Abstract
Identification of the auditory hair cell mechano-electrical transduction (hcMET) channel has been a major focus in the hearing research field since the 1980s when direct mechanical gating of a transduction channel was proposed (Corey and Hudspeth J Neurosci 3:962–976, 1983). To this day, the molecular identity of this channel remains controversial. However, many of the hcMET channel’s properties have been characterized, including pore properties, calcium-dependent ion permeability, rectification, and single channel conductance. At this point, elucidating the molecular identity of the hcMET channel will provide new tools for understanding the mechanotransduction process. This review discusses the significance of identifying the hcMET channel, the difficulties associated with that task, as well as the establishment of clear criteria for this identification. Finally, we discuss potential candidate channels in light of these criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanosensation is a primordial sense. Because sensing ones environment is fundamental to an organism’s survival, several sensory modalities have developed based on mechanosensation. Touch, proprioception, perception of osmotic stresses, and, of course, hearing are a few examples of such sensory modalities. As we understand it, all of those sensations employ directly mechanically gated transduction channels, the mechano-electrical transduction (MET) channels. Some of these MET channels have been identified, such as the osmotic stress detectors MscL [15, 82, 83, 101] and MscS [6] in Escherichia coli or the MEC-4 touch receptor in Caenorhabditis elegans [13, 39, 73]. The channels piezo [24, 25], TREK-1 [1], and TRAAK [72] have been implicated in mechanosensitivity of mammalian dorsal root ganglia. None of these channels have to operate at frequencies required for mammalian hearing [93], which can reach as high as 150 kHz in dolphins [46]. As for MET channels implicated in hearing, NOMPC (TRPN1) in Drosophila melanogaster [29, 30, 112, 116] is currently supported by the strongest evidence. However, even though NOMPC fulfills all criteria for a bona fide transduction channel involved in gentle touch sensation [112], its function in hearing is still a matter of debate [60]. Several MET channel candidates important for mammalian hearing have been proposed, but none meets all requirements, as will be discussed later in this review.
In recent decades, many proteins required for hearing and more specifically hair cell mechanotransduction have been identified; among those identified are the tip link proteins (protocadherin 15 PCDH15 and cadherin 23 CDH23) [54]. Tip links connect the shorter rows of stereocilia to their next taller neighbor and relay mechanical forces resulting from a sheering motion between those stereocilia to the hair cell MET (hcMET) channels. Other proteins identified include harmonin [5], myosin VIIa [41], myosin 3 [87], whirlin [109], and numerous actin-binding proteins (see review [81] and proteomics screen from chicken utricle [99]). The loss of these molecules results in compromised mechanotransduction and a deterioration or even complete loss of hearing. Yet the molecular identity of the hcMET channel remains unknown, even after three decades of intensive search. Many consider the hcMET channel as the “holy grail” of the hearing field. But is this molecule more important than other components such as the tip link proteins or any of the other molecules known to underlie genetic human diseases resulting in hearing loss? We would argue that the significance of the final identification of the hcMET channel has grown more because of the immense amount of effort and degree of difficulty in resolving the question than due to its pure scientific impact. That said, identifying the channel is important, as it will make a plethora of new experiments possible. For example, it will allow us to determine how the channel is gated and where specifically the channel is located to more directly probe the functioning of numerous accessory proteins thus far identified as well as to provide insight into how MET channels of different sensory modalities are related to one another. Assuming that the hcMET channels did not develop de novo but from existing MET channels of, i.e., touch, we could investigate how (if) those channels changed during evolution to achieve ever higher frequency sensitivity.
The auditory field has been searching for the molecular identity of the hcMET channel for more than three decades, and although a number of candidates have been proposed, the channel’s identity remains elusive. Assessment of channel candidacy has varied based on each investigator’s approach; some perceive localization and molecular interactions as critical, whereas others regard the biophysical correlation to native properties as the linchpin for identification. Attesting to the difficulty of identifying the unequivocal experiment is the fact that there are currently three molecules where data is remarkably similar in support of their involvement in mechanotransduction, transmembrane channel-like (TMC [10, 53, 57, 69, 76]), LHFPL5 (formerly known as TMHS [67, 98, 111]), and transmembrane inner ear (TMIE [38, 78, 100, 113]). Mutation or knockout of two of these molecules (TMC [76], LHFPL5 [111]) results in changes in single channel conductance, a property typically considered intrinsic to the channel, yet it is unclear whether these molecules can form an ion channel by themselves or interact with the hcMET channel. Thus, even a property typically considered intrinsic to the channel protein, like single channel conductance, must be interpreted carefully. This illustrates the technical difficulty and molecular complexity of hair cell mechanotransduction that makes it difficult for a single piece of data to conclusively prove that a given molecule is the transduction channel (see [21] and [7, 59]). It is much more likely that a series of investigations using multiple approaches will be necessary to substantiate channel identity. The following discusses the potential criteria necessary to identify this channel, how these criteria could be weighted, and the reasons why these criteria have been so difficult to meet.
Why is the hcMET channel so hard to find?
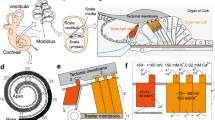
The sensory auditory organelle, the hair bundle, consists of specialized microvilli called stereocilia, connected by tip links at their top and side links along their side [50, 81]. Under normal conditions, hcMET channels are opened when stereocilia are deflected towards the tallest row [84, 85] (Fig. 1) of stereocilia. The resulting sheering motion between stereocilia is transferred into a force at the top of the next shorter row, mediated by the tip link [54, 84, 85]. The location of tip links and Ca2+ imaging suggest that functional hcMET channels are located near stereocilia tops (in all but the tallest row), thereby placing it in close proximity to PCDH15 at the bottom end of the tip link [9]. Whether the channel is directly or indirectly coupled to PCDH15 is unknown, making it difficult to design or interpret data that uses protein interactions assays. For example, can PCDH15 be used as bait to pull down the channel? This example exemplifies a common theme when it comes to hcMET channel identification, which is that there is so little data at the functional molecular level that critical assumptions need to be made at both the design and interpretation levels. The underlying assumptions required for data interpretation are discussed below along with each of the selected criteria for identification.
MET current activation. The mammalian hcMET channels are thought to be located to the tip of stereocilia. Forces resulting from a deflection towards the shortest row (blue arrow) are thought to close hcMET channels while deflections towards the tallest row (red arrow) opens hcMET channels, allowing for the inflow of cations. Displacements of different amplitudes elicit currents of different sizes (lower panel). The tip link, comprised of protocadherin 15 and cadherin 23, is thought to be essential for the force relay to the hcMET channel
Further exacerbating channel identification, electrophysiological data indicates that there are as few as two functional channel proteins per stereocilium [8, 9, 26]. Consequently, there may only be about 200 active hcMET channel proteins in a given hair cell hair bundle. With approximately 16,000 hair cells per mammalian cochlea, this amounts to about 3.2 million proteins. Compared to the 640 billion copies of γ actin [99] and hundreds of billions of other proteins in the hair cell hair bundles alone, isolating the hcMET channel proteins is similar to finding a needle in a hay stack. If a large number of nonfunctional channels are present, the problem might not be quite as bad. However, if the channel’s molecular components vary with frequency, as does the single channel conductance [8, 90], then the problem of identifying those varying components may become considerably more difficult. Thus, the limited data regarding the molecular interactions underlying mechanotransduction and the limited protein availability severely hamper attempts to identify the hcMET channel.
Is the hcMET channel unique to the ear?
Not knowing the molecular identity of the channel inspires much discussion as to how unique an entity this channel might be. Will it only be found in the ear? Does it have only one function? Is it inherently mechanosensitive? Properties of the hcMET channel suggest that it is specialized to operate at high frequencies [81] as compared to other mechanosensitive channels [15, 30, 39, 73, 83, 101, 112]. Other mechanosensory modalities operate at low frequency, at most a couple of hundred Hertz; the hcMET channel can detect signals over 100 kHz [46]. Are the kinetic differences between systems intrinsic to the MET channels or imparted by the microenvironment in which they reside? If the former is the case, then the hcMET channels likely represent a novel class of mechanosensitive channels; if the latter is the case, it is possible that a specialized environment surrounding a more conventional MET channel is responsible for the frequency sensitivities. Hereto, the answer has consequences towards channel identification.
If the microenvironment dictates the channel properties, then its removal from the native stereocilia environment may result in a functionally different hcMET channel, making identification much more difficult. If so, methods could be devised in which “hair cell-like” cells (where morphology, proteome, and functionality resemble auditory hair cells) are used as the template from which to investigate hcMET channel candidates. These cells would emulate the natural microenvironment more closely, perhaps providing as yet undescribed factors to allow functional expression. Previous attempts to generate hair cell-like cells resulted in very low yields, rendering them presently nonpractical [44, 63, 75]. Alternatively, cell lines such as COS-7 [97] could be used. Compounding this problem is the possibility that the channel is a heteromeric complex of different proteins so that expressing a single subunit in a heterologous system will not produce native channel properties [27, 28]. Not knowing these answers makes designing experiments and interpreting results difficult. How much one values these questions and associated criteria will shape how one interprets data regarding potential channel identity.
What does it take to validate a potential hcMET channel candidate?
A hcMET channel candidate must fulfill several criteria. In the following sections, we separate those criteria into four categories: channel localization, possible interaction partners, intrinsic channel properties, and genetic considerations (Fig. 2).
Criteria flow chart for identifying the hcMET channel. These flow charts show different experimental criteria that a candidate must pass to fit our current understanding of the characteristics and physiology of the hcMET channel using immunohistochemistry, electrophysiology, pharmacology, and genetic techniques. However, there are instances where a candidate may fail the set criterion but still be the hcMET channel, as is explained in the boxes leading to the next criteria
Localization criteria
The expectations as to channel localization for a given candidate would be the following: (i) expression in auditory hair cells but not necessarily exclusively, (ii) localization to the tips of stereocilia but not necessarily exclusively, (iii) localization to the membrane, and (iv) overlap between temporal expression of candidate and onset of mechanosensitivity. We will expand on those requirements and caveats in the following paragraphs.
In situ hybridization allows for cellular localization of a channel candidate’s mRNA and is used to determine if the candidate protein can be expressed in hair cells. This does not, however, mean that the protein need only be in hair cells as it may serve multiple functions and so be found in a variety of cell types. An appropriately localized signal should also appear prior to the onset of mechanosensitivity. With only a small number of channel proteins present in a given hair cell, and possible low protein turnover, there could be only a very small amount of coding mRNA present. It is possible that the in situ technique would not be sensitive enough to detect this low signal leading to false-negative results. Alternatively, one could employ several PCR methods on isolated hair cells, which should allow for the detection of very low levels of mRNA. Also, RNA fluorescence in situ hybridization methods are sensitive enough to detect single RNA copies in a given cell [61]. Some progress has been made in recent years to identify the transcriptome of cochlea hair cells [65] and the proteome of the hair bundles of chicken utricle hair cells [99]. This data is of high importance as it helps in establishing a functional network of proteins involved in hair cell function. However, we are still at the very beginning of solving this particular puzzle, although knowing its pieces is a good start. The leading MET channel candidate, the TMC proteins, shows an appropriate temporal and spatial distribution that supports their candidacy as hcMET channels [53]. Care must be taken though as a previous candidate TRPA1 [7, 21, 59] as well as several other transient receptor potential (TRP) channels show a similar temporal [3] or spatial [4, 51, 79, 86, 105, 106] expression pattern.
The next step after cellular localization of the channel candidate is the subcellular localization. Immunohistochemistry can be used to locate a candidate protein subcellularly. To be the hcMET channel, the candidate protein must be a transmembrane protein that localizes to stereocilia, specifically near to the stereocilia top. In addition, an antibody to localize a channel candidate must also be tested on hair cells that are missing the targeted protein (knockout animals for example) to avoid false-positive results (hair bundles are notoriously sticky and tend to bind antibodies nonspecifically). However, if the channel turns over regularly, the protein may be more distributed throughout the cell. As a result, the major immunohistochemical signal might not be at the location of functional relevance, similar to CDH23, a tip link protein, which is most highly expressed in the hair cell body and supporting cells [115]. If hcMET channel protein turnover is limited, then there may only be two functional channels per stereocilium, likely resulting in a very low immunohistochemical signal. Finally, if the hcMET channel is part of a complex protein network and membrane bound, antigenicity might be compromised, leading to a false-negative result. Antigen retrieval technology is available to overcome these difficulties, so it is likely an answer will be obtained, but it is also likely that the road to this answer will not be straightforward.
Overexpression of a fluorescently tagged candidate protein is often used for localization. However, these data may be difficult to interpret as both tagging proteins and overexpressing proteins can lead to mislocalization [64, 66]. Additionally, expression of proteins in the bundle does not imply a functional relevance for mechano-electrical transduction. For example, other channels are associated with the stereocilia including ATP-gated channels [47], TRPML3 [35, 51], TRPV4 [51, 62], and TRPC3 and 6 [86] (for review, see [81]). Thus, localization data, though necessary for identifying the hcMET channel, must be interpreted carefully.
Besides the spatial expression pattern, temporal expression of a candidate protein should also match the onset of mechano-electrical transduction, which can be used to infer when hcMET channel protein is present and correctly localized [107]. However, a positive match of this onset and candidate expression can still be misleading (see TRPA1 developmental expression pattern [21]). Also, maturation might involve different channel isoforms being differentially expressed in time and location along the cochlea. This argument was used for the most recent candidates TMC1/2 [53, 76]. Hence, a match between the candidate’s temporal expression pattern and the onset of mechano-electrical transduction does not necessarily validate the candidate, though in the end, the protein must be expressed prior to the channel being functionally mature.
All of the above assume that the investigated candidate is the hcMET channel. However, the candidate could also be a nonchannel protein necessary for correct channel localization or for channel transport and have no functional role for mechanosensitivity, yet provide positive results to each of the localization criteria listed.
Channel interaction partners
Some hcMET channel-interacting partners are predicted, based on the proposed molecular mechanisms involved in the mechano-electrical transduction process and also, as in the case of LHFPL5, by the unexpected finding of changes in hcMET single channel properties when mutated [111].
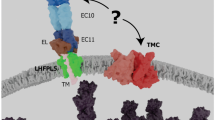
Since the existence of a directly mechanically gated transduction channel was proposed [23, 48, 49], the relay of forces to the channel has been a matter of debate. There is, to date, no conclusive evidence of direct coupling between the tip link and hcMET channel, so four mechanisms of force relay between tip link and channel are possible. First, the PCDH15 portion of the tip link directly or indirectly (possibly involving additional proteins, like LHFPL5 [111]) connects to the hcMET channel, which is tethered to the cytoskeleton [81]. Second, the hcMET channel is directly or indirectly coupled to PCDH15 but does not have a connection to the cytoskeleton. Third, forces are relayed to the channel from the insertion point of PCDH15 via the membrane, with or without intermediate proteins, and the channel is tethered intracellularly to the cytoskeleton. Fourth, the channel is tethered neither to the cytoskeleton nor to PCDH15 but completely regulated by membrane lipid properties (Fig. 3; see also [81]).
Channel tethering. A Model showing protein-protein interactions both intra- and extracellularly. The tip link pulls directly on the channel, possibly through accessory proteins, which is itself attached to the actin cytoskeleton. Red arrows show the direction of force during bundle deflection. B Model showing extracellular tethering only. The tip link pulls directly on the channel, possibly through accessory proteins. C Model showing intracellular tethering only. The tip link pulls on the stereocilia membrane, and the channels are attached to the actin cytoskeleton. D Model showing no tethering. The tip link pulls on the stereocilia membrane only
Keeping these models in mind is important when interpreting results of experiments involving the expression of a hcMET channel candidate in vitro because expected interactions are quite different between models. For example, a candidate interacting with PCDH15 might support a tethering model and the conclusion that the candidate was indeed the hcMET channel. Unfortunately, other channels like HCN1 [88, 89] can interact with PCDH15, and so this conclusion needs to be tempered. Given that a tethered model is not proven, a negative result regarding the candidate interacting with PCDH15 is uninterpretable.
From a functional perspective, if the hcMET channel requires tethering, then its in vitro expression may require interaction partners in order to mimic native mechanosensitive responses. Eventually, the necessity of accessory proteins could be used to identify possible channel interaction partners but in the short term may lead to expression of a channel candidate that cannot be activated mechanically. For example, in the leading hcMET channel candidate, the mammalian TMC proteins neither localize to plasma membrane nor act as an ion channel in any expression system. The C. elegans homologue tmc-1 functions as a Na+-sensitive channel [20] but only possesses about 25 % amino acid homology to the mammalian TMCs [58]. What does this mean? If tethering is not required, hcMET channel in vitro expression and activation may be achievable; however, recent data suggest an additional problem. TREK1 can be gated by alterations of the lipid, showing that a mammalian MET channel can be activated/regulated by its lipid environment [17] (see review [2]). If this were true for the hcMET channel, then the expression system would also need to provide the appropriate lipid environment for channel activation.
Intrinsic channel properties
To validate a candidate as the hcMET channel, one needs to discriminate between intrinsic channel properties and properties that could be due to accessory partner proteins. To test the electrophysiological properties of a candidate channel, one must be able to express the protein in a heterologous system, locate it to the membrane, and then open it. As previously discussed, this is a nontrivial undertaking. For example, whether TMC proteins are mechanosensitive or act as ion channels, with properties similar to the native hcMET channel, remains to be determined due to the difficulties in localization and activation in heterologous systems (see above for potential reasons).
In addition, ion selectivity, single channel conductance, and even force sensing may not necessarily be intrinsic or exclusive properties of the hcMET channel. Accessory proteins interacting with different parts of the channel may impart these properties. For example, the channel’s ion selectivity could be altered by proteins interacting with its vestibule, as is the case in the MEC complex [18] or recently suggested for the hcMET channel [10]. An accessory protein that alters the structure of the channel could impact the pore diameter or the residues facing the pore lumen, which is one possible explanation for the phenotype seen in LHFPL5 knockout [111] or the TMC1 Beethoven mutant mice [10, 76]. Thus, even with properties traditionally considered intrinsic to the channel, care must be taken when interpreting data in order to account for the potential role of extrinsic factors.
