Abstract
Background
Despite improved surgical techniques, anastomotic leakage is still a serious complication that can occur after colon cancer resection, resulting in increased morbidity and mortality. The aim of this study was to evaluate the risk factors for anastomotic leakage after colon cancer surgery, provide a theoretical basis for reducing its occurrence, and guide the practice of clinicians.
Methods
A systematic review of PubMed, Ovid, Web of Science and Cochrane Central Register of Controlled Trials databases was conducted by using a combination of subject terms and free words for online searches. The databases were searched from their inception to 31 March 2022, and all cross-sectional, cohort or case‒control studies examining the risk factors for the development of anastomotic fistula after surgery for colon cancer were identified.
Result
A total of 2133 articles were searched for this study, and 16 publications were ultimately included, all of which were cohort studies. A total of 115,462 subjects were included, and a total of 3959 cases of anastomotic leakage occurred postoperatively, with an incidence of 3.4%. The odds ratio (OR) and 95% confidence interval (CI) were used for evaluation. Male sex (OR = 1.37, 95% CI: 1.29–1.46, P < 0.00001), BMI (OR = 1.04, 95% CI: 1.00–1.08, P = 0.03), diabetes (OR = 2.80, 95% CI: 1.81–4.33, P < 0.00001), combined lung disease (OR = 1.28, 95% CI: 1.15–1.42, P < 0.00001), anaesthesia ASA score (OR = 1.35, 95% CI: 1.24–1.46, P < 0.00001), ASA class ≥ III (OR = 1.34, 95% CI: 1.22–1.47, P < 0.00001), emergency surgery (OR = 1.31, 95% CI: 1.11–1.55, P = 0.001), open surgery (OR = 1.94, 95% CI: 1.69–2.24, P < 0.00001) and type of surgical resection (OR = 1.34, 95% CI: 1.12–1.61, P = 0.002) are risk factors for anastomotic leakage after colon cancer surgery. There is still a lack of strong evidence on whether age (OR = 1.00, 95% CI: 0.99–1.01, P = 0.36) and cardiovascular disease (OR = 1.18, 95% CI: 0.94–1.47, P = 0.16) are factors influencing the occurrence of anastomotic leakage after colon cancer surgery.
Conclusions
Male sex, BMI, obesity, coexisting pulmonary disease, anaesthesia ASA score, emergency surgery, open surgery and type of resection were risk factors for anastomotic leakage after colon cancer surgery. The effect of age and cardiovascular disease on postoperative anastomotic leakage in patients with colon cancer needs further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2020 Global Cancer Statistics show that there are an estimated 19.3 million new cancer cases and approximately 10 million cancer deaths worldwide [1]. Research shows [2] that the number of new cases of colorectal cancer in EU countries increased from 261,306 in 1990 to 444,872 in 2019, a growth rate of 70.2%, and the death toll rose from 155,823 to 213,174, an increase of 36.8%. The increased incidence of tumours and the associated increase in patient mortality rates have led to malignant tumours becoming a major public health problem in the world, with colon cancer being one of the most common types of gastrointestinal cancers that cause patient death. Approximately 800,000 people die from colon cancer each year worldwide [3]. Surgery is still the main and most effective method of treatment for colon cancer [4], and colectomy is reported to be one of the most common major abdominal surgeries in the USA [5]. An anastomotic leak (AL) is caused by an intra- and extratubular communication resulting from a defect in the integrity of the bowel wall at the anastomosis. A pelvic abscess in close proximity to the anastomosis should be considered a leak, even without any apparent communication with the colonic lumen, and is one of the most fatal postoperative complications for patients [6]. Postoperative AL in surgical patients prolongs the postoperative recovery time, increases the rate of distant tumour recurrence [7] and affects the overall survival (OS) and disease-free survival (DFS) rate [8-11]. The incidence of anastomotic fistula in postoperative colon cancer patients has been reported to range from 3 ~ 5.6% [5, 12, 13], with approximately 12% of colon cancer patients dying after the development of AL [14] and a significantly lower 5-year postoperative survival rate [12].
Several previous studies [15-21] have reported a range of risk factors associated with AL after colon cancer resection, including smoking, male sex, preoperative serum protein concentration, emergency surgery, BMI, duration of surgery and ASA score. However, these studies are either from a single institution, a small cohort or a large database, and the conclusions of the studies may be influenced by limitations in study design, potential confounding factors, etc. Preoperative gastrointestinal assessment of patient-related risk factors can help reduce the incidence of anastomotic fistula, but to date, there has been no systematic review or meta-analysis involving the risk factors associated with the development of AL after colon cancer resection. To help clinicians better identify patients at risk and provide a valid basis for targeted interventions, we conducted a meta-analysis.
Methods
The review protocol was registered with the International Prospective Register of Systematic Reviews (registration number CRD42022332432). The study was reported in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis [22] and the Meta-Analysis of Observational Studies in Epidemiology guidelines [23].
Information sources and search strategy
PubMed, Ovid, Web of Science and Cochrane Central Register of Controlled Trials databases were searched for literature on the risk factors for AL after colon cancer surgery. The databases were searched from their inception to March 31, 2022, and the language was limited to English. The references in the relevant literature were also traced and supplemented. The search terms included “colonic neoplasms”, “anastomotic leakage” and “risk factor”. The specific search strategy is shown in Tables 1, 2, 3, and 4.
Study selection
Studies that met the following criteria were included:
-
Prospective or retrospective cohort studies, case‒control studies and randomized controlled trials that focused on risk factors or predictors of anastomotic leakage after colorectal cancer resection
-
Studies of patients (18 years or older) who were diagnosed with colon cancer and treated with surgery
-
Studies in which researchers used definite standards to assess the outcomes or risk factors for anastomotic leakage, which could be variant but acceptable
-
Studies with available full text that were published in English
-
Studies in which researchers provided odds ratios (OR) and 95% confidence interval (CI) values of the factors related to anastomotic leaks that could be used to calculate the statistics
Study selection and data extraction
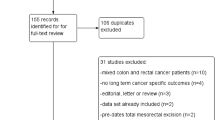
Two members who were trained to perform systematic evidence-based reviews independently searched the literature, used EndNote X9 to manage the literature and assessed the eligibility of the titles and abstracts. Then, the full text of eligible studies was read and checked with respect to the inclusion and exclusion criteria to determine the final included literature. Two members extracted and recorded the data from the included studies using a standardized approach. The extracted data included the first author, publication year, publication country, study design, sample size, data source, study duration, number/incidence of AL and research factors. In the process of literature screening and data extraction, disagreements were resolved through discussion or third-party adjudication, and the PRISMA [24] flowchart (Fig. 1) was used to illustrate the process of screening and inclusion/exclusion of articles.
Risk of bias assessment
The quality of the literature was evaluated independently by two members, and the process and results were recorded. In case of disagreements, consensus was reached through discussion or third-party adjudication. All the literature included in this study were cohort studies, which were evaluated using the Newcastle‒Ottawa Scale (NOS) [25, 26], including study population selection, comparability between groups, exposure evaluation or outcome evaluation. The total score of the table is 9 points, and the higher the score, the better the quality of the literature. Literature with a quality score > 6 points was included in this study.
Data synthesis and analysis
Statistical analysis was performed using Review Manager 5.4 software. The I2 statistic was used to evaluate the heterogeneity of the study results. When I2 ≤ 50%, the homogeneity among the studies was good, and the fixed-effects model was used to merge and analyse the data. Otherwise, a random-effects model was used, the odds ratio (OR) was used to combine statistics, and the corresponding 95% confidence interval (CI) was calculated. When more studies (≥ 10) were included, a funnel plot was used to analyse publication bias. A P value < 0.05 indicated that the difference was statistically significant.
Results
The study flow chart (Fig. 1) outlines the literature screening process for this study and the reasons for inclusion and exclusion. From the titles and abstracts of 2133 articles that were screened, 274 articles were selected for full-text reading, and 16 [5, 16, 18, 20, 21, 26-36] studies with a total of 115,462 patients were ultimately included. There were 3959 cases of postoperative AL, the proportion of AL ranged from 1.29% [30] to 8.7% [33], and the combined incidence of AL was 3.4%. Of the included studies, 1 [20] study was conducted in Japan, 1 [32] study was conducted in Korea, 2 [5, 30] studies were conducted in the USA, and 3 [34-36] studies were conducted in the Netherlands. One [16] study was conducted in Australia. One [28] study was conducted in Iran, 1 [27] study was conducted in France, 5 [18, 21, 26, 31, 33] studies were conducted in Spain, and 1 [29] study was conducted in Germany. The top ten research factors (patient gender, age, BMI, obesity, lung disease, cardiovascular disease, anaesthetic ASA score classification, emergency surgery, open surgery, type of surgical resection) were eventually included in the meta-analysis. They were divided into two categories: patient factors and surgical factors. The quality of the included studies was good (median score: 8, interquartile range (IQR): 7–9). See Supplementary Table 5 for general information and NOS scores. All meta-analyses are presented in Supplementary Table 6 and Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, and 11.
Patient factors
Men
Eleven [5, 18, 20, 21, 28, 30-35] studies reported on the effect of male sex on the occurrence of AL after surgery for colon cancer. Figure 2 shows the meta-analysis of these 11 studies, with the combined effect sizes of the 11 studies showing no significant heterogeneity on the heterogeneity test (P = 0.32, I2 = 13%) and a meta-analysis under a fixed effects model showing that male sex was a risk factor for postoperative AL in patients with colon cancer [OR = 1.37, (95% CI: 1.29–1.46), P < 0.00001].
Age
Six [5, 20, 21, 29, 30, 35] studies reported on the effect of age on the occurrence of AL after surgery for colon cancer. Figure 3 shows a meta-analysis of these six studies, with no significant heterogeneity in the combined effect sizes of the six studies on a test of heterogeneity (P = 0.08, I2 = 48%), and a meta-analysis under a fixed effects model showing that age may not be an influential factor in postoperative AL in patients with colon cancer [OR = 1.00, (95% CI: 0.99–1.01), P = 0.36].
BMI
Five [5, 18, 30, 33, 35] studies reported on the effect of BMI on the occurrence of AL after colon cancer surgery. Figure 4 shows the meta-analysis of these five studies, with the combined effect sizes of the five studies showing no heterogeneity in the test of heterogeneity (P = 0.61, I2 = 0%) and a meta-analysis under a fixed effects model showing that BMI was a risk factor for postoperative AL in patients with colon cancer [OR = 1.04, (95% CI: 1.00–1.08), P = 0.03].
Obesity
Four [27, 31, 33, 36] studies reported on the effect of obesity on the occurrence of AL after colon cancer surgery. Figure 5 shows the meta-analysis of these 4 studies, with the combined effect size showing no heterogeneity in the test of heterogeneity (P = 0.76, I2 = 0%), and the results of the meta-analysis under a fixed effects model showing that obesity leads to a higher incidence of anastomotic fistula [OR = 2.80, (95% CI: 1.81–4.33), P < 0.00001].
Pulmonary disease
Five studies [5, 18, 30, 33, 35] examined the effect of lung disease on the occurrence of AL after colon cancer surgery. Figure 6 shows the meta-analysis of these five studies, with the combined effect size showing no significant heterogeneity in the test of heterogeneity (P = 0.12, I2 = 45%) and the results of the meta-analysis under a fixed effects model showing that lung disease was a risk factor for postoperative AL in patients with colon cancer [OR = 1.28, (95% CI: 1.15–1.42); P < 0.00001].
Cardiovascular disease
Five [5, 30, 31, 33, 35] studies reported on the effect of comorbid cardiovascular disease on the occurrence of AL after surgery for colon cancer. Figure 7 shows the meta-analysis of these five studies, with the combined effect size showing significant heterogeneity in the test for heterogeneity (P = 0.07, I2 = 53%), and a meta-analysis under a random effects model, which showed that cardiovascular disease had no effect on the occurrence of AL after colon cancer surgery [OR = 1.18, (95% CI: 0.94–1.47); P = 0.16].
Anaesthesia ASA score
Seven [5, 20, 21, 29, 33-35] studies reported on the effect of anaesthesia ASA scores on the occurrence of AL after colon cancer surgery. Figure 8 shows the meta-analysis of these seven studies, with the combined effect size showing no significant heterogeneity in the test of heterogeneity (P = 0.14, I2 = 38%), and the results of the meta-analysis under a fixed effects model, which showed that anaesthesia ASA score was a risk factor for postoperative AL in patients with colon cancer [OR = 1.35, (95% CI:1.24–1.46); P < 0.00001]; four of these [5, 21, 33, 34] reported an ASA score ≥ III, and the combined effect size showed no significant heterogeneity in the test of heterogeneity (P = 0.64, I2 = 0%), and a meta-analysis under a fixed-effects model showed that anaesthesia ASA score ≥ III was a risk factor for postoperative AL in patients with colon cancer [OR = 1.34, (95% CI:1.22–1.47); P < 0.00001] (Fig. 8).
Surgical factors
Emergency surgery
Five studies [21, 31-34] reported on the effect of emergency surgery on the occurrence of AL after surgery for colon cancer. Figure 9 shows the meta-analysis of these five studies, with the combined effect size showing no heterogeneity in the heterogeneity test (P = 0.53, I2 = 0%), and the results of the meta-analysis under a fixed effects model showing that emergency surgery was a risk factor for the occurrence of postoperative AL in patients with colon cancer [OR = 1.31, (95% CI: 1.11–1.55); P = 0.001].
Open surgery
Three studies [5, 20, 21] reported the effect of open surgery on the occurrence of postoperative AL in colon cancer. Figure 10 shows the meta-analysis of these three studies, with the combined effect size showing no heterogeneity (P = 0.89, I2 = 0%), and a meta-analysis under a fixed effects model, which showed that open surgery was a risk factor for postoperative AL in patients with colon cancer [OR = 1.94, (95% CI: 1.69–2.24), P < 0.00001].
Type of resection
Five studies [5, 26, 30, 34, 35] reported on the effect of resection type on the occurrence of AL after surgery for colon cancer. Figure 11 shows the meta-analysis of these five studies, with the combined effect size showing significant heterogeneity in the test for heterogeneity (P < 0.00001, I2 = 84%), and a random effects model combined effect [OR = 1.34, (95% CI: 1.12–1.61); P = 0.002] was used to find the reasons for the large heterogeneity and to exclude each for sensitivity analysis. There was no significant change in the heterogeneity test (I2 = 77–88%), indicating that the findings were robust, and there was a significantly high association between resection type and AL (P = 0.002), suggesting that resection type is a risk factor for postoperative AL in patients with colon cancer.
Publication bias
Publication bias was analysed for possible risk factors, and no significant publication bias was found. Figure 12 is a funnel plot for male publication bias testing.
Results
The funnel plot was symmetrical on both sides, suggesting no significant publication bias.
Discussion
Cancer is a major public health problem worldwide, and colon cancer is one of the most common gastrointestinal malignancies, with an increasing incidence in recent years [1, 37]. In recent years, with improvements in clinical practice, surgical techniques have improved significantly, but AL is still a serious and unavoidable complication for postoperative patients. Identifying which factors are associated with the development of postoperative AL is essential to better identify patients at high risk of AL, as well as to predict the occurrence of AL and develop strategies to reduce its occurrence in clinical practice. In this study, 16 studies were included for systematic review and meta-analysis to identify the risk factors for postoperative AL in patients with colon cancer.
The analysis of this study found that male patients had a higher risk of postoperative AL, which may be related to men having a triangular and narrow pelvis [10, 38]. A clearly visible operative field is necessary, but intraoperative separation is difficult; thus, the incidence of anastomotic leakage is relatively high. It may also be related to androgen-related differences in intestinal microcirculation, as found in an animal study [39]. In male rats, the onset of collagen synthesis at the anastomotic stoma is delayed, and the levels of total and soluble collagen in the anastomotic stoma are lower than those in female rats. Collagen metabolism in colonic anastomotic stoma is poor in the early stage, and wound healing is slower. It may also be related to the expression of inflammatory substances during wound healing. It was found [40] that compared with men, women had lower expressions of inflammatory and proapoptotic markers, less Paneth cell necrosis and thus greater resistance to ischaemia‒reperfusion-induced intestinal injury. Studies have shown [41] that Paneth cells play an important role in regulating the balance of intestinal flora, and the occurrence of AL is related to general intestinal flatulence, abdominal pