild-type mice show highly aversive behavior towards caffeine in drinking water, but this aversion is much less pronounced in mice not expressing TRPA1. This raises the possibility that the detection of this compound occurs via a trigeminal-dependent pathway. Interestingly, caffeine has a suppressing action on human TRPA1, and mutation of the amino acid residue methionine in the position 268 to proline was shown to be responsible for the differences between species (Fig. 7) [149]. Since the M268 residue is conserved in rodents, it is possible that, in addition to its bitterness, caffeine has also a pungent character that may enhance its ability to evoke aversive behavior in these animals. Conversely, the presence of a proline at this position in primate TRPA1 may allow intake of caffeine, with the consequent beneficial pharmacological effects. The bitter perception of caffeine in mammals is attributed to the family of taste receptors that are evolutionary similar to insect gustatory receptors [36, 37, 181].
Differential effect of TRPA1 activation by caffeine. a, b Current recordings using two-electrode voltage clamp (− 100 to + 100 mV) from Xenopus oocytes expressing mouse TRPA1 (a) or human TRPA1 (b). Agonist application is indicated by the bars. Right panels represent an expanded view of the boxed region in a and b. Reproduced with permission from Nagatomo and Kubo [150] “Copyright (2008) National Academy of Sciences, U.S.A.”
In insects, TRPA1 was found to be expressed in bitter-sensing GRNs responsible for aversion behaviors. Although this channel is not directly activated by caffeine, it is clear that it contributes to aversive behavior in insects. In Drosophila, several gustatory receptors such as Gr66a, Gr93a, and Gr32a are implicated in caffeine-evoked action potentials [122, 146]. Accordingly, aversive behavior towards the bitter aristolochic acid was reported to be TRPA1-dependent. However, dTRPA1 is not directly activated by aristolochic acid, but via a phospholipase C (PLC) signaling cascade [104].
Cannabinoids—addictive guardians
The Cannabis genus contains multiple species of flowering plants as Cannabis sativa, Cannabis ruderalis, or Cannabis indica, producing more than 100 different cannabinoids along with the primary psychoactive component, Δ9-tetrahydrocannabinol (Δ9-THC) [9, 198]. These compounds ensure plant survival in many different climates, from hot, tropical regions to very cold and harsh conditions in mountains. Cannabinoids such as tetrahydrocannabinolic acid (THCA) are toxic to cells and are produced in the extracellular compartment of the trichomes, hair-like growths on the plant leaves and buds [9], where they protects plant tissues from insects and herbivores [197]. THCA, together with other chemicals including terpenes and flavonoids, cause cannabis plant extracts to have bitter, unpleasant taste, strong aroma, as well as antimicrobial activity [12]. THCA is a precursor of Δ9-THC, which is formed during progressive decarboxylation in the course of drying process [81]. In contrast to Δ9-THC, THCA is a non-psychoactive chemical that have been found to have multiple health benefits. Consumption of THC may induce hyperemesis syndrome, impaired coordination and performance, anxiety, suicidal ideations/tendencies, and psychotic symptoms [117, 198]. Cannabinoids actions have been attributed to activation of metabotropic cannabinoid receptors CB1 and CB2. These receptors are present in the central and peripheral nervous system and are endogenously activated by endocannabinoid neurotransmitters such as N-arachidonoylethanolamine (anandamide) and sn-2-arachidonoylglycerol (2-AG) [198]. The endocannabinoid system (ECS) is involved in many physiological and cognitive processes, including appetite, pain, mood, and memory. The ECS functions in exercise-induced euphoria, as well as in the modulation of locomotor activity and motivational salience for rewards. Thus, due its structural similarity to ECS signaling molecules, Δ9-THC mimics rewords correlated with fitness, which may explain the addictive effects of the compound [203].
Further, cannabinoids induce supraspinal, spinal, and peripheral antinociception and antihyperalgesia in different pain syndromes [30, 31, 44]. Moreover, chemicals belonging to the aminoalkylindole family of cannabinoids produce peripherally mediated antinociception in acute pain models [135] and alleviate hyperalgesia/allodynia induced by capsaicin [82, 89], heat [90], inflammation [148, 234], and nerve injury [65, 184, 227]. ∆9-THC and cannabinol were shown to relax hepatic and mesenteric arteries, via activation of CGRP-containing sensory nerve endings that innervate the vascular smooth muscle. This effect was independent of cannabinoid receptors and TRPV1 [235]. The population of capsaicin-sensitive TG neurons was responsive to both AITC and ∆9-THC, suggesting a contribution of TRPA1 to cannabinoid-induced inflammatory hypersensitivity and vasodilation [92].
Phytocannabinoids such as cannabichromene (CBC), cannabigerol (CBG), cannabidiol (CBD), ∆9-THC, THCA, or CBDA were shown to influence the TRPA1- and TRPM8-mediated elevation of Ca2+ in DRG sensory neurons and in an overexpression system [49, 51]. The potency of these compounds followed the strength of their electrophilic nature, suggesting the formation of covalent bonds with cysteine residues as underlying mechanism of channel activation [51, 80, 131]. Although, cells expressing TRPA1 responded to multiple phytocannabinoids, the responses in TRPA1 expressing DRG neurons were lower. This could be attributed to the lower expression levels in comparison with the overexpression system, as well presence of other channels. Both human and rat recombinant TRPV1 were shown to be insensitive to most of phytocannabinoids, with only CBD, CBG, and CBDA being TRPV1 activators [124]. On the other hand, pre-incubation with CBC and CBG inhibited Ca2+ elevation in cells overexpressing TRPA1 and in DRG neurons. Moreover, in TG neurons, the synthetic cannabinoids WIN and AM1241 inhibit the responses to capsaicin and AITC, via activation of TRPA1 expressed in the sensory neurons [3]. Finally, TRPM8 responses to menthol and icillin were inhibited by some phytocannabinoids [51], as well as endocannabinoids such as anandamide and N-arachidonoyldopamine [49, 50].
Potential roles of reactive oxygen and nitrogen species
Many Cannabis, Solanum, and Arabidopsis plants are able to generate hydrogen peroxide (H2O2), in response to plant wounding by herbivores and pathogens. The production of reactive oxygen species (ROS) such as O2−, OH−, or H2O2, also called the oxidative burst [6, 56, 115, 169], is induced by activation of a membrane-bound NADPH oxidase [54, 103]. ROS plays several function during pathogen infection, being direct antimicrobial agents, activator of several defense genes, inducer of the hypersensitive response (HR), cell death, salicylic acid production, and systemic acquired resistance (SAR) [56, 115]. ROS can be sensed by multiple TRP channels in sensory neurons, including TRPA1, TRPV1, TRPV4, TRPC3–5, TRPM2, TRPM7, and TRPM8 [7, 142, 152, 153, 160, 191, 216].
Indisputably, TRPA1 acts as a one of the major sensors of ROS and reactive nitrogen species (RNS), including nitric oxide (NO) and peroxynitrite (ONOO−) [144, 205, 208]. It is known that these chemicals produce covalent S-nitrosylation of TRPA1, but the full mechanism of activation by reactive species is still unclear. It is possible that channel is directly gated by these compounds [8] or an indirect activation mechanism could be involved induced by for instance generation of lipid peroxidation products [202]. TRPA1 activation is also essential in responses to hypoxic conditions, resulting in cardiovascular and respiratory regulatory reflexes [111]. In Trpa1 KO mice, VG neurons responses to hyperoxia and mild hypoxia were abolished. TRPA1 may be activated by hypoxia via the reduction of proline hydroxylase activity, leading to decreased hydroxylation of P394 within the channel ankyrin repeats relieving inhibition. On the other hand, hyperoxia induces channels C633 and C856 oxidation [204]. Taken together, the recent literature on the interactions between reactive molecules and TRPA1 or other sensory TRP channels suggest their roles as alarm systems protecting herbivores from harmful plant defenses.
Vanilloids
Belonging to the plant genus Capsicum (Greek kapto, “to bite”), chili peppers are well-known for their pungency. Fruits of the plants from this genus contain the chemical capsaicin (8-methyl-N-vanillyl-6-nonenamide) and other structurally related compounds, called capsaicinoids, such as dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin [147]. Capsaicin belongs to the vanilloid family, which also includes vanillin (vanilla bean), eugenol (bay leaves or cloves oil), and zingerone (ginger) (Fig. 8d). Capsaicin is highly lipophilic, which explains the lack of pungency alleviation by drinking water after oral intake. The main function of these chemicals is to discourage consumption by plant herbivores, but on the other hand, the primary function of the plant fruit should be facilitation of the seed distribution. Without doubt, Capsicum plants evolved a specific strategy through which mammals are repelled, but birds are not and serve as main seed distributors [210]. It has been observed that consumption by birds is advantageous to these plants, as these animals excrete the seeds in places convenient for germination, such as under other plants, assuring shade beneficial for seed survival and shelter from consumers [209, 210]. Interestingly, seeds excreted by birds are able to germinate, but no germination occurs after ingestion by mice. This was attributed to the distinct ways in which the seeds are ingested by those animals. In birds, seeds are not chewed on, pass the gut very quickly and come out intact. In contrast rodents and other mammals damage the seeds by chewing and by the effects of acidic juices during food digestion. For example, it was found that seeds of Capsicum chacoense (native to Bolivia, Argentina, and Paraguay) that pass through the digestive system of the small-billed Elaenia, a bird of the family Tyrannidae had less pathogens (Fusarium fungi) and were devoid of volatile chemicals that attract granivorous ants. This resulted in a 370% rise in seed survival rate [67]. Reduction in fungi load on C. chaocense seeds is extremely important because it represents a major seed mortality factor [67, 211]. A key question is why mammals, but not birds, display such strong aversion towards hot peppers? The answer lays in the different sensitivity of nociceptive nerve fibers of these animals towards capsaicin, a specific agonist of TRPV1 (Fig. 8). It was shown that bird TRPV1 orthologs lack sensitivity to capsaicin and other vanilloids [93]. Comparison of mammalian and different avian TRPV1 sequences revealed only 68% amino acid sequence conservation [93]. The capsaicin-TRPV1 interactions have been studied extensively by creation of mammalian channels chimeras with vanilloid-insensitive TRPV2 or avian or rabbit capsaicin-insensitive TRPV1. These studies identified essential residues in the TM2 and TM3 necessary for capsaicin binding with a single, evolutionary conserved amino acid (Y511), crucial for vanilloid sensitivity [93]. The sensitivity differences between species could be attributed to I550 residue as substitution of this amino acid to threonine rendered rabbit TRPV1 vanilloid sensitive [72]. Moreover, the inverse substitution, T550I, produced reduction of capsaicin responses in human and rat TRPV1 [72]. Taken together, these results suggested that the hydrophobic tail of capsaicin interacts with aromatic Y511 residue and that several polar amino acids including M547, S512, and R491 are important in accommodating its vanilloid moiety [72, 93]. Multiple molecular dynamics simulations showed “tail-up, head-down” configurations of capsaicin in the vanilloid pocket (Fig. 8c), where the aliphatic “tail” forms van der Waals interaction with the channel and contributes to binding affinity. The vanillyl “head” and amide “neck” of the molecule interact with the channel via hydrogen bonds, which grant specificity for the capsaicin binding [46, 75, 119, 162]. The cryo-EM confirmed the localization of the vanilloid binding pocket, where interactions occur between the vanillyl group of capsaicin and Y511, while the adjacent S512 also participates via hydrogen bonds [33, 119]. Moreover, depending on the presence of vanilloids, Y511 assumes two distinct rotamers, suggesting an “induced fit” mechanism [33]. TRPV1 opening, upon capsaicin binding, is associated with major structural rearrangements of the pore helix and selectivity filter, in addition to distinct dilation of a hydrophobic constriction at the lower gate [33]. Therefore, capsaicin sensitivity that greatly varies between animals has important implications for understanding the complexity of the interactions between plant survival strategies and plant consumers. For birds, lack of sensitivity to capsaicin is beneficial as they can forge on this food source. On the other hand, the high sensitivity of other species, in particular mammals, acts and allows plants to use capsaicin as a strong repellent. Overall, these mechanisms are highly beneficial for the plants, as they ensure that their seeds are spread by the optimal vector and protect the plant from overconsumption.
TRPV1 activation by capsaicin. a Effect of peppers extracts with different pungencies on currents recorded in a Xenopus laevis oocyte expressing TRPV1 channels. Reproduced with permission from Caterina et al. [35]. b Concentration-response curve (top) and single channel recordings (bottom) of TRPV1 upon application of capsaicin. c Localization of the binding site of capsaicin (orange) in the TRPV1 structure. Reproduced with permission from Yang et al. [232]. d Chemical structures of capsaicin and structurally related vanilin, eugenol and zingerone.
Vanillin, a volatile chemical structurally similar to capsaicin, is produced by approximately 110 species of the vanilla plants, including Vanilla planifolia, Vanilla tahitensis, and Vanilla pompon [69]. This primary component of the vanilla bean extract is largely used in foods, beverages, cosmetics, and pharmaceutical industries as a flavoring agent. Vanillin has antimicrobial activity [129] and is a biologically active attractant of orchid bees [62]. Together with vanillin, more than 40 other chemicals such as 8-cineole, eugenol, or linalool are present in bees attracting extracts [62]. Vanillin has mild CNS effects and is often regarded as an aphrodisiac. Although, vanillin acts mainly on the olfactory system, this compound was shown to act also on TG neurons. In mammals, this odorant was shown to activate TRPV3 at rather high concentrations (above 10 mM) [228]. Later, it was shown to activate trigeminal TRPA1 and TRPV1 and inhibit TASK1 channels [130]. Since TG were activated with lower vanillin concentrations (1 mM) and TRPV3 expression in trigeminal neurons is arguable, TRPA1 and TRPV1 activation was linked with the observed activity.
Eugenol is a volatile chemical produced by various plants, where it functions as a floral attractant for pollinators as well as an antimicrobial agent [107]. Eugenol and its derivatives are present in essential clove and clocimum oils obtained from Eugenia carophyllata and Ocimum gratissimum, nutmeg, cinnamon, basil, and pimento berry [102]. This compound contributes the specific aroma of many fruits, as in the case of ripe strawberries [13]. Eugenol acts as repellent of beetles, inhibits egg and larvae development of the greater grain weevil Sitophilus zeamais [163], and can be toxic for humans, inducing liver damage and failure, respiratory distress syndrome, and CNS depression [60]. The structural similarity between eugenol and capsaicin suggests analogous molecular mechanisms of action. Indeed, it has been shown that it possesses antinociceptive properties similar to once induced by capsaicin, attributed to TRPV1 activation [228, 231]. However, eugenol-induced responses were slower and characterized by smaller amplitudes, than those to capsaicin [173, 231]. Additionally, eugenol shares many properties with local anesthetics, such as the ability to alleviate tooth pain, which relate to the inhibitions of voltage-gated Ca2+ and Na+ channels [38, 121, 174]. Eugenol can also block production of inflammatory pain-metabolites as prostaglandins through inhibition of cyclooxygenase-2 and lipoxygenase activity [55, 213]. Other TRP channels, namely, TRPA1, TRPM8, and TRPV3 are also activated by this volatile chemical [17, 228]. Oral application of eugenol induces pungent sensations as warmth and thermal pain, which were proposed to be mediated by TRPV3 and TRPV3-mediated enhancement of thermal gating of TRPV1 expressed in lingual polymodal nociceptors [105].
Zingerone is a crucial contributor to the specific flavor of dried and cooked ginger (Zingiber officinale). It is produced by heat conversion of gingerol, another active ginger chemical [88]. It is structurally similar to capsaicin, suggesting that it may act on TRPV1, but has a shorter hydrophobic moiety and lacks an acyl-amide moiety. These structural differences are likely to be responsible for a more rapid onset and a faster decay of zingerone’s gustatory and trigeminal responses, compared to those of capsaicin [127]. On the other hand, zingerone induces TG desensitization and tachyphylaxis, similarly to capsaicin [127]. As described above, the aromatic portion of the Y511 residue in TRPV1 is necessary for the interaction with the vanilloid moiety of capsaicin. It was proposed that zingerone and other capsaicin-related chemicals can interact only partially with Y511, resulting in weaker TRPV1 agonist activity [72].
Another target of zingerone is TRPA1, acting through a mechanism distinct from that of AITC. Instead, it was proposed that TRPA1 activation by zingerone involves intracellular Ca2+ mobilization from internal stores through Ca2+-induced Ca2+-release mechanisms [112]. Finally, different fruit fly species are distinctly attracted to plants producing zingerone, eugenol, and its derivatives, suggesting for a differential expression of the relevant chemosensory TRP channels in these species [207].
Plants from the Piper genus, such as black pepper and long pepper, produce another pungent vanillamide, piperine. In addition, pepper plants contain roughly 145 different chemicals, including chavicine, piperettine, piperiline, piperlonguminine, 4,5-dihydropiperlongumine, pellitorine, pipercide, guineensine, and sylvatine, all contributing to the characteristic spicy, tingling, and warming sensations induced by black pepper [48]. These compounds are all deterrent to many animals. In nature, these chemicals act as insecticides, exhibiting low levels of threat to the environment or to human health. In fact, pepper is broadly used as flavoring agent having many beneficial effects as an antioxidant, anti-inflammatory, and antimicrobial agent [141]. Although at first glance piperine seems to be tasteless, this impression is quickly replaced by a burning sensation attributed to activation of TRPV1 [140]. Another member of the TRP superfamily, TRPA1, is also activated by pepper chemicals including piperine, isopiperine, isochavicine, piperanine, piperolein A, piperolein B, and N-tetra, although at concentrations higher than those required to activate TRPV1 [166]. Therefore, the pungent flavor of pepper may be attributed to the activation of both channels.