Pore properties
A channel’s pore is arguably its most defining part; it allows ions to flow and thus for fundamental channel function. The outer and inner faces of the hcMET channel pore are asymmetric. The outer face minimum diameter is estimated at ~1.25 nm and the inner face at ~0.6 nm [31, 77], a difference considered the result of Ca2+ binding inside the membrane electrical field partially occluding the pore [77]. These estimates are based on pharmacological data that tested the pore size with molecules of different size, charge, and form. A channel candidate could be tested the same way. However, this approach has similar issues to single channel conductance measurements in terms of tonotopic variations and whether the properties are truly intrinsic, again making this measurement less than ideal as a sole criterion for identifying the hcMET channel. Nonetheless, this approach is useful because it uses macroscopic currents that may be easier to elicit in a heterologous system compared to the single channel measurements.
There is a growing body of literature to demonstrate that hcMET channels’ single channel properties vary with frequency location [8, 92] ranging between 145 and 210 pS for OHCs and being approximately 260 pS for IHCs throughout the organ of Corti (in 20 μM external Ca2+) [10–12]. The underlying mechanism for this tonotopic arrangement remains unknown, though splice variants or posttranslational modifications of a single protein could be responsible [96]. The hcMET channel may also be heteromeric with different subunit distributions giving rise to the differences in single channel conductance. If true, it is unlikely that homomeric expression of a single subunit will recapitulate native properties. If the hcMET channel is comprised of different subunits, the loss of one subunit does not necessarily render the channel complex nonfunctional as remaining subunits might compensate (compare to [53, 76, 111]). Given that we do not know the function of any particular subunit, whether it be to impart mechanosensitivity, to regulate trafficking and insertion, or control pore properties, it is complicated to predict outcomes from expression systems. Nonetheless, these experiments are critical, though likely frustrating, for the complete reconstruction of the hair cell MET complex. While heteromeric composition of the hcMET channel increases the difficulty of identifying channel components, it can be used as a tool to probe for channel constituents, similar to the discovery of different subunits in cyclic nucleotide-gated ion channels [14, 32, 52, 108, 114]. Here, the mismatch between native channel properties and those found in expression systems was critical for the identification of accessory subunits [36, 43].
The native hcMET channel has a nonspecific inwardly rectified cation conductance, where rectification is based on the ability of external Ca2+ to limit current flow [22, 74, 77, 91]. The hcMET channel not only exhibits a 10× higher permeability for Ca2+ over Na+ [68, 91] but also shows a mole fraction effect which alters ion selectivity and permeability based on external Ca2+ concentration (compare to [37, 45]). It is generally accepted that Ca2+ alters the permeation properties at all frequencies and regulates both conductance and rectification [33, 90, 93]. Thus, investigating candidate channels for their Ca2+ permeation could be quite telling. Furthermore, as Ca2+ binding sites are well documented in other channel classes (see [19, 34, 40, 103]), genetically modifying the Ca2+ binding site of a candidate and measuring changes in permeation in the native channel would be definitive in identifying the hcMET channel.
Genetics
If there is only one essential channel protein, its loss would have a significant impact on hearing performance and one might expect it to have been discovered by now in mutational screens investigating hearing loss. That no such protein is known suggests two possibilities: (a) loss of the hcMET channel function is a lethal mutation, indicating that the channel is essential for more than just hearing, and (b) there is redundancy; in this case, another protein could compensate for the loss of a given channel component. Tonotopic variations in hcMET channel properties suggest that no single protein comprises the channel. Therefore, redundancy could compensate for a knockout of a channel protein, though some subtle phenotype might be expected [73]. For example, it was recently demonstrated that TMC proteins compensate for one another and only loss of both TMC1 and 2 results in a loss of mechano-electrical transduction and thus hearing [53, 76]. Therefore, hearing loss as a screen for identifying the hcMET channel may not be sufficiently stringent to identify these elusive molecules.
Genetic manipulation allows for a detailed investigation of channel candidates where the most common manipulation is the knockdown or knockout of the protein of interest. A loss of mechano-electrical transduction in these experiments suggests that the gene product is part of the MET cascade and, possibly, though not necessarily, the hcMET channel. For example, the candidate protein may be important for hcMET channel trafficking or force relay. Also, the results of a knockdown (with siRNA for example) and those of a knockout can be quite different. Although siRNA knockdown of TRPA1 in cultured organ of Corti showed a loss of mechanosensitivity [21], the knockout of TRPA1 in mice resulted in no hearing loss [7, 16]. Thus, specificity of the genetic manipulation as well as possible compensation by other proteins need to be carefully considered when drawing conclusions from these data types.
Discussion of current and previous channel candidates
Over the past 30 years, several hcMET channel candidates have arisen. In the following paragraphs, we revisit some of these candidates and discuss why they were candidates, what led to their exclusion, and if revisiting those channels could be worthwhile.
ENaC
Epithelial sodium channels (ENaC) are implicated in mechanosensation of C. elegans and had been discussed as a possible candidate for the mammalian auditory hcMET channel. Interest in ENaCs as a hcMET channel candidate was due to the amiloride sensitivity of the proteins and their role as a mechanotransduction channel in C. elegans [73]. An antibody raised against the purified ENaC channel of the bovine renal papilla [104] was used in an immunogold labeling of hair cells, which showed labeling close to the tip of stereocilia but below the tip link insertion point and thus closer to the shaft links [42]. However, subsequent studies showed that the amiloride sensitivity of the hcMET channel is about 100-fold higher than that of ENaCs, and the single channel conductance was about 10-fold higher for the auditory hcMET channel (~150–250 pS for the hcMET channel and ~10–15 pS for ENaCs). Further electrophysiological examinations also revealed that ENaCs have a higher Na+ selectivity (PNa/PK = 5–100) and a lower Ca2+ selectivity (PCa/PK < 0.4) than the hcMET channel [55]. Although the α-ENaC subunit and another member of the DEG/ENaC family, ASICS, have been shown to be expressed in chick cochlea, the knockdown of those genes in postnatal mice did not reveal a loss of mechanosensitivity [80, 94, 95, 110]. As a result, the ENaC family was abandoned as possible candidates for the mammalian auditory hcMET channel. As discussed earlier, none of the listed experiments completely disqualified ENaC, but the sum of evidence makes it rather unlikely.
TRPA1
The TRP family of ion channels was and remains a focus of interest as some of its members showed expression patterns in the cochlea that matched requirements for the hcMET channel [21, 51, 102]. The most prominent of those TRPs was TRPA1, whose developmental expression pattern matched that of the onset of mechanosensitivity [21]. Also, the localization of TRPA1 to the tips of stereocilia fulfilled one of the criteria for being the hcMET channel. The immunohistochemical data was supported by electrophysiological data from a siRNA knockdown in E18-cultured vestibular hair cells that showed an almost complete loss of hcMET current. Also, the high conductance, Ca2+ permeability, and nonspecificity of the TRP pore made it an interesting candidate, though the outward channel rectification never matched that of the native channel. This combined data made TRPA1 a strong hcMET channel candidate. However, subsequent pharmacological and electrophysiological investigation of TRPA1 showed that it was different from the auditory hcMET channel. TRPA1 was 10–20 times less sensitive to amiloride block than the hcMET channel and ~100 times more sensitive to Gd3+ [71]. TRPA1 was dropped as a candidate after the TRPA1 knockout mouse, missing essential parts of the protein, showed normal hearing and unchanged hcMET currents [7, 59]. The problem with this, though, was the nonconditional nature of the knockout. As discussed before, the hcMET channel complex might be able to compensate for the systemic loss of TRPA1 while it could not in the siRNA knockdown situation. Although TRPA1 was quickly discarded as a hcMET channel candidate, it is still possible that it is part of the channel complex.
TMC1/2
TMC1 and TMC2 are the latest addition to the list of auditory hcMET channel candidates. Loss of TMCs results in profound hearing loss [53, 57]. TMCs 1 and 2 are the genes involved in previously described dominant and recessive nonsyndromic hearing loss, associated with the DFNA36 and DFNB7/B11 loci. How mutating these genes causes hearing loss remained unknown until recent findings suggest that TMC1 and 2 are important for a functional hvMET channel complex [53, 56, 76]. However, it is currently a matter of debate as to whether these proteins constitute (at least in part) the hcMET channel proper or are involved in trafficking/regulating the channel in some other way. While hair cells of single knockout mutants of either TMC1 or 2 retained MET currents, currents were lost in double knockouts [76]. Measurements of single channel conductance of either TMC1 or 2 knockouts were similar to native hcMET channels. Also, in situ hybridization data showed an expression of TMC1/2 in the hair cells of the organ of Corti and utricle. Immunohistochemical data of cultured utricle hair cells that overexpress a TMC2::AcGFP construct showed a labeling not only at the tip of stereocilia but also throughout the rest of the hair bundle [53]. Probably, the strongest data derived from investigating a point mutation of TMC1 (Beethoven) in a TMC2 null background showed an effect on the Ca2+-dependent block of MET current in 1.3 mM external Ca2+. While mutant mice possessing one allele of TMC1 in a TMC2 null background would show a reduction of ~40 % MET current, compared between 50 μM and 1.3 mM external Ca2+, the TMC1-Bth mutant mice would only show 30 % reduction [76]. This difference as well as an apparent reduction in single channel current (from 12.4pA for one copy of TMC1 to 8.4 in the TMC1-Bth mutant, both on a TMC2 null background) led the authors to argue that TMC1 forms part of the pore complex of the hcMET channel [76]. Most recently, an interaction between TMCs and PCDH15 [69] was identified. The interaction of TMCs and PCDH15 seems to be based on a common sequence at the C-terminus of different PCDH15 isoforms. Loss of that common sequence abolishes interaction. Overexpression of TMCs in zebrafish hair cells reduced mechanically evoked Ca2+ inflow into the cells, indicating that alterations of the ratio between TMCs and PCDH15 alter mechanotransduction. Thus, although TMCs have not been convincingly shown to localize at the tip of hair bundles, this possible interaction with PCDH15 infers that they will localize to the right spot. Therefore, localization and timing of expression as well as genetic and functional assays support an argument for TMCs as components of the hcMET channel.
What issues remain to be resolved? TMCs have not been shown to localize to plasma membrane, a requirement for any MET channel. Localization at the stereocilia tips of endogenous proteins remains to be elucidated (but see above regarding molecular interactions). Mammalian TMCs have not been demonstrated to be ion channels or to be mechanically sensitive, clearly important attributes of the hcMET channel (but as described herein, arguments also exist as to why these data may be difficult to generate). Although it has been shown that C. elegans TMC1 forms Na+-sensitive ion channels [20], the experiments have not been repeated for the mammalian TMCs and there is only about 25 % sequence homology between mammalian and C. elegans [58]. Recent data showing that a hcMET current with reversed mechanosensitivity persists in TMC1 and 2 double knockouts further clouds the issue [56]. The pharmacology of this anomalous MET current in TMC1 and 2 knockouts was comparable to that of the control hcMET channel [56], prompting authors to suggest that TMCs are not forming the channel proper but might be involved in trafficking, localization, or functionality of the hcMET channel [56]. They suggest a similar role for the TMCs, as was suggested for LHFPL5 (formerly known as TMHS) [111], and argue against TMCs being integral to the hcMET channel pore. The basic argument being, if MET currents persist in the double knockout, then the knocked out proteins cannot be the channel proper but likely serve some other capacity needed to localize and position the actual hcMET channel to its native location. However, an independent study suggests that the anomalous MET currents were two orders of magnitude less sensitive to a block by the permeable aminoglycoside blockers and that the blocking effect was not released at large negative holding potentials [70], suggesting they were impermeable. Thus, these authors argue that the anomalous current represents a novel channel that is not identical with the normal, tip link-dependent hcMET channel [70], but a new channel unmasked by loss of normal mechanotransduction (either with the knockout animals or also with treatments that break tip links). Hereto, care must be taken when interpreting dose response curves with aminoglycosides as the efficacy is dependent on channel open time which might be very different under conditions where the anomalous currents are produced [90]. Most recent evidence revisits both the pharmacology and the single channel data, concluding that TMCs may provide an accessory subunit to the channel that confers both single channel properties and pharmacological sensitivities but is not in itself the pore-forming subunit [10]. Clarifying the role and molecular distinction of the anomalous current from the native MET current is important for interpreting all existing data. It is clear that additional work is required to determine whether TMCs are pore-forming mechanosensitive ion channels in hair cells or whether they play some other critical role in the mechanotransduction process. It is also clear that regardless of molecular role, the TMCs are an important component of the mechanotransduction machinery.
LHFPL5 (formerly known as TMHS)
One hcMET channel candidate, LHFPL5, is present at the top of stereocilia, exhibits a large reduction in MET currents when knocked out, and shows a decreased single channel conductance and a slowing of activation and adaptation MET current kinetics [111]. Mutation in LHFPL5 also underlies the human deafness locus DFNB67 and the deafness in hurry-scurry mice [67, 98]. The hair bundles of LHFPL5 null mutants showed mild differences to control hair bundles, and the developmental expression pattern of LHFPL5 correlated to the maturation of MET currents [67, 111]. LHFPL5 interacts with PCDH15 (immunoprecipitation), one component of the tip link, suggesting a linker role for LHFPL5 between the force-relaying tip link and the hcMET channel [111]. Coexpression of LHFPL5 and PCDH15 leads to a localization of PCDH15 to the membrane, while PCDH15 stayed cytosolic when expressed without LHFPL5. This also indicated a possible reason for the deafness phenotypes of hurry-scurry mice, as PCDH15 mislocalization would affect tip link formation. The phenotype could be rescued by postnatal expression of the wild-type protein in cultured cochlea explants. The loss of LHFPL5 also led to a significant decrease of MET currents in whole cell voltage-clamp recordings of mechanically stimulated hair cells, as well as a slowing down of activation kinetics [111]. In summary, the effect on PCDH15 localization, the immunoprecipitation data, the effect on MET currents, and the effect on hair bundle morphology led the authors to hypothesize that LHFPL5 is a part of the hcMET channel complex, although not the pore-forming component of the channel itself, perhaps functioning as a linker of PCDH15 and the hcMET channel.
Conclusion
Finding a channel candidate is difficult, and proving that it is the hcMET channel is even more difficult. So much remains unknown about the mechano-electrical transduction process that interpretation of data regarding channel identity often depends on the perspective of the investigator. Additionally, we do not know if there is just one unique hcMET channel protein or multiple heteromeric forms. Criteria for identification typically include the following: localization to the membrane, localization to the tip of stereocilia, developmental expression comparable to the onset of mechano-electrical transduction, specific ion selectivity and permeability, differences in ion selectivity relative to the Ca2+ concentration of the surrounding solutions, ability to express the candidate in a heterologous system while maintaining mechanosensitivity, and possible interaction with tip link proteins or possible accessory proteins like LHFPL5. Each criterion has advantages and disadvantages. So far, no candidate has fulfilled all criteria. As discussed though, a candidate might not fulfill all criteria and still be a part of the channel complex. Additionally, only a few criteria are decisive, while the majority only hint at confirming or disproving a candidate. Identifying the hcMET channel is a decade-old challenge that tests the limits of technology and rigor. A great deal has been learned about hair cell mechanotransduction during this search. Perhaps in this case, the journey is as relevant as the destination.
References
Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M (2006) TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25(11):2368–2376
Anishkin A, Loukin SH, Teng J, Kung C (2014) Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A 111(22):7898–7905
Asai Y, Holt JR, Géléoc GSG (2010) A quantitative analysis of the spatiotemporal pattern of transient receptor potential gene expression in the developing mouse cochlea. J Assoc Res Otolaryngol 11(1):27–37
Atiba-Davies M, Noben-Trauth K (2007) TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta 1772(8):1028–1031
Bahloul A, Michel V, Hardelin J-P, Nouaille S, Hoos S, Houdusse A, England P, Petit C (2010) Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet 19(18):3557–3565
Bass RB, Strop P, Barclay M, Rees DC (2002) Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298(5598):1582–1587
Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124(6):1269–1282
Beurg M, Evans MG, Hackney CM, Fettiplace R (2006) A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26(43):10992–11000
Beurg M, Fettiplace R, Nam J-H, Ricci AJ (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12(5):553–558
Beurg M, Kim KX, Fettiplace R (2014) Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 144(1):55–69
Beurg M, Nam J-H, Chen Q, Fettiplace R (2010) Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104(1):18–34
Beurg M, Nam J-H, Crawford A, Fettiplace R (2008) The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94(7):2639–2653
Bianchi L (2007) Mechanotransduction: touch and feel at the molecular level as modeled in Caenorhabditis elegans. Mol Neurobiol 36(3):254–271
Biel M, Zong X, Ludwig A, Sautter A, Hofmann F (1999) Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135:151–171
Blount P, Sukharev S, Kung C (1997) A mechanosensitive channel protein and its gene in E. coli. Gravit Space Biol Bull 10(2):43–47
Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R (2006) A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26(18):4835–4840
Brohawn SG, Su Z, MacKinnon R (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A 111(9):3614–3619
Brown AL, Liao Z, Goodman MB (2008) MEC-2 and MEC-6 in the Caenorhabditis elegans sensory mechanotransduction complex: auxiliary subunits that enable channel activity. J Gen Physiol 131(6):605–616
Catterall WA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3(8):a003947
Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR (2013) tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 494(7435):95–99
Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin S-Y, Vollrath MA, Amalfitano A, Cheung EL-M, Derfler BH, Duggan A, Géléoc GSG, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang D-S (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432(7018):723–730
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281(5733):675–677
Corey DP, Hudspeth AJ (1983) Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3(5):962–976
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330(6000):55–60
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483(7388):176–181
Denk W, Holt JR, Shepherd GM, Corey DP (1995) Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15(6):1311–1321
Dzeja C, Hagen V, Kaupp UB, Frings S (1999) Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J 18(1):131–144
Eastwood AL, Goodman MB (2012) Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda) 27(5):282–290
Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Göpfert MC (2012) Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci 15(9):1198–1200
Effertz T, Wiek R, Göpfert MC (2011) NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol 21(21):592–597
Farris HE, LeBlanc CL, Goswami J, Ricci AJ (2004) Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558(Pt 3):769–792
Fesenko EE, Kolesnikov SS, Lyubarsky AL (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313(6000):310–313
Fettiplace R (2009) Defining features of the hair cell mechanoelectrical transducer channel. Pflugers Arch 458(6):1115–1123
Frings S, Seifert R, Godde M, Kaupp UB (1995) Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron 15(1):169–179
Gerka-Stuyt J, Au A, Peachey NS, Alagramam KN (2013) Transient receptor potential melastatin 1: a hair cell transduction channel candidate. PLoS One 8(10):e77213
Gerstner A, Zong X, Hofmann F, Biel M (2000) Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. J Neurosci 20(4):1324–1332
Gillespie D, Boda D (2008) The anomalous mole fraction effect in calcium channels: a measure of preferential selectivity. Biophys J 95(6):2658–2672
Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, Hudspeth AJ (2009) The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci U S A 106(50):21347–21352
Goodman MB, Schwarz EM (2003) Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65:429–452
Gradogna A, Babini E, Picollo A, Pusch M (2010) A regulatory calcium-binding site at the subunit interface of CLC-K kidney chloride channels. J Gen Physiol 136(3):311–323
Grati M, Kachar B (2011) Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci U S A 108(28):11476–11481
Hackney CM, Furness DN, Benos DJ, Woodley JF, Barratt J (1992) Putative immunolocalization of the mechanoelectrical transduction channels in mammalian cochlear hair cells. Proc Biol Sci 248(1323):215–221
Haynes LW, Yau KW (1990) Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J Physiol 429:451–481
Helyer R, Cacciabue-Rivolta D, Davies D, Rivolta MN, Kros CJ, Holley MC (2007) A model for mammalian cochlear hair cell differentiation in vitro: effects of retinoic acid on cytoskeletal proteins and potassium conductances. Eur J Neurosci 25(4):957–973
Hess P, Tsien RW (1984) Mechanism of ion permeation through calcium channels. Nature 309(5967):453–456
Houser DS, Finneran JJ (2006) A comparison of underwater hearing sensitivity in bottlenose dolphins (Tursiops truncatus) determined by electrophysiological and behavioral methods. J Acoust Soc Am 120(3):1713–1722
Housley GD, Greenwood D, Ashmore JF (1992) Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci 249(1326):265–273
Howard J, Hudspeth AJ (1987) Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog’s saccular hair cell. Proc Natl Acad Sci U S A 84(9):3064–3068
Howard J, Hudspeth AJ (1988) Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1(3):189–199
Hudspeth AJ (2005) How the ear’s works work: mechanoelectrical transduction and amplification by hair cells. C R Biol 328(2):155–162
Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M (2008) Expression of transient receptor potential vanilloid (TRPV) 1, 2, 3, and 4 in mouse inner ear. Acta Otolaryngol 128(12):1286–1293
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82(3):769–824
Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ (2011) Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 31(34):12241–12250
Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449(7158):87–91
Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82(3):735–767
Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R (2013) The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol 142(5):493–505
Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PSN, Deshmukh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Deininger PL, Hampton LL, Sullivan SL, Battey J, James F, Keats BJB, Wilcox ER, Friedman TB, Griffith AJ (2002) Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30(3):277–284
Kurima K, Yang Y, Sorber K, Griffith AJ (2003) Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics 82(3):300–308
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50(2):277–289
Lehnert BP, Baker AE, Gaudry Q, Chiang A-S, Wilson RI (2013) Distinct roles of TRP channels in auditory transduction and amplification in drosophila. Neuron 77(1):115–128
Levsky JM, Singer RH (2003) Fluorescence in situ hybridization: past, present and future. J Cell Sci 116(Pt 14):2833–2838
Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103(3):525–535
Lin Z, Perez P, Sun Z, Liu J-J, Shin JH, Hyrc KL, Samways D, Egan T, Holley MC, Bao J (2012) Reprogramming of single-cell-derived mesenchymal stem cells into hair cell-like cells. Otol Neurotol 33(9):1648–1655
Lisenbee CS, Karnik SK, Trelease RN (2003) Overexpression and mislocalization of a tail-anchored GFP redefines the identity of peroxisomal ER. Traffic 4(7):491–501
Liu H, Pecka JL, Zhang Q, Soukup GA, Beisel KW, He DZZ (2014) Characterization of transcriptomes of cochlear inner and outer hair cells. J Neurosci 34(33):11085–11095
Lo P-K, Lee JS, Chen H, Reisman D, Berger FG, Sukumar S (2013) Cytoplasmic mislocalization of overexpressed FOXF1 is associated with the malignancy and metastasis of colorectal adenocarcinomas. Exp Mol Pathol 94(1):262–269
Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR (2005) A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102(22):7894–7899
Lumpkin EA, Marquis RE, Hudspeth AJ (1997) The selectivity of the hair cell’s mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci U S A 94(20):10997–11002
Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T (2014) Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci USA
Marcotti W, Corns LF, Desmonds T, Kirkwood NK, Richardson GP, Kros CJ (2014) Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci 34(16):5505–5514
Nagata K, Duggan A, Kumar G, García-Añoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25(16):4052–4061
Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M (2009) The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 28(9):1308–1318
O’Hagan R, Chalfie M, Goodman MB (2005) The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8(1):43–50
Ohmori H (1985) Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359:189–217
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S (2010) Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141(4):704–716
Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron
Pan B, Waguespack J, Schnee ME, Leblanc C, Ricci AJ (2012) Permeation properties of the hair cell mechanotransducer channel provide insight into its molecular structure. J Neurophysiol 107(9):2408–2420
Park S, Lee J-H, Cho H-J, K-y L, Kim MO, Yun B-W, Ryoo Z (2013) tmie is required for gentamicin uptake by the hair cells of mice. Comp Med 63(2):136–142
Pedersen SF, Owsianik G, Nilius B (2005) TRP channels: an overview. Cell Calcium 38(3–4):233–252
Peng B-G, Ahmad S, Chen S, Chen P, Price MP, Lin X (2004) Acid-sensing ion channel 2 contributes a major component to acid-evoked excitatory responses in spiral ganglion neurons and plays a role in noise susceptibility of mice. J Neurosci 24(45):10167–10175
Peng AW, Salles FT, Pan B, Ricci AJ (2011) Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction. Nat Commun 2:523
Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B (2002) Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418(6901):942–948
Perozo E, Rees DC (2003) Structure and mechanism in prokaryotic mechanosensitive channels. Curr Opin Struct Biol 13(4):432–442
Pickles JO, Brix J, Comis SD, Gleich O, Köppl C, Manley GA, Osborne MP (1989) The organization of tip links and stereocilia on hair cells of bird and lizard basilar papillae. Hear Res 41(1):31–41
Pickles JO, Comis SD, Osborne MP (1984) Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15(2):103–112
Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, Raouf R, Gringhuis M, Sexton JE, Abramowitz J, Taylor R, Forge A, Ashmore J, Kirkwood N, Kros CJ, Richardson GP, Freichel M, Flockerzi V, Birnbaumer L, Wood JN (2012) TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2(5):120068
Quintero OA, Moore JE, Unrath WC, Manor U, Salles FT, Grati M, Kachar B, Yengo CM (2010) Intermolecular autophosphorylation regulates myosin IIIa activity and localization in parallel actin bundles. J Biol Chem 285(46):35770–35782
Ramakrishnan NA, Drescher MJ, Barretto RL, Beisel KW, Hatfield JS, Drescher DG (2009) Calcium-dependent binding of HCN1 channel protein to hair cell stereociliary tip link protein protocadherin 15 CD3. J Biol Chem 284(5):3227–3238
Ramakrishnan NA, Drescher MJ, Khan KM, Hatfield JS, Drescher DG (2012) HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actin-binding filamin A or can interact with HCN2. J Biol Chem 287(45):37628–37646
Ricci AJ, Crawford AC, Fettiplace R (2003) Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40(5):983–990
Ricci AJ, Fettiplace R (1998) Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol 506(Pt 1):159–173
Ricci AJ, Gray-Keller M, Fettiplace R (2000) Tonotopic variations of calcium signalling in turtle auditory hair cells. J Physiol 524(Pt 2):423–436
Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R (2005) The transduction channel filter in auditory hair cells. J Neurosci 25(34):7831–7839
Roza C, Puel J-L, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R (2004) Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol 558(Pt 2):659–669
Rüsch A, Hummler E (1999) Mechano-electrical transduction in mice lacking the alpha-subunit of the epithelial sodium channel. Hear Res 131(1–2):170–176
Saleem F, Rowe ICM, Shipston MJ (2009) Characterization of BK channel splice variants using membrane potential dyes. Br J Pharmacol 156(1):143–152
Salles FT, Merritt J, Raymond C, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dosé AC, Kachar B (2009) Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol 11(4):443–450
Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, Friedman TB, Riazuddin S (2006) Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43(8):634–640
Shin J-B, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, Wilmarth PA, Choi D, David LL, Auer M, Barr-Gillespie PG (2013) Molecular architecture of the chick vestibular hair bundle. Nat Neurosci 16(3):365–374
Shin MJ, Lee J-H, Yu DH, Kim HJ, Bae KB, Yuh HS, Kim MO, Hyun B-H, Lee S, Park R, Ryoo ZY (2010) Spatiotemporal expression of tmie in the inner ear of rats during postnatal development. Comp Med 60(4):288–294
Sukharev SI, Blount P, Martinac B, Guy HR, Kung C (1996) MscL: a mechanosensitive channel in Escherichia coli. Soc Gen Physiol Ser 51:133–141
Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M (2009) Expression of transient receptor potential channel melastin (TRPM) 1–8 and TRPA1 (ankyrin) in mouse inner ear. Acta Otolaryngol 129(10):1050–1060
Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA (2014) Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505(7481):56–61
Tousson A, Alley CD, Sorscher EJ, Brinkley BR, Benos DJ (1989) Immunochemical localization of amiloride-sensitive sodium channels in sodium-transporting epithelia. J Cell Sci 93(Pt 2):349–362
van Aken AFJ, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ (2008) TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol 586(Pt 22):5403–5418
Voets T, Talavera K, Owsianik G, Nilius B (2005) Sensing with TRP channels. Nat Chem Biol 1(2):85–92
Waguespack J, Salles FT, Kachar B, Ricci AJ (2007) Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27(50):13890–13902
Wang Z, Jiang Y, Lu L, Huang R, Hou Q, Shi F (2007) Molecular mechanisms of cyclic nucleotide-gated ion channel gating. J Genet Genom 34(6):477–485
Wang L, Zou J, Shen Z, Song E, Yang J (2012) Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II. Hum Mol Genet 21(3):692–710
Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ (2003) ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89(5):2459–2465
Xiong W, Grillet N, Elledge HM, Wagner TFJ, Zhao B, Johnson KR, Kazmierczak P, Müller U (2012) TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151(6):1283–1295
Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN (2013) Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493(7431):221–225
Yang J-J, Su M-C, Chien K-H, Hsin C-H, Li S-Y (2010) Identification of novel variants in the TMIE gene of patients with nonsyndromic hearing loss. Int J Pediatr Otorhinolaryngol 74(5):489–493
Yau KW, Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12:289–327
Zallocchi M, Meehan DT, Delimont D, Rutledge J, Gratton MA, Flannery J, Cosgrove D (2012) Role for a novel Usher protein complex in hair cell synaptic maturation. PLoS One 7(2):e30573
Zhang W, Yan Z, Jan LY, Jan YN (2013) Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci U S A 110(33):13612–13617
Acknowledgments
This work was supported by DAAD (German academic exchange service) to T.E., by the NSF-GRFP to A.L.S., and by RO1 DC003896 from NIDCD to A.J.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Effertz, T., Scharr, A.L. & Ricci, A.J. The how and why of identifying the hair cell mechano-electrical transduction channel. Pflugers Arch - Eur J Physiol 467, 73–84 (2015). https://doi.org/10.1007/s00424-014-1606-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1606-z