Abstract
Background
Wailitst lost is an critical issue and we investigated the long-term effect of insufficient liver functional reserve at liver transplantation evaluation on waitlist outcomes in patients with hepatocellular carcinoma (HCC).
Methods
Clinical data of patients with HCC waitlisted for liver transplantation were retrospectively collected from a single hospital cohort during the period from 2014 to 2021. Parameters of liver reserve, including cirrhosis, Child–Pugh grade, and Model for End-Stage Liver Disease (MELD) scores, were analyzed for patient survival, after adjustment for tumor factors.
Results
Of 292 eligible patients, 94.2% had cirrhosis, 55.8% had Child–Pugh grade B or C, and the median MELD score was 13.2. The median follow-up time was 2.2 years, with a dropout rate of 62.7%. Eighty-nine candidates (30.5%) eventually received liver transplant, including 67 from live donors. The estimated 1-year mortality rate reached 40.6% in 203 patients who remained on the waitlist without receiving a transplant, of whom 143 died. Most deaths were attributed to liver failure (37.1%) and cancer death (35.7%). After we adjusted for tumor confounders, including alpha fetoprotein, primary HCC stage, tumor number at evaluation, and sequential cancer treatment before and while waiting, hazard ratios (HRs) for patient survival were 1.69 (95% confidence interval, 1.18–2.41) for cirrhotic stage B or C, 1.07 (1.04–1.10) for MELD scores, and 1.14 (1.04–1.25) for tumor size at transplant evaluation. Transplantation was a protective disease modifier with adjusted HR 0.22 (0.14–0.33).
Conclusion
Insufficient liver functional reserve poses more risk than expected to liver transplant waitlist outcomes with HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocarcinogenesis results from a permissive microenvironment created by chronic liver disease [1]. Hepatocellular carcinoma (HCC), the most common form of primary liver cancer, typically develops against a background of chronic liver disease, with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol abuse, and nonalcoholic fatty liver disease being the major etiologies [2]. Apart from the cancer field effect, background liver functional reserve, which hosts HCC, largely determines HCC treatment choices and posttreatment recurrence, thus influencing survival [3,4,5]. Clearly, background liver condition plays a critical role in HCC outcomes.
Although HCC therapeutics depends largely on a relatively well-preserved liver to yield long-term survival, treatment options become considerably limited when liver reserve diminishes [3]. One curative treatment for HCC is liver transplantation, which also replaces the sick background liver. In HCC therapeutics, liver transplantation can be performed at an early stage of HCC (USA) [6], intermediate stage of HCC (Barcelona Clinic Liver Cancer classification) [7], and even at an advanced stage with poor liver reserve (Taiwan) [8]. The latter case, in which transplantation recommendation is shifted toward a later stage in the management spectrum of HCC therapeutics, [8] is usually preferred in situations of limited deceased donor livers to maximize overall transplant utility and individual benefit–risk ratio.
Although liver transplantation is a flexible and viable option for HCC cases with adequate liver reserve, it is limited by organ shortage and prolonged or unpredictable waiting times, thereby causing patient dropout due to tumor progression [1]. The effect of poor liver reserve on waitlist and survival outcomes has not been fully explored, although limited tumor progression beyond the Milan criteria did not result in irreversible impairment of survival in patients on the waiting list [9]. In this study, we examined the prognostic effect of background liver function in transplant candidates diagnosed as having HCC.
Methods
Patients
This study cohort included waitlisted adult patients who received a diagnosis of HCC in a university hospital between January 2014 and October 2021. HCC was diagnosed when imaging revealed a new lesion with features of HCC, either by pathology or by typical imaging of background liver in chronic hepatitis or cirrhosis [10]. The management policy of primary or recurrent HCC, including local treatment and surveillance, have been previously described and audited by our multidisciplinary tumor board [10, 11].
Liver transplantation was considered for either of the following conditions: resistance to an adequate local treatment modality against HCC or deterioration of liver function due to liver cirrhosis or its complications [5]. The University of California San Francisco (UCSF) criteria (single tumor < 6.5 cm, maximum of 3 total tumors with none > 4.5 cm, or cumulative tumor size < 8 cm without vascular invasion) were used for determining waitlist eligibility [5, 11, 12].