Garlic, onion, and mustard: organosulfur-based defense systems
Plants from the Brassica genus, also known as mustard plants (mustard, broccoli, Brussels sprouts, watercress, wasabi, and cauliflower), produce the highly pungent compound allyl isothiocyanate (AITC) upon disruption of the plant tissue by pathogens or herbivores. Isothiocyanates (ITC), and several other compounds such as nitriles, thiocyanates, oxazolidine-2-thiones, and epithionitriles, are produced from glucosinolates that are hydrolyzed by the enzyme myrosinase [175]. These volatile compounds reduce oviposition and feeding activities of insects, and their strong pungency induces aversion behavior in other herbivores [68]. The pungent sensation that AITC produces in mammals was initially attributed to a specific activation of TRPA1 expressed in nociceptive neurons [20]. ITCs are highly electrophilic chemicals and can form conjugates with accessible thiol groups in proteins. In the case of human TRPA1, these were reported to be cysteines at positions C619, C639, and C663 and to a lesser extent lysine 708, all located in the cytoplasmic N-terminal tail of the channel between the last ankyrin repeat and the first transmembrane segment [80]. It was also shown that immunoprecipitated mouse TRPA1 can be covalently modified by electrophilic agonists and at least 14 other cysteine residues can contribute to channels activation [131]. The key cysteine residues of mouse TRPA1 (C415, C422, and C622) are close and within the ankyrin repeats presenting intriguing differences between two species [131]. Moreover, cysteines located in the mouse TRPA1 N-terminal can form disulfide bridges that stabilize channel structure [236]. Actually, residues can be shared to form bridges as for example in mouse TRPA1 C666–C622, C666–C463, C666–C193, and C622–C609, suggesting that channel activation may involve different N-terminal conformations [220].
Our later studies show that some TRPA1 electrophilic agonists such as AITC and cinnamaldehyde activate the channel at low concentrations but also have an inhibitory effect at high, but pharmacologically relevant, concentrations [5, 61]. In addition, although TRPA1 is a main target of ITCs, it has been demonstrated that trpa1 knockout mice display residual aversive behavior against AITC [113]. More detailed investigations revealed that AITC also activates TRPV1 and that this channel contributes to the nociceptive effects of AITC [61, 73, 164].
In insects, AITC exposure induces mortality, adult and immature malformation, repellency, and altered development [66, 85, 189]. The mechanisms of AITC action in insects are still unresolved. However, it may involve disrupting the activity of the mitochondrial complex IV and complex I, leading to tissue dysfunction [136, 233]. Insect feeding is also inhibited by ITC and related chemicals via a mechanism involving TRPA1 expressed in GRNs in the insects mouthparts [4, 97]. This further points to evolutionarily conserved molecular pathways involving sensory TRP channels that are implicated in chemical nociception between different organisms. Detection of harmful, electrophilic chemicals by TRPA1 emerged over 500 million years ago in a common vertebrate/invertebrate ancestor [97]. Interestingly, sensing noxious chemicals was a “secondary” function as different TRPA clades express highly temperature-sensitive channels, indicating thermosensitivity to be ancestral. In addition, the channel N-terminal region, which is highly variable in the sequence between different animals, even within same class as insects, indicates specialization of the channel properties [74, 97]. For instance, different mosquito species, express two TRPA1 isoforms (TRPA1(A) and TRPA1(B)) that not only vary in thermal sensitivity but also differently respond to electrophilic agonists [97]. This conserved, functional diversity of TRPA1 between different host-feeding species provides a clue for how this insects sense and select their host.
Furthermore, extracts from Brassica plants induce genotoxicity in bacteria, in fungi, as well as in mammalian cells. Different chemical substitutions of ITC have variations in their DNA-damaging capacities, as for instance, benzyl-ITC has much more effect than AITC or phenethyl-ITC [99].
Garlic (Allium sativum) has also adapted to deter foragers by inducing burning, irritating sensation in the mouth when cloves are consumed. Sensations elicited by garlic are attributed to activation of TRPA1 and TRPV1 channels [132, 187]. It has been shown that approximately 30% of rodent TG neurons responds to garlic extract [20]. Diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) activate both channels (Fig. 9b), but display higher affinity for TRPA1 [20, 109, 132]. Although garlic consumption has been shown to be highly beneficial for health [10, 18, 26, 168] and garlic is used as natural remedy for many illnesses, overconsumption is greatly toxic. Garlic extracts are mutagenic, induce anemia by lysing red blood cells, and even induce death in rats (above 5 ml of garlic juice per kg) [18, 110]. Garlic components, mainly DADS, allyl propyl disulfide, and allicin, can also induce vomiting, heartburn, diarrhea, allergic contact dermatitis, asthma, hypoglycemia, flatulence, tachycardia, and insomnia [18, 32, 71, 172]. One of the pests of garlic and other Allium plants is Ditylenchus dipsaci. This bloat nematode lives between the cells of bulbs, stems, and leaves feeding on the sap. A question is then, how can this animal live and feed on garlic juices containing so many harmful chemicals. Part of the answer may lay in the TRP channel expression patterns, as most nematode parasites possess fewer genes, often lacking TRPA, TRPM, or TRPP sequences [224]. This may lead to a reduction of noxious sensory inputs recognition, which is consistent with these animals residing in environments that others would perceive as harmful.
Chemical structures of produced by garlic defense chemicals is associated with TRPA1 activation. a Two classes of organosulfur chemicals are present in whole garlic cloves: L-cysteine sulfoxides (alliin) and γ-glutamyl-L-cysteine peptides. Alliin, which accounts for about 80% of cysteine sulfoxides in garlic, is transformed to allicin by alliinase enzymes within a few seconds after clove integrity disruption [26, 219]. This short-lived chemical is further transformed into more stable sulfur compounds as DAS, DADS, and DATS [20, 26]. In fact, garlic contains more than 33 different organosulfur chemicals, as well as many other chemicals whose functions remain unknown [138, 168, 219]. b Intracellular Ca2+ responses of rat TG primary sensory neurons expressing TRPA1 channels exposed to raw garlic extracts (1:10.000 dilution, top panel) and its derivatives 40 μM allicin (middle panel) and 200 μM DADS (bottom panel). Subsequently, all AITC-responsive neurons were also activated by garlic compounds, pointing to a role of TRPA1 in sensing these chemicals. Reproduced with permission from Bautista et al. [20] “Copyright (2005) National Academy of Sciences, U.S.A.”
Other plants of the Allium genus such as onions, shallots, chives, and leeks also produce pungent thiosulfinates and disulfides. Onion (Allium cepa), similarly to garlic, generates many toxic compounds causing hemolytic anemia in dogs, cats, cattle, and horses [188]. These plants contain S-alk(en)yl-cysteine sulfoxides (ACSO), whose content in different crops is regulated by genetic and environmental factors. There are six different ASCOs produced, including S-methylcysteine sulfoxide (methiin), S-propylcysteine sulfoxide (propiin), trans-S-(1-propenyl)cysteine sulfoxide (isoalliin), S-butylcysteine sulfoxide (butiin), S-ethylcysteine sulfoxide (ethiin), and the above-mentioned S-(2-propenyl)cysteine sulfoxide (alliin) [128]. Upon cell integrity disruption by foragers, short-lived ASCOs degrade, resulting in a pungent, irritating aroma. Onion produces methiin, propiin, and isoalliin [128], resulting in the onion lachrymatory factor attributed to formation of propanthial S-oxide, 1-propenyl methane thiosulfinate, and di-propyl disulfide [22, 58, 84]. In domestic animals, consumption of 5–30 g/kg of onion can result in red cell damage [188], while humans, sheep, goats, and rodents are more tolerant. All chemicals mentioned here are highly potent electrophiles, activating both insect and vertebrate TRPA1 via covalent modification. Channel opening by these compound results in avoidance, as for instance, escape behavior, due to induction of pain, coughing, and lachrymation.
Terpenoids—etheric frontiers
Terpenoids form a group of highly important plant secondary metabolites having multiple functions in primary plant physiology (photopigments, electron carriers, membrane function modifiers, etc.), as well as in the protection against abiotic and biotic stress [1, 212]. This group contains over 40,000 structurally different chemicals derived from isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP) [212]. Plants produce terpenoid precursors via two independent, genetically regulated pathways. One is the cytosolic mevalonic acid (MVA) pathway, involved in the synthesis of sesquiterpenoids, polyprenols, phytosterols, and brassinosteroids, and the second is the plastidial methylerythritol phosphate (MEP) pathway, implicated in the synthesis of hemiterpenoids, monoterpenoids, diterpenoids, carotenoids, cytokinins, gibberellins, chlorophyll, tocopherols, and plastoquinones [77, 78, 212]. Many terpenoids are highly volatile chemicals associated with plant fragrance, acting as insect attractants or repellants [190]. For instance, a monoterpenoids mix containing d-limonene, β-myrcene, and E-β-ocimene, released by flowering plants such as the monkeyflowers (Mimulus), is crucial to attract specific pollinators [28]. Plants from the orchid genus (Satyrium) emit fragrance mixtures composed of at least 70 different compounds, of which many are monoterpenoids, sesquiterpenoids, and benzenoids. The specific scent of these plants is necessary for attracting only pollinating Atrichelaphinus tigrina beetles and Hemipepsis hilaris wasps [91]. The most abundant in the orchid fragrance mixtures are linalool, eucalyptol, elemicin, α-pinene, myrcene, and 2,6-dimethyl-1,5(Z),7-octatrien-3-ol [91]. Many of these compounds, especially linalool, are able to directly induce electrophysiological responses in antennae of the pollinating beetle species, making them generalists visiting these plant species. Linalool (3,7-dimethyl-1,6-octadien-3-ol) (Fig. 10a) has a sweet, pleasant scent, occurring widely among diverse monocot and dicot plant families [106]. Remarkably, it has been shown that linalool chirality determines the specific interactions between bees and orchids [91]. Similarly, bees could respond contrarily to the linalool enantiomers, as this compound is dominant in secretion of receptive females [25]. Another theory states that the scent emitted by orchids repels other pollinating insects and animals, as absence or reduced concentration of the compound attracted bats and flies. Linalool was shown to activate members of mammalian TRP channel superfamily, TRPA1 (EC50 117 μM) (Fig. 10b) [185] and TRPM8 (EC50 6.7 mM) [21]. Interestingly, in sensory trials, linalool failed to induce the burning, painful feeling associated with TRPA1 activation [185], which may be explained by its analgesic properties via interaction with other targets. Linalool enantiomers were shown to reduce peak amplitudes of fast-conducting compound action potentials of frog sciatic nerve fibers [165], to inhibit action potentials of rat dorsal root ganglion neurons [118], and to inhibit voltage-gated Na+ channels expressed in newt olfactory receptor cells [151].
Structures of linalool and citral and their activity on TRP channels. a Monoterpenoid alcohol, linalool, has chiral properties and is present in two enantiomeric forms; (R) and (S)-linalool. (R)-linalool is found in many other plants, such as camphor, rosewood trees, thyme, basil, lavender, and bergamot. The (S) enantiomer is found in black locust and plants from Coriandrum genus, palmarosa and sweet orange [24, 123, 183]. b Dose-response relationships for linalool, 6-shagol and 6-paradol and TRPA1 activation. c Chemical structures of citral and its enantiomers. d Currents through the TRP channels rTRPV1, rTRPV3, rTRPM8, and rTRPA1 are increased upon application of citral. Inward currents are increased for TRPV1 by 14-fold, TRPV3 by 38-fold, TRPM8 by 2-fold, and TRPA1 3-fold (top panel). Dose-response curves (bottom panel) for citral activation of TRPV1, TRPV3, TRPM8, and TRPA1. Reproduced with permission from b Riera et al. [185] and d Stotz et al. [200]
Citronella essential oil is a pale to dark yellow liquid with woody, grassy, or lemony scent, produced by the plants Corymbia citriodora, Cymbopogon nardus, Java citronella, and more than 50 other plants. This oil is well-known by its insect repellent properties (especially mosquito) and antimicrobial functions, and it is often used in ancient Indian and South-east Asian traditional medicines. Originally, citronella oil was purified for use in perfumery and its repellent properties were exploited as early as the beginning of the twentieth century [134]. Nowadays, the citronella essential oil is used in cosmetic, pharmaceutic, flavor, and fragrance industry. It contains more than 80 chemicals, including as main components the monoterpenes citronellal (3,7-dimethyl-6-octenal), citronellol (3,7-dimethyloct-6-en-1-ol), citral (3,7-dimethyl-2,6-octadienal), and geraniol ((2E)-3,7-dimethyl-2,6-octadien-1-ol). It has been shown that aversion to citronellal in Drosophila melanogaster involves two distinct olfactory pathways. The first implicates insect-specific olfactory receptors and the second involves the function of Drosophila TRPA1(A) isoform downstream of a GPCR. Moreover, human and mosquito TRPA1 are activated by citronellal, with higher sensitivity than for dTRPA1(A). Although citronella is a well-known bug repellent, it should not be used at concentrations above 10% due to induction of severe skin reactions.
Citral (Fig. 10c), characterized by its bittersweet, lemony flavor and odor, is an active ingredient of lemongrass oil, lemon peel, citronella, and palmarosa grass and has been characterized as insect repellant [76]. Citral, in nature found as two more stable isomers, E-citral (neral) and Z-citral (geranial) [178], acts via GPCRs in olfactory epithelia, but was also shown to be an agonist of TRPV3 and partial agonist of TRPM8, TRPV1, and TRPA1 (Fig. 10d) [200]. Citral is an α,β-unsaturated aldehyde, so it may well activate TRP channels by electrophilic modification of cysteine and lysine residues. As mentioned above, detection of aversive electrophiles is one of the ancestral defense mechanisms conserved in invertebrates and vertebrates. Furthermore, citral was shown to interact with the transmembrane segments TM2-TM4 of TRP channels [200], which contain residues crucial for TRPV1 activation by capsaicin and TRPM8 activation by menthol [16, 87, 217]. However, a capsaicin-insensitive TRPV1 point mutant at residue Tyr 511 is activated by citral, suggesting that the binding sites for these two compounds are different [200].
Conclusions
Plants produce many aversive compounds serving to protect themselves from herbivores. This urges plant consumers to evolve specific mechanisms of chemicals recognition by specialized receptors. The analysis of the feeding ecology of different organisms demonstrates that aversive behaviors play crucial roles in the fitness of herbivores. TRP channels are one group of receptors specialized in detecting harmful stimuli and triggering protective responses in organisms. Whereas much progress has been made in the last decade in the understanding of the structure and functions of TRP channels, the evolutionary link between bypassing plant defense mechanisms and TRP channel functional expression in the animal kingdom is largely unexplored. Summarizing, in this review, we illustrate the involvement of TRP channels in detection of harmful stimuli and the evolutionary adaptation of organisms consuming plants that use TRP channel agonists as defense mechanisms. Since the expression pattern and ligand sensitivity of TRP channels varies between species, this presents an intriguing evolutionary adaptation to their specific habitat and life cycles.
References
Abbas F, Ke Y, Yu R, Yue Y, Amanullah S, Jahangir MM, Fan Y (2017) Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 246:803–816. https://doi.org/10.1007/s00425-017-2749-x
Afroz A, Howlett N, Shukla A, Ahmad F, Batista E, Bedard K, Payne S, Morton B, Mansfield JH, Glendinning JI (2013) Gustatory receptor neurons in Manduca sexta contain a TrpA1-dependent signaling pathway that integrates taste and temperature. Chem Senses 38:605–617. https://doi.org/10.1093/chemse/bjt032
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci 28:1064–1075. https://doi.org/10.1523/JNEUROSCI.1565-06.2008
Al-Anzi B, Tracey WD Jr, Benzer S (2006) Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol 16:1034–1040. https://doi.org/10.1016/j.cub.2006.04.002
Alpizar YA, Gees M, Sanchez A, Apetrei A, Voets T, Nilius B, Talavera K (2013) Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Pflugers Arch 465:853–864. https://doi.org/10.1007/s00424-012-1204-x
Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784. https://doi.org/10.1016/S0092-8674(00)81405-1
Anderson RL, Minton KW, Li GC, Hahn GM (1981) Temperature-induced homeoviscous adaptation of Chinese hamster ovary cells. Biochim Biophys Acta 641(2):334–48
Andersson DA, Gentry C, Moss S, Bevan S (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494. https://doi.org/10.1523/JNEUROSCI.5369-07.2008
Andre CM, Hausman JF, Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 7:19. https://doi.org/10.3389/fpls.2016.00019
Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1:125–129
Appel HM, Martin MM (1992) Significance of metabolic load in the evolution of host specificity of Manduca sexta. Ecology 73:216–228. https://doi.org/10.2307/1938733
Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM (2008) Antibacterial cannabinoids from Cannabis sativa: a structure–activity study. J Nat Prod 71:1427–1430. https://doi.org/10.1021/np8002673
Aragüez I, Osorio S, Hoffmann T, Rambla JL, Medina-Escobar N, Granell A, Botella MÁ, Schwab W, Valpuesta V (2013) Eugenol production in achenes and receptacles of strawberry fruits is catalyzed by synthases exhibiting distinct kinetics. Plant Physiol 163:946–958. https://doi.org/10.1104/pp.113.224352
Ashihara H, Kato M, Crozier A (2011) Distribution, biosynthesis and catabolism of methylxanthines in plants. Handb Exp Pharmacol 11–31. https://doi.org/10.1007/978-3-642-13443-2_2
Ashihara H, Suzuki T (2004) Distribution and biosynthesis of caffeine in plants. Front Biosci 9:1864–1876
Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A (2006) High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 9:493–500. https://doi.org/10.1038/nn1665
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Banerjee SK, Mukherjee PK, Maulik SK (2003) Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res 17:97–106. https://doi.org/10.1002/ptr.1281
Barbosa P, Saunders JA, Kemper J, Trumbule R, Olechno J, Martinat P (1986) Plant allelochemicals and insect parasitoids effects of nicotine onCotesia congregata (say) (Hymenoptera: Braconidae) andHyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae). J Chem Ecol 12:1319–1328. https://doi.org/10.1007/BF01012351
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102:12248–12252. https://doi.org/10.1073/pnas.0505356102
Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. In: Br J Pharmacol, vol 141. vol 4. England, pp 737-745. https://doi.org/10.1038/sj.bjp.0705652
Block E (1985) The chemistry of garlic and onions. Sci Am 252:114–119
Bonilla M, Cunningham KW (2002) Calcium release and influx in yeast: TRPC and VGCC rule another kingdom. Sci STKE \:pe17. https://doi.org/10.1126/stke.2002.127.pe17
Boonen B, Startek JB, Talavera K (2017) Chemical activation of sensory TRP channels. In: Krautwurst D (ed) Taste and smell. Springer International Publishing, Cham, pp 73–113. https://doi.org/10.1007/7355_2015_98
Borg-Karlson AK, Tengö J, Valterová I, Unelius CR, Taghizadeh T, Tolasch T, Francke W (2003) (S)-(+)-linalool, a mate attractant pheromone component in the bee Colletes cunicularius. J Chem Ecol 29:1–14
Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ (2014) Allicin: chemistry and biological properties. Molecules 19:12591–12618. https://doi.org/10.3390/molecules190812591
Bouron A, Kiselyov K, Oberwinkler J (2015) Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Archiv 467:1143–1164. https://doi.org/10.1007/s00424-014-1590-3
Byers KJ, Bradshaw HD, Riffell JA (2014) Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). J Exp Biol 217:614–623. https://doi.org/10.1242/jeb.092213
Cai B, Jack AM, Lewis RS, Dewey RE, Bush LP (2013) (R)-nicotine biosynthesis, metabolism and translocation in tobacco as determined by nicotine demethylase mutants. Phytochemistry 95:188–196. https://doi.org/10.1016/j.phytochem.2013.06.012
Calignano A, La Rana G, Giuffrida A, Piomelli D (1998) Control of pain initiation by endogenous cannabinoids. Nature 394:277–281. https://doi.org/10.1038/28393
Calignano A, La Rana G, Loubet-Lescoulié P, Piomelli D (2000) A role for the endogenous cannabinoid system in the peripheral control of pain initiation. Prog Brain Res 129:471–482
Canduela V, Mongil I, Carrascosa M, Docio S, Cagigas P (1995) Garlic: always good for the health? Br J Dermatol 132:161–162
Cao E, Liao M, Cheng Y, Julius D (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504:113–118. https://doi.org/10.1038/nature12823
Cappelletti S, Daria P, Sani G, Aromatario M (2015) Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 13:71–88. https://doi.org/10.2174/1570159X13666141210215655
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444:288–294. https://doi.org/10.1038/nature05401
Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ (2000) T2Rs function as bitter taste receptors. In: Cell, vol 100. vol 6. United States, pp 703-711
Chung G, Rhee JN, Jung SJ, Kim JS, Oh SB (2008) Modulation of CaV2.3 calcium channel currents by eugenol. J Dent Res 87:137–141. https://doi.org/10.1177/154405910808700201
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524. https://doi.org/10.1038/nature02196
Clapham DE, Runnels LW, Strübing C (2001) The TRP ion channel family. Nat Rev Neurosci 2:387–396. https://doi.org/10.1038/35077544
Cook D, Ralphs MH, Welch KD, Stegelmeier BL (2009) Locoweed poisoning in livestock. Rangelands 31:16–21. https://doi.org/10.2111/1551-501X-31.1.16
Coren S (2013) Are some dogs getting addicted to hallucinogens? Psychology Today. https://www.psychologytoday.com/intl/blog/canine-corner/201312/are-some-dogs-getting-addicted-hallucinogens. Accessed 7 May 2018
Crew B (2015) Bennett’s wallabies get high on poppy seeds. Australian Geographic, Australian Geographic. https://www.australiangeographic.com.au/blogs/creatura-blog/2015/02/bennetts-wallabies-get-high/. Accessed 7 May 2018
Croxford JL (2003) Therapeutic potential of cannabinoids in CNS disease. CNS Drugs 17:179–202
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729. https://doi.org/10.1146/annurev.pharmtox.47.120505.105214
Darré L, Domene C (2015) Binding of capsaicin to the TRPV1 Ion Channel. Mol Pharm 12:4454–4465. https://doi.org/10.1021/acs.molpharmaceut.5b00641
Davis AP, Govaerts R, Bridson DM, Stoffelen P (2006) An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot J Linn Soc 152:465–512. https://doi.org/10.1111/j.1095-8339.2006.00584.x
Dawid C, Henze A, Frank O, Glabasnia A, Rupp M, Büning K, Orlikowski D, Bader M, Hofmann T (2012) Structural and sensory characterization of key pungent and tingling compounds from black pepper (Piper nigrum L.). J Agric Food Chem 60:2884–2895. https://doi.org/10.1021/jf300036a
De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V (2011) Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163:1479–1494. https://doi.org/10.1111/j.1476-5381.2010.01166.x
De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V (2007) Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB1 receptors and endovanilloids. Exp Cell Res 313:1911–1920. https://doi.org/10.1016/j.yexcr.2007.01.008
De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015. https://doi.org/10.1124/jpet.107.134809
Denis V, Cyert MS (2002) Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol 156:29–34. https://doi.org/10.1083/jcb.200111004
Denoeud F, Carretero-Paulet L, Dereeper A, Droc G, Guyot R, Pietrella M, Zheng C, Alberti A, Anthony F, Aprea G, Aury JM, Bento P, Bernard M, Bocs S, Campa C, Cenci A, Combes MC, Crouzillat D, Da Silva C, Daddiego L, De Bellis F, Dussert S, Garsmeur O, Gayraud T, Guignon V, Jahn K, Jamilloux V, Joët T, Labadie K, Lan T, Leclercq J, Lepelley M, Leroy T, Li LT, Librado P, Lopez L, Muñoz A, Noel B, Pallavicini A, Perrotta G, Poncet V, Pot D, Priyono, Rigoreau M, Rouard M, Rozas J, Tranchant-Dubreuil C, VanBuren R, Zhang Q, Andrade AC, Argout X, Bertrand B, de Kochko A, Graziosi G, Henry RJ, Jayarama, Ming R, Nagai C, Rounsley S, Sankoff D, Giuliano G, Albert VA, Wincker P, Lashermes P (2014) The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 345:1181–1184. https://doi.org/10.1126/science.1255274
Desikan R, Hancock JT, Coffey MJ, Neill SJ (1996) Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett 382:213–217. https://doi.org/10.1016/0014-5793(96)00177-9
Dohi T, Anamura S, Shirakawa M, Okamoto H, Tsujimoto A (1991) Inhibition of lipoxygenase by phenolic compounds. Jpn J Pharmacol 55:547–550
Doke N, Miura Y, Sanchez LM, Park HJ, Noritake T, Yoshioka H, Kawakita K (1996) The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defence—a review. Gene 179:45–51. https://doi.org/10.1016/S0378-1119(96)00423-4
Dussor GO, Leong AS, Gracia NB, Kilo S, Price TJ, Hargreaves KM, Flores CM (2003) Potentiation of evoked calcitonin gene-related peptide release from oral mucosa: a potential basis for the pro-inflammatory effects of nicotine. Eur J Neurosci 18:2515–2526
Eady CC, Kamoi T, Kato M, Porter NG, Davis S, Shaw M, Kamoi A, Imai S (2008) Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol 147:2096–2106. https://doi.org/10.1104/pp.108.123273
Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A (2010) Caffeine’s vascular mechanisms of action. International Journal of Vascular Medicine 2010:834060. https://doi.org/10.1155/2010/834060
Eisen JS, Koren G, Juurlink DN, Ng VL (2004) N-acetylcysteine for the treatment of clove oil induced fulminant hepatic failure: case report and review of the literature. J Toxicol Clin Toxicol 42:89–92. https://doi.org/10.1081/CLT-120028751
Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K (2011) The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 21:316–321. https://doi.org/10.1016/j.cub.2011.01.031
Faria LRR, Zanella FCV (2015) Beta-ionone attracts Euglossa mandibularis (Hymenoptera, Apidae) males in western Paraná forests. Revista Brasileira de Entomologia 59:260–264. https://doi.org/10.1016/j.rbe.2015.05.003
Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ (2006) A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 127:621–633. https://doi.org/10.1016/j.cell.2006.09.035
Flockerzi V, Nilius B (2014) TRPs: truly remarkable proteins. Handb Exp Pharmacol 222:1–12. https://doi.org/10.1007/978-3-642-54215-2_1
Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I (2001) The role of central and peripheral cannabinoid 1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 92:91–100
Freitas RCP, Faroni LRDA, Haddi K, Viteri Jumbo LO, Oliveira EE (2016) Allyl isothiocyanate actions on populations of Sitophilus zeamais resistant to phosphine: toxicity, emergence inhibition and repellency. J Stored Prod Res 69:257–264. https://doi.org/10.1016/j.jspr.2016.09.006
Fricke EC, Simon MJ, Reagan KM, Levey DJ, Riffell JA, Carlo TA, Tewksbury JJ (2013) When condition trumps location: seed consumption by fruit-eating birds removes pathogens and predator attractants. Ecol Lett 16:1031–1036. https://doi.org/10.1111/ele.12134
Fürstenberg-Hägg J, Zagrobelny M, Bak S (2013) Plant defense against insect herbivores. Int J Mol Sci 14:10242–10297. https://doi.org/10.3390/ijms140510242
Gallage NJ, JØrgensen K, Janfelt C, Nielsen AJZ, Naake T, Duński E, Dalsten L, Grisoni M, MØller BL (2018) The intracellular localization of the vanillin biosynthetic machinery in pods of Vanilla planifolia. Plant Cell Physiol 59:304–318. https://doi.org/10.1093/pcp/pcx185
Gartrell BD, Reid C (2007) Death by chocolate: a fatal problem for an inquisitive wild parrot. N Z Vet J 55:149–151. https://doi.org/10.1080/00480169.2007.36759
Garty BZ (1993) Garlic burns. Pediatrics 91:658–659
Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ (2004) Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem 279:20283–20295. https://doi.org/10.1074/jbc.M312577200
Gees M, Alpizar YA, Boonen B, Sanchez A, Everaerts W, Segal A, Xue F, Janssens A, Owsianik G, Nilius B, Voets T, Talavera K (2013) Mechanisms of transient receptor potential vanilloid 1 activation and sensitization by allyl isothiocyanate. Mol Pharmacol 84:325–334. https://doi.org/10.1124/mol.113.085548
Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA (2008) An internal thermal sensor controlling temperature preference in Drosophila. Nature 454:217. https://doi.org/10.1038/nature07001
Hanson SM, Newstead S, Swartz KJ, Sansom MSP (2015) Capsaicin interaction with TRPV1 channels in a lipid bilayer: molecular dynamics simulation. Biophys J 108:1425–1434. https://doi.org/10.1016/j.bpj.2015.02.013
Hao H, Wei J, Dai J, Du J (2008) Host-seeking and blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) exposed to vapors of geraniol, citral, citronellal, eugenol, or anisaldehyde. J Med Entomol 45:533–539