Demographic parameters
We collected demographic information, namely sex, age, body mass index, underlying liver diseases and comorbidities (presence HBV or HCV), alcohol use, presence of cirrhosis and cirrhotic grades, HCC status (primary TNM stages, tumor number, and largest tumor size at transplant evaluation), diabetes mellitus, hypertension, Model for End-Stage Liver Disease (MELD) scores [13], serum alpha fetoprotein (AFP), and other clinical variables at the time of transplant evaluation for the preclaim review. History of sequential HCC treatment, including resection, radiofrequency ablation (RFA), and transarterial chemoembolization (TACE), was coded before transplant evaluation and after patients were waitlisted.
Primary HCC TNM stages were charted according to the American Joint Committee on Cancer Staging Manual applicable at the time of diagnosis. Cirrhotic stage was graded using the Child–Turcotte–Pugh scoring system [14, 15]. Acute-on-chronic liver failure (ACLF) was defined according to the consensus recommendations of the Asian Pacific Association for the Study of the Liver [16], namely the presence of acute hepatic insult, jaundice (bilirubin ≥ 5 mg/dL), and coagulopathy (international normalized ratio ≥ 1.5) complicated by ascites or encephalopathy or both within 4 weeks, with previously diagnosed or undiagnosed chronic liver disease [16, 17]. Antiviral therapy for HBV (nucleoside/nucleotide inhibitors of HBV polymerase) and HCV (interferon) were administered as per doctors’ prescription. Transplant candidates were excluded from the waiting list when contraindications emerged or experts deemed the prospect of transplant as futile [18]. The transplantation panel retained the final decision for waitlist exclusion [18].
Outcome measurement
Patients were followed up until their death or April 2022, and the cause of death was recorded. The index date was the date of waitlisting. The event date was the date of death, waitlist exclusion, liver transplantation, or last follow-up. The primary outcome was overall survival, and the secondary outcome was survival of patients on the waiting list.
Statistical analysis
Descriptive statistics are expressed as means ± standard deviation, medians (interquartile range [IQR]), or numbers (percentages) where appropriate. The Student t test, χ2 test, Mann–Whitney U test, or Fisher exact test was used, where appropriate, to compare variables. Cumulative survival rates were estimated using the Kaplan–Meier method and compared using the log-rank test. Cox regression modeling was employed for univariable and multivariable analyses. Sensitivity analysis was conducted in the subgroup of patients who did not undergo liver transplantation until the end of the study period. Statistical significance was indicated by a two-sided p value of < 0.05. Analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Demographics
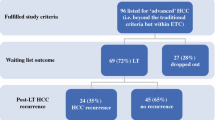
Among 891 adult transplant candidates waitlisted during the study period, 292 candidates diagnosed as having HCC were included in the analysis (Fig. 1). Their clinical features are summarized in Table 1. Most of the candidates were male patients in their late fifties (71.9%) and HBV carriers (60.3%), had cirrhosis (94.2%) and esophageal varices (EV) (63.7%), had primary HCC stage 1 (56.1%), had underwent TACE (73.3%), had low (< 400 ng/mL) AFP level (90.1%), and had a low tumor burden at evaluation. Compared with patients with less advanced cirrhosis (Child A or less (no cirrhosis)), those with advanced cirrhosis (Child B or C) were frequently characterized with alcoholic cirrhotic liver, EV, ascites, and encephalopathy; had higher MELD scores; underwent more TACE and less curative treatments (resection or RFA) in previous cancer treatments; received less cancer treatment while waiting; and had higher rates of waitlist and overall mortality (all p < 0.05).
Among HBV carriers (n = 176), 108 received anti-HBV medications. Among candidates with positive anti-HCV serology (n = 97), 25 received interferon and 11 received direct-acting antiviral agents. Seven patients had malignancies in addition to HCC: two had lung adenocarcinoma; one, breast cancer; one, thyroid cancer; one, gastric B-cell lymphoma; one, buccal cancer; and one, transitional bladder cancer (developed while waiting). Furthermore, 81 patients (27.7%) had no viable HCC as revealed by imaging at evaluation, 27 (27/81, 33.3%) developed HCC recurrence while waiting, and 22 received at least one session of cancer treatment while waiting. Among 21 candidates who were tumor-free at evaluation but eventually received liver transplants, three received local treatment while waiting and all three had recurrent HCC in liver explants. Fourteen of the other 18 explants had recurrent HCC.
Eighty-nine candidates (30.5%) eventually received liver transplants, including 22 deceased donor liver transplants (DDLTs) and 67 live donor liver transplants (LDLTs). Compared with those with Child A cirrhosis, candidates with Child B or C cirrhosis had a higher tendency of receiving LDLT (p = 0.07, Table 1).
Survivals
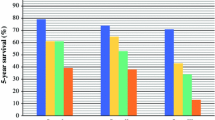
The median survival time among all 292 candidates was 2.2 (IQR 0.7–NA) years, and among waitlisted patients who had not received a transplant, 1.4 (0.4–4.1) years. For all 292 candidates, the 6-month, 1-year, 2-year, and 3-year overall survival rates after placement on the waiting list were 79.5%, 69.9%, 53.0%, and 40.8%, respectively. Both overall and waitlist survival rates in patients with advanced cirrhosis (Child B or C) were inferior to those in the less advanced cirrhosis group (Child A or less) (p < 0.001, Figs. 2A and B).
Survivals in patients with hepatocellular carcinoma waitlisted for liver transplantation. Child B or C vs. Child A or less in overall (A) and waitlist survival (B). Probability of transplant (C) and overall survival (D) in candidates who eventually received liver transplantation. Overall survival in patients with hepatocellular carcinoma who did not undergo liver transplantation (E). HBV (F) and HCV (G) subgroups. IFN, interferon
The median time for liver transplantation after placement on the waiting list was 4.3 (2.8–8.7) months. Furthermore, although the advanced cirrhosis group was more likely to receive a liver transplant (p = 0.08, Fig. 2C), the overall survival between the advanced and less advanced cirrhosis group was not significant (Fig. 2D).
For those who did not undergo liver transplantation, the 6-month, 1-year, 2-year, and 3-year estimated mortality rates were 28.2%, 40.6%, 60.7%, and 71.8%, respectively. The advanced cirrhosis group had a lower overall survival rate than the less advanced group (p < 0.001, Fig. 2E). In the subgroups of HBV carriers and patients with positive anti-HCV serology, the use of antiviral agents did not produce any difference in survival (Fig. 2F and G) or cirrhotic stages (data not shown).
Cause of death
A total of 175 patients died in our study cohort, among whom 32 died after liver transplantation. Seventy-one patients died of cancer: 51 patients on the waitlist (including one patient with rectal cancer and one with lung cancer) and 20 recipients after transplantation. Among the 143 candidates who died while on the waiting list, 53 deaths (53/143, 37.1%) were attributed to liver failure, and most deaths (43/53) were from the advanced cirrhosis group. Other causes included infection (n = 19), bleeding (n = 5), and nonliver etiologies (n = 15).
Dropout due to other reasons
Apart from the 143 candidates who died while waiting, 40 patients dropped out for other reasons. Of these, four patients exceeded the UCSF criteria during waitlist follow-up. One patient developed tuberculosis; one, cryptococcosis; one, total portal vein thrombosis; and one, bladder cancer. The remaining 32 were lost to follow-up. The overall dropout rate in this cohort was 62.7% (183/292).
Univariable and multivariable risk factor analyses of overall survival
Our univariable analysis revealed that HBV carrier state, presence of cirrhosis, advanced cirrhosis (Child B or C), increased MELD scores, ACLF, larger tumor size at evaluation, AFP and levels > 400 ng/mL, and encephalopathy were risk factors associated with poor survival (Table 2). By contrast, RFA before transplant evaluation or after placement on the waiting list and liver transplantation (LDLT or DDLT) were protective factors. In the multivariable analysis with backward selection of variables and p < 0.1, advanced cirrhosis (adjusted hazard ratio [aHR], 1.69; 95% confidence interval [CI], 1.18–2.41), increased MELD scores (aHR, 1.07; 95% CI, 1.04–1.10), and larger tumor size at evaluation (aHR, 1.14; 95% CI, 1.04–1.25) remained risk factors, and only transplantation (aHR, 0.22; 95% CI, 0.14–0.33) remained associated with superior patient survival (Table 2). ALCF and encephalopathy were borderline risk factors after adjustment.
Further sensitivity analysis of overall survival in waitlist patients who did not receive a liver transplant revealed that advanced cirrhosis (aHR, 1.95; 95% CI, 1.31–2.89), ACLF (aHR, 3.91; 95% CI, 1.56–9.84), increased MELD scores (similar effect size to that in Table 2), larger tumor size (similar effect size), and encephalopathy (adjusted HR, 1.68; 95% CI, 1.13–2.48) were associated risk factors (Table 3).
Discussion
The present cohort study yielded four main findings. First, the median survival time after placement on the waiting list was 2.2 years, and overall dropout rate was 62.7%. For patients who did not receive a transplant, the 1-year estimated mortality rate was 40.6%. Nearly 40% of deaths on the waiting list were due to liver failure. Second, although 81 patients (27.7%) had no viable HCC as revealed by imaging at evaluation, one-third developed HCC recurrence while waiting, and 22 received at least one session of cancer treatment. Third, both overall and waitlist survival in the advanced cirrhosis group (Child B or C) were inferior to those in the less advanced group (Child A or less). Finally, advanced cirrhotic stage, increased MELD scores, and larger tumor size at evaluation were adjusted risk factors associated with overall survival.
This study highlighted the role of poor liver functional reserve in waitlisted patients with HCC. Previous studies have similarly reported that cirrhosis and its parallel markers were risk factors for poor prognosis in nontransplant patients with HCC and that severe cirrhosis adversely affects long-term outcomes in HCC patients after hepatectomy or TACE [4, 5, 19,20,21,22,23]. MELD score and Child–Pugh (CP) grade were significant risk factors after other confounders were controlled for. Other noninvasive markers such as the albumin-bilirubin (ALBI) grade have demonstrated prognostic significance and performed as well as CP grade in HCC [24, 25]. The major advantage of the ALBI grade is that the Child A score in the ALBI grade comprises two classes with clearly different prognoses [24]. Compared with CP grade and MELD scores, the survival difference in the ALBI grade was not particularly obvious in patients with HCC receiving TACE who had less liver reserve than surgical patients [22]. Our waitlist cohort spanned a wide range of liver functional reserves; therefore, CP grade was used favorably to triage candidates according to liver reserve.
Our study also revealed that evidence of no tumor recurrence in imaging studies did not guarantee long-term free of recurrence, and most candidates (17/21, 81%) who eventually received transplants had HCC on explants. This temporary “cancer-free” status either may be due to previous noncurative treatments (inadequate treatment) or may be true, with new HCC developing later. Nonetheless, this finding echoes the fact that HCC carcinogenesis may occur in an altered microenvironment despite local tumor treatment. The interplay of various factors initiates the early steps of hepatocyte malignant transformation and HCC development [1]. These factors include genetic predisposition (genomic instability), reciprocal interactions between viral and nonviral risk factors, cellular microenvironment and various immune cells (cancer-associated fibroblast remodeling and immunoediting), and severity of underlying chronic liver disease [1, 2, 26]. Reliable evidence demonstrates that tumor clonal composition changes over time and after exposure to different treatments [2]. Currently, providing adequate liver cancer care without sequential evaluation of tumor response with imaging techniques or analysis of liver functional reserve with biochemical blood tests would be unrealistic [2]. Moreover, different background livers may involve different drivers and prognoses [1, 2, 27,28,29]. To fully implement precision medicine, cancer specialists require new methods to sequentially monitor molecular alterations in cancer [2]. Whatever the case, liver transplantation can be the all-in-one solution to both HCC and microenvironmental deviation.
The present study had a few limitations. We conducted this study in areas with low deceased organ donation and high LDLT, thereby limiting the generalizability of our results. External validation with application in areas with high organ donation rates and high prioritization of patients with HCC in the organ sharing allocation policy is required. Although baseline AFP alone was not a risk to survival after adjustment of other variables, data regarding the changing trend of AFP were not available, as it has only recently been identified as a prognostic factor in waitlist and posttransplant survival [30, 31]. Nevertheless, it still may not greatly modify the effect of the “nontumor” background liver, which we have emphasized.
In conclusion, advanced liver cirrhosis poses a substantial risk to the survival of waitlisted transplant candidates with HCC, even adjusting tumor burdens. Issues of proper management of this subgroup are critical to reducing patient dropout, especially when cancer treatment is inadequate and timely transplant is not available.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ABI:
-
Albumin-Bilirubin index
- ACLF:
-
Acute-on-chronic liver failure;
- AFP:
-
Alpha fetoprotein
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DDLT:
-
Deceased donor liver transplantation
- DM:
-
Diabetes mellitus
- EV:
-
Esophageal varices
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HR:
-
Hazard ratio
- IQR:
-
Interquartile ranges
- LDLT:
-
Living donor liver transplantation
- MELD:
-
Model for End-Stage Liver Disease
- RFA:
-
Radiofrequency ablation
- TACE:
-
Trans-arterial chemoembolization
- USCF:
-
University of California San Francisco
References
Llovet JM, Kelley RK, Villanueva A et al (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7(1):6
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A (2020) Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 17(3):139–152
Granito A, Bolondi L (2017) Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol 18(2):e101–e112
Hoshida Y, Villanueva A, Kobayashi M et al (2008) Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 359(19):1995–2004
Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH (2012) Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol 19(3):826–833
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380(15):1450–1462
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76(3):681–693
Shao YY, Wang SY, Lin SM, Diagnosis Group,; Systemic Therapy Group (2021) Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 120(4):1051–60
Ferrer-Fàbrega J, Sampson-Dávila J, Forner A et al (2021) Limited tumour progression beyond Milan criteria while on the waiting list does not result in unacceptable impairment of survival. J Hepatol 75(5):1154–1163
Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH (2012) Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery 151(5):700–709
Ho CM, Lee CH, Lee MC et al (2021) Survival after treatable hepatocellular carcinoma recurrence in liver recipients: a nationwide cohort analysis. Front Oncol 10:616094
Yao FY, Ferrell L, Bass NM et al (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33(6):1394–1403
Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC (2000) A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31(4):864–871
Child C, Turcotte J (1964) The liver and portal hypertension. In: Child CI (ed) Surgery and Portal Hypertension. W. B. Saunders, Philadelphia, pp 50–58
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60(8):646–649
Sarin SK, Choudhury A, Sharma MK et al (2019) Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL): an update. Hepatol Int 13(4):353–390
Lu CY, Chen CL, Ho CM et al (2020) Dynamic prognostication in transplant candidates with acute-on-chronic liver failure. J Pers Med 10(4):230
Lin HY, Ho CM, Hsieh PY et al (2021) Circuitous path to live donor liver transplantation from the coordinator’s perspective. J Pers Med 11(11):1173
Kim SU, Jung KS, Lee S et al (2014) Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int 34(7):1008–1017
Huang ZY, Liang BY, Xiong M et al (2016) Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function. Surgery 159(2):621–631
Gu J, Liang BY, Zhang EL, Huang ZY (2022) Prognostic nomograms based on the cirrhotic severity scoring for preoperative prediction of long-term outcomes in patients with HBV-related hepatocellular carcinoma and Child-Pugh Grade A liver function. J Oncol 2022:7031674. https://doi.org/10.1155/2022/7031674
Ho SY, Liu PH, Hsu CY et al (2017) Prognostic role of noninvasive liver reserve markers in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. PLoS One 12(7):e0180408
Cucchetti A, Ercolani G, Vivarelli M et al (2006) Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl 12(6):966–971
Johnson PJ, Berhane S, Kagebayashi C et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33(6):550–558
Fagenson AM, Gleeson EM, Pitt HA et al (2020) Albumin-bilirubin score vs Model for End-Stage Liver Disease in predicting post-hepatectomy outcomes. J Am Coll Surg 230(4):637–645
Budhu A, Forgues M, Ye QH et al (2006) Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 10(2):99–111
Tan DJH, Ng CH, Lin SY et al (2022) Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 23(4):521–530
Kelley RK, Greten TF (2021) Hepatocellular carcinoma - origins and outcomes. N Engl J Med 385(3):280–282
Pfister D, Núñez NG, Pinyol R et al (2021) NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592(7854):450–456
Halazun KJ, Tabrizian P, Najjar M et al (2018) Is it time to abandon the Milan criteria?: Results of a bicoastal US collaboration to redefine hepatocellular carcinoma liver transplantation selection policies. Ann Surg 268(4):690–699
Halazun KJ, Rosenblatt RE, Mehta N et al (2021) Dynamic α-fetoprotein response and outcomes after liver transplant for hepatocellular carcinoma. JAMA Surg 156(6):559–567
Acknowledgements
We thank the coordinators (Hui-Ying Lin and Min-Heuy Lin) for their helpful efforts in data collection.
Funding
None.
Author information
Authors and Affiliations
Contributions
HCM, CHY, HCY, and HRH collected the data, HCM drafted the manuscript, and HCM, HRH, and LPH designed the study. HCM, WYM, and HMC conducted data processing, and HCM and LPH performed data analysis. HCM and HRH were the directors responsible for general organization and instruction. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of National Taiwan University Hospital approved this study (NTUH REC: 201701044RIND and 202004053RINB). Because this study retrospectively analyzed data through a chart review, the Institutional Review Board of National Taiwan University Hospital waived the need for informed consent. The research was conducted in accordance with both the Declarations of Helsinki and Istanbul.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ho, CM., Lee, PH., Cheng, HY. et al. Longitudinal analysis of liver transplant candidates for hepatocellular carcinoma in a single center. Langenbecks Arch Surg 409, 143 (2024). https://doi.org/10.1007/s00423-024-03336-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03336-6