Hemmerlin A (2013) Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci 203-204:41–54. https://doi.org/10.1016/j.plantsci.2012.12.008
Hemmerlin A, Harwood JL, Bach TJ (2012) A raison d'être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog Lipid Res 51:95–148. https://doi.org/10.1016/j.plipres.2011.12.001
Hindorf H, Omondi CO (2011) A review of three major fungal diseases of Coffea arabica L. in the rainforests of Ethiopia and progress in breeding for resistance in Kenya. J Adv Res 2:109–120. https://doi.org/10.1016/j.jare.2010.08.006
Hinman A, Chuang HH, Bautista DM, Julius D (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 103:19564–19568. https://doi.org/10.1073/pnas.0609598103
Hirst RA, Lambert DG, Notcutt WG (1998) Pharmacology and potential therapeutic uses of cannabis. Br J Anaesth 81:77–84
Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A (2004) Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther 308:446–453. https://doi.org/10.1124/jpet.103.060079
Hollingsworth RG, Armstrong JW, Campbell E (2002) Caffeine as a repellent for slugs and snails. Nature 417:915–916. https://doi.org/10.1038/417915a
Imai S, Tsuge N, Tomotake M, Nagatome Y, Sawada H, Nagata T, Kumagai H (2002) Plant biochemistry: an onion enzyme that makes the eyes water. Nature 419:685. https://doi.org/10.1038/419685a
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66. https://doi.org/10.1146/annurev.ento.51.110104.151146
Jacquot L, Monnin J, Lucarz A, Brand G (2005) Trigeminal sensitization and desensitization in the nasal cavity: a study of cross interactions. Rhinology 43:93–98
Janssens A, Voets T (2011) Ligand stoichiometry of the cold- and menthol-activated channel TRPM8. J Physiol 589:4827–4835. https://doi.org/10.1113/jphysiol.2011.216523
Jesudoss VAS, Victor Antony Santiago S, Venkatachalam K, Subramanian P (2017) Chapter 21—zingerone (ginger extract): antioxidant potential for efficacy in gastrointestinal and liver disease. In: Gracia-Sancho J, Salvadó J (eds) Gastrointestinal tissue. Academic Press, Cambridge, pp 289–297. https://doi.org/10.1016/B978-0-12-805377-5.00021-7
Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA (2001) Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain 93:303–315
Johanek LM, Simone DA (2004) Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain 109:432–442. https://doi.org/10.1016/j.pain.2004.02.020
Johnson SD, Ellis A, Dötterl S (2007) Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae). Am J Bot 94:47–55. https://doi.org/10.3732/ajb.94.1.47
Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260. https://doi.org/10.1038/nature02282
Jordt SE, Julius D (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108:421–430
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384. https://doi.org/10.1146/annurev-cellbio-101011-155833
Junca P, Sandoz JC (2015) Heat perception and aversive learning in honey bees: putative involvement of the thermal/chemical sensor AmHsTRPA. Front Physiol 6:316. https://doi.org/10.3389/fphys.2015.00316
Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K (2001) Molecular bases of odor discrimination: reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci 21:6018–6025
Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA (2010) Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464:597–600. https://doi.org/10.1038/nature08848
Kapsimali M, Barlow LA (2013) Developing a sense of taste. Semin Cell Dev Biol 24:200–209. https://doi.org/10.1016/j.semcdb.2012.11.002
Kassie F, Parzefall W, Musk S, Johnson I, Lamprecht G, Sontag G, Knasmüller S (1996) Genotoxic effects of crude juices from Brassica vegetables and juices and extracts from phytopharmaceutical preparations and spices of cruciferous plants origin in bacterial and mammalian cells. Chem Biol Interact 102:1–16
Katoh A, Hashimoto T (2004) Molecular biology of pyridine nucleotide and nicotine biosynthesis. Front Biosci 9:1577–1586
Katoh A, Shoji T, Hashimoto T (2007) Molecular cloning of N-methylputrescine oxidase from tobacco. Plant Cell Physiol 48:550–554. https://doi.org/10.1093/pcp/pcm018
Kaufman T (2015) The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals, vol 26. https://doi.org/10.5935/0103-5053.20150086
Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with ca2+ binding motifs. Plant Cell 10:255
Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C (2010) Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A 107:8440–8445. https://doi.org/10.1073/pnas.1001425107
Klein AH, Carstens MI, Carstens E (2013) Eugenol and carvacrol induce temporally desensitizing patterns of oral irritation and enhance innocuous warmth and noxious heat sensation on the tongue. Pain 154:2078–2087. https://doi.org/10.1016/j.pain.2013.06.025
Knudsen JT, Tollsten L, Bergström LG (1993) Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280. https://doi.org/10.1016/0031-9422(93)85502-I
Koeduka T, Fridman E, Gang DR, Vassão DG, Jackson BL, Kish CM, Orlova I, Spassova SM, Lewis NG, Noel JP, Baiga TJ, Dudareva N, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc Natl Acad Sci U S A 103:10128–10133. https://doi.org/10.1073/pnas.0603732103
Kohno K, Sokabe T, Tominaga M, Kadowaki T (2010) Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential channel genes. J Neurosci 30:12219–12229. https://doi.org/10.1523/JNEUROSCI.2001-10.2010
Koizumi K, Iwasaki Y, Narukawa M, Iitsuka Y, Fukao T, Seki T, Ariga T, Watanabe T (2009) Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem Biophys Res Commun 382:545–548. https://doi.org/10.1016/j.bbrc.2009.03.066
Kovalkovičová N, Sutiaková I, Pistl J, Sutiak V (2009) Some food toxic for pets. Interdiscip Toxicol 2:169–176. https://doi.org/10.2478/v10102-009-0012-4
Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR (2006) Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101(2):618–627
Kumamoto E, Fujita T, Jiang C-Y (2014) TRP channels involved in spontaneous l-glutamate release enhancement in the adult rat spinal substantia gelatinosa. Cells 3:331–362. https://doi.org/10.3390/cells3020331
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289. https://doi.org/10.1016/j.neuron.2006.03.042
Labbé R, Caveney S, Donly C (2011) Expression of multidrug resistance proteins is localized principally to the Malpighian tubules in larvae of the cabbage looper moth, Trichoplusia ni. J Exp Biol 214:937–944. https://doi.org/10.1242/jeb.051060
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275. https://doi.org/10.1146/annurev.arplant.48.1.251
Laska M, Distel H, Hudson R (1997) Trigeminal perception of odorant quality in congenitally anosmic subjects. Chem Senses 22:447–456
Laurent K, Perrine R, Benjamin R, Amine B, Michel R, Aubin H-J, Christophe L (2014) Acute and long-term effects of cannabis use: a review. Curr Pharm Des 20:4112–4118. https://doi.org/10.2174/13816128113199990620
Leal-Cardoso JH, da Silva-Alves KS, Ferreira-da-Silva FW, dos Santos-Nascimento T, Joca HC, de Macedo FH, de Albuquerque-Neto PM, Magalhães PJ, Lahlou S, Cruz JS, Barbosa R (2010) Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur J Pharmacol 645:86–93. https://doi.org/10.1016/j.ejphar.2010.07.014
Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S (2011) Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J Comput Aided Mol Des 25:317–327. https://doi.org/10.1007/s10822-011-9421-5
Lee LY, Gu Q (2009) Cough sensors. IV. Nicotinic membrane receptors on cough sensors. Handb Exp Pharmacol 77–98. https://doi.org/10.1007/978-3-540-79842-2_5
Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, Lee JH, Kim JS, Oh SB (2005) Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res 84:848–851. https://doi.org/10.1177/154405910508400913
Lee Y, Moon SJ, Montell C (2009) Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A 106:4495–4500. https://doi.org/10.1073/pnas.0811744106
Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E (2001) Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol 127:1256–1265
Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, Laezza C, Portella G, Bifulco M, Di Marzo V (2006) Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 318:1375–1387. https://doi.org/10.1124/jpet.106.105247
Liman ER (2007) The Ca2+-Activated TRP Channels: TRPM4 and TRPM5. https://www.ncbi.nlm.nih.gov/books/NBK5257/
Liu D, Liman ER (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A 100:15160–15165. https://doi.org/10.1073/pnas.2334159100
Liu L, Welch JM, Erickson RP, Reinhart PH, Simon SA (2000) Different responses to repeated applications of zingerone in behavioral studies, recordings from intact and cultured TG neurons, and from VR1 receptors. Physiol Behav 69:177–186. https://doi.org/10.1016/S0031-9384(00)00200-6
Lundegårdh B, Botek P, Schulzov V, Hajslov J, Strömberg A, Andersson HC (2008) Impact of different green manures on the content of S-alk(en)yl-L-cysteine sulfoxides and L-ascorbic acid in leek (Allium porrum). J Agric Food Chem 56:2102–2111. https://doi.org/10.1021/jf071710s
López-Malo A, Alzamora SM, Argaiz A (1995) Effect of natural vanillin on germination time and radial growth of moulds in fruit-based agar systems. Food Microbiol 12:213–219. https://doi.org/10.1016/S0740-0020(95)80100-6
Lübbert M, Kyereme J, Schöbel N, Beltrán L, Wetzel CH, Hatt H (2013) Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons. PLoS One 8:e77998. https://doi.org/10.1371/journal.pone.0077998
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545. https://doi.org/10.1038/nature05544
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934. https://doi.org/10.1016/j.cub.2005.04.018
Maddrell SH, Gardiner BO (1976) Excretion of alkaloids by malpighian tubules of insects. J Exp Biol 64:267–281
Maia MF, Moore SJ (2011) Plant-based insect repellents: a review of their efficacy, development and testing. Malar J 10(Suppl 1):S11. https://doi.org/10.1186/1475-2875-10-S1-S11
Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A (2001) CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 93:239–245
Mansour EE, Mi F, Zhang G, Jiugao X, Wang Y, Kargbo A (2012) Effect of allylisothiocyanate on Sitophilus oryzae, Tribolium confusum and Plodia interpunctella: toxicity and effect on insect mitochondria. Crop Prot 33:40–51. https://doi.org/10.1016/j.cropro.2011.11.010
Marieb EN, Hoehn K (2009) Human anatomy and physiology. In., 8th edn. Pearson Benjamin Cummings, pp 547–589
Martins N, Petropoulos S, Ferreira IC (2016) Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chem 211:41–50. https://doi.org/10.1016/j.foodchem.2016.05.029
McIndoo NE (1937) Quantitative injection and effects of nicotine in insects., vol 55. Journal of Agricultural Research
McNamara FN, Randall A, Gunthorpe MJ (2005) Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol 144:781–790. https://doi.org/10.1038/sj.bjp.0706040
Meghwal M, Goswami TK (2013) Piper nigrum and piperine: an update. Phytother Res 27:1121–1130. https://doi.org/10.1002/ptr.4972
Miller BA, Zhang W (2011) TRP channels as mediators of oxidative stress. In: Islam MS (ed) Transient receptor potential channels. Springer Netherlands, Dordrecht, pp 531–544. https://doi.org/10.1007/978-94-007-0265-3_29
Miwa JM, Freedman R, Lester HA (2011) Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 70:20–33. https://doi.org/10.1016/j.neuron.2011.03.014
Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A (2009) TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 4:e7596. https://doi.org/10.1371/journal.pone.0007596
Montell C (2005, 2005) The TRP superfamily of cation channels. Sci STKE:re3. https://doi.org/10.1126/stke.2722005re3
Moon SJ, Lee Y, Jiao Y, Montell C (2009) A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol 19:1623–1627. https://doi.org/10.1016/j.cub.2009.07.061
Mueller-Seitz E, Hiepler C, Petz M (2008) Chili pepper fruits: content and pattern of capsaicinoids in single fruits of different ages. In: J Agric Food Chem, vol 56. vol 24. United States, pp 12114-12121. https://doi.org/10.1021/jf802385v
Nackley AG, Makriyannis A, Hohmann AG (2003) Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 119:747–757
Nagatomo K, Ishii H, Yamamoto T, Nakajo K, Kubo Y (2010) The Met268Pro mutation of mouse TRPA1 changes the effect of caffeine from activation to suppression. Biophys J 99:3609–3618. https://doi.org/10.1016/j.bpj.2010.10.014
Nagatomo K, Kubo Y (2008) Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A 105:17373–17378. https://doi.org/10.1073/pnas.0809769105
Narusuye K, Kawai F, Matsuzaki K, Miyachi E (2005) Linalool suppresses voltage-gated currents in sensory neurons and cerebellar Purkinje cells. J Neural Transm (Vienna) 112:193–203. https://doi.org/10.1007/s00702-004-0187-y
Nazıroğlu M, Çiğ B, Özgül C (2013) Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242:151–160. https://doi.org/10.1016/j.neuroscience.2013.03.032
Nicholas S, Yuan SY, Brookes SJH, Spencer NJ, Zagorodnyuk VP (2016) Hydrogen peroxide preferentially activates capsaicin-sensitive high threshold afferents via TRPA1 channels in the guinea pig bladder. Br J Pharmacol 174:126–138. https://doi.org/10.1111/bph.13661
Nilius B, Appendino G (2013) Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol 164:1–76. https://doi.org/10.1007/112_2013_11
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 464:425–458. https://doi.org/10.1007/s00424-012-1158-z
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12:218. https://doi.org/10.1186/gb-2011-12-3-218
Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T (2005) The selectivity filter of the cation channel TRPM4. J Biol Chem 280:22899–22906. https://doi.org/10.1074/jbc.M501686200
Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX (2005) Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280:6423–6433. https://doi.org/10.1074/jbc.M411089200
Nilius B, Voets T, Peters J (2005) TRP channels in disease. Sci STKE 2005:re8
Nocchi L, Daly DM, Chapple C, Grundy D (2014) Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell 13:540–550. https://doi.org/10.1111/acel.12208
Nuwer R (2013) Dolphins seem to use toxic pufferfish to get high. Smithsonian, smithsonianmag.com
Ohbuchi K, Mori Y, Ogawa K, Warabi E, Yamamoto M, Hirokawa T (2016) Detailed analysis of the binding mode of vanilloids to transient receptor potential vanilloid type I (TRPV1) by a mutational and computational study. PLoS One 11:e0162543. https://doi.org/10.1371/journal.pone.0162543
Ohkubo T, Shibata M (1997) The selective capsaicin antagonist capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J Dent Res 76:848–851
Ohta T, Imagawa T, Ito S (2007) Novel agonistic action of mustard oil on recombinant and endogenous porcine transient receptor potential V1 (pTRPV1) channels. Biochem Pharmacol 73:1646–1656. https://doi.org/10.1016/j.bcp.2007.01.029
Ohtsubo S, Fujita T, Matsushita A, Kumamoto E (2015) Inhibition of the compound action potentials of frog sciatic nerves by aroma oil compounds having various chemical structures. Pharmacol Res Perspect 3:e00127. https://doi.org/10.1002/prp2.127
Okumura Y, Narukawa M, Iwasaki Y, Ishikawa A, Matsuda H, Yoshikawa M, Watanabe T (2010) Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem 74:1068–1072
Olender T, Lancet D, Nebert DW (2008) Update on the olfactory receptor (OR) gene superfamily. Hum Genomics 3:87–97
Omar SH, Al-Wabel NA (2010) Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J 18:51–58. https://doi.org/10.1016/j.jsps.2009.12.007
Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci 96:6553
Owsianik G, Talavera K, Voets T, Nilius B (2006) Permeation and selectivity of TRP channels. Annu Rev Physiol 68:685–717. https://doi.org/10.1146/annurev.physiol.68.040204.101406
Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y (2001) A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A 98:7801–7805. https://doi.org/10.1073/pnas.141036198
Parish RA, McIntire S, Heimbach DM (1987) Garlic burns: a naturopathic remedy gone awry. Pediatr Emerg Care 3:258–260
Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB (2009) Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. In: Pain, vol 144. vol 1-2. Netherlands, pp 84-94. https://doi.org/10.1016/j.pain.2009.03.016
Park CK, Li HY, Yeon KY, Jung SJ, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB (2006) Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res 85:900–904. https://doi.org/10.1177/154405910608501005
Parker JK (2015) 1—introduction to aroma compounds in foods. In: Parker JK, Elmore JS, Methven L (eds) Flavour development, analysis and perception in food and beverages. Woodhead Publishing, Sawston, pp 3–30. https://doi.org/10.1016/B978-1-78242-103-0.00001-1
Pedersen SF, Owsianik G, Nilius B (2005) TRP channels: an overview. Cell Calcium 38:233–252. https://doi.org/10.1016/j.ceca.2005.06.028
Peteresen OH (2006) Special senses. In: Petersen OH (ed) Lecture notes: human physiology. 5 edn. Blackwell Sciences, pp 138-184
Pihlasalo J, Klika KD, Murzin DY, Nieminen V (2007) Conformational equilibria of citral. J Mol Struct THEOCHEM 814:33–41. https://doi.org/10.1016/j.theochem.2007.02.031
Pinto JM (2011) Olfaction. Proc Am Thorac Soc 8:46–52. https://doi.org/10.1513/pats.201005-035RN
Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. In: Proc Natl Acad Sci U S A, vol 100. vol 25. United States, pp 15166-15171. https://doi.org/10.1073/pnas.2334624100
Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176. https://doi.org/10.1038/nn952
Pérez CA, Margolskee RF, Kinnamon SC, Ogura T (2003) Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 33:541–549
Raguso RA, Pichersky E (1999) New perspectives in pollination biology: floral fragrances. A day in the life of a linalool molecule: chemical communication in a plant-pollinator system. Part 1: linalool biosynthesis in flowering plants. Plant Species Biology 14:95–120. https://doi.org/10.1046/j.1442-1984.1999.00014.x
Rahn EJ, Hohmann AG (2009) Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 6:713–737. https://doi.org/10.1016/j.nurt.2009.08.002
Riera CE, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, Vogel H, Simon SA, le Coutre J (2009) Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol 157:1398–1409. https://doi.org/10.1111/j.1476-5381.2009.00307.x
Roper SD (2014) TRPs in taste and chemesthesis. Handb Exp Pharmacol 223:827–871. https://doi.org/10.1007/978-3-319-05161-1_5
Salazar H, Llorente I, Jara-Oseguera A, García-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T (2008) A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci 11:255–261. https://doi.org/10.1038/nn2056
Salgado BS, Monteiro LN, Rocha NS (2011) Allium species poisoning in dogs and cats. JVATiTD 17:4–11
Santos JC, Faroni LRA, Sousa AH, Guedes RNC (2011) Fumigant toxicity of allyl isothiocyanate to populations of the red flour beetle Tribolium castaneum. J Stored Prod Res 47:238–243. https://doi.org/10.1016/j.jspr.2011.03.004
Sharma E, Anand G, Kapoor R (2017) Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann Bot 119:791–801. https://doi.org/10.1093/aob/mcw263
Shimizu S, Takahashi N, Mori Y (2014) TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handb Exp Pharmacol 223:767–794. https://doi.org/10.1007/978-3-319-05161-1_3
Sidi S, Friedrich RW, Nicolson T (2003) NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301:96–99. https://doi.org/10.1126/science.1084370
Silver WL, Clapp TR, Stone LM, Kinnamon SC (2006) TRPV1 receptors and nasal trigeminal chemesthesis. Chem Senses 31:807–812. https://doi.org/10.1093/chemse/bjl022
Silver WL, Finger TE (2009) The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N Y Acad Sci 1170:202–205. https://doi.org/10.1111/j.1749-6632.2009.03894.x
Simons CT, Boucher Y, Carstens MI, Carstens E (2006) Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol 96:1877–1886. https://doi.org/10.1152/jn.00345.2006
Simons CT, Sudo S, Sudo M, Carstens E (2003) Mecamylamine reduces nicotine cross-desensitization of trigeminal caudalis neuronal responses to oral chemical irritation. Brain Res 991:249–253. https://doi.org/10.1016/S0006-8993(03)03539-X
Sirikantaramas S, Taura F, Tanaka Y, Ishikawa Y, Morimoto S, Shoyama Y (2005) Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol 46:1578–1582. https://doi.org/10.1093/pcp/pci166
Solymosi K, Köfalvi A (2016) Cannabis: a treasure trove or pandora’s box? vol 16. https://doi.org/10.2174/1389557516666161004162133
Stephenson D, Halpern BP (2009) No oral-cavity-only discrimination of purely olfactory odorants. Chem Senses 34:121–126. https://doi.org/10.1093/chemse/bjn063
Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE (2008) Citral sensing by transient [corrected] receptor potential channels in dorsal root ganglion neurons. PLoS One 3:e2082. https://doi.org/10.1371/journal.pone.0002082
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S (2015) Localized TRPA1 channel Ca(2+) signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8:ra2–ra2. https://doi.org/10.1126/scisignal.2005659
Sullivan RJ, Hagen EH, Hammerstein P (2008) Revealing the paradox of drug reward in human evolution. Proc R Soc B Biol Sci 275:1231–1241. https://doi.org/10.1098/rspb.2007.1673
Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y (2011) TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7:701–711. https://doi.org/10.1038/nchembio.640
Takahashi N, Mori Y (2011) TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol 2:58. https://doi.org/10.3389/fphar.2011.00058
Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T (2009) Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12:1293–1299. https://doi.org/10.1038/nn.2379
Tan KH, Nishida R (2012) Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 12:56. https://doi.org/10.1673/031.012.5601
Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ (2009) Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol 75:820–829. https://doi.org/10.1124/mol.108.054445
Tewksbury JJ, Nabhan GP (2001) Seed dispersal. Directed deterrence by capsaicin in chilies. Nature 412:403–404. https://doi.org/10.1038/35086653
Tewksbury JJ, Nabhan GP, Norman D, Suzán H, Tuxill J, Donovan J (1999) In situ conservation of wild chiles and their biotic associates. Conserv Biol 13:98–107. https://doi.org/10.1046/j.1523-1739.1999.97399.x
Tewksbury JJ, Reagan KM, Machnicki NJ, Carlo TA, Haak DC, Peñaloza AL, Levey DJ (2008) Evolutionary ecology of pungency in wild chilies. Proc Natl Acad Sci U S A 105:11808–11811. https://doi.org/10.1073/pnas.0802691105
Tholl D (2015) Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol 148:63–106. https://doi.org/10.1007/10_2014_295
Thompson D, Eling T (1989) Mechanism of inhibition of prostaglandin H synthase by eugenol and other phenolic peroxidase substrates. Mol Pharmacol 36:809–817
Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A (1993) Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57:845–859. https://doi.org/10.1016/0306-4522(93)90029-F
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417. https://doi.org/10.1146/annurev.biochem.75.103004.142819
Viana F (2016) TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol 594:4151–4169. https://doi.org/10.1113/JP270935
Voets T, Owsianik G, Janssens A, Talavera K, Nilius B (2007) TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3:174. https://doi.org/10.1038/nchembio862
Walker RG, Willingham AT, Zuker CS (2000) A Drosophila mechanosensory transduction channel. Science 287:2229–2234
Wallock-Richards D, Doherty CJ, Doherty L, Clarke DJ, Place M, Govan JR, Campopiano DJ (2014) Garlic revisited: antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS One 9:e112726. https://doi.org/10.1371/journal.pone.0112726
Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY (2012) Identification of in vivo disulfide conformation of TRPA1 ion channeL. J Biol Chem 287:6169–6176. https://doi.org/10.1074/jbc.M111.329748
Weigmann R (1958) Die Wirkung von Substanzen auf den Netzbau der Spinne als biologischer Test. Von P. N. Witt. Springer-Verlag, Berlin 1956. III, 79 S. mit 49 Abb. Preis DM 15,60. Arch Pharm 291:54–55. https://doi.org/10.1002/ardp.19582910113
Wescott SA, Rauthan M, Xu XZ (2013) When a TRP goes bad: transient receptor potential channels in addiction. Life Sci 92:410–414. https://doi.org/10.1016/j.lfs.2012.07.008
Wink M, Theile V (2002) Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae). Chemoecology 12:29–46. https://doi.org/10.1007/s00049-002-8324-2
Wolstenholme AJ, Williamson SM, Reaves BJ (2011) TRP channels in parasites. Adv Exp Med Biol 704:359–371. https://doi.org/10.1007/978-94-007-0265-3_20
Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC (2013) Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 339:1202–1204. https://doi.org/10.1126/science.1228806
Xiao R, Xu XZ (2009) Function and regulation of TRP family channels in C. elegans. Pflugers Arch 458:851–860. https://doi.org/10.1007/s00424-009-0678-7
Xiong W, Cui T, Cheng K, Yang F, Chen S-R, Willenbring D, Guan Y, Pan H-L, Ren K, Xu Y, Zhang L (2012) Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J Exp Med 209:1121–1134. https://doi.org/10.1084/jem.20120242
Xu H, Delling M, Jun JC, Clapham DE (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635. https://doi.org/10.1038/nn1692
Xu XZ, Sternberg PW (2003) A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell 114:285–297
Xue H-H, Zhao D-M, Sucla T, Uchida C, Oda T, Chida K, Ichiyama A, Nakamura H (2000) Store depletion by caffeine/ryanodine activates capacitative Ca<sup>2+</sup> entry in nonexcitable A549 cells. The Journal of Biochemistry 128:329–336
Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82:781–785
Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, Yarov-Yarovoy V, Zheng J (2015) Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol 11:518–524. https://doi.org/10.1038/nchembio.1835
Zhang C, Wu H, Zhao Y, Ma Z, Zhang X (2016) Comparative studies on mitochondrial electron transport chain complexes of Sitophilus zeamais treated with allyl isothiocyanate and calcium phosphide. Pestic Biochem Physiol 126:70–75. https://doi.org/10.1016/j.pestbp.2015.07.009
Zurier RB, Burstein SH (2016) Cannabinoids, inflammation, and fibrosis. FASEB J 30:3682–3689. https://doi.org/10.1096/fj.201600646R
Zygmunt PM, Andersson DA, Högestätt ED (2002) Δ9-Tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci 22:4720
Zygmunt PM, Högestätt ED (2014) TRPA1. In: Nilius B, Flockerzi V (eds) Mammalian transient receptor potential (TRP) cation channels: volume I. Springer Berlin Heidelberg, Heidelberg, pp 583–630. https://doi.org/10.1007/978-3-642-54215-2_23
Acknowledgments
We thank Dr. Y. A. Alpizar for his helpful comments on the manuscript. JChem for Office was used for drawing and displaying chemical structures, JChem for Office 17.3.2700.1567, ChemAxon (http://www.chemaxon.com).
Funding
This work was supported by grants from KU Leuven Research Council (PF-TRPLe and C1-TRPLe to T.V.) and the Queen Elisabeth Medical Foundation for Neurosciences (to T.V.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Startek, J.B., Voets, T. & Talavera, K. To flourish or perish: evolutionary TRiPs into the sensory biology of plant-herbivore interactions. Pflugers Arch - Eur J Physiol 471, 213–236 (2019). https://doi.org/10.1007/s00424-018-2205-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2205-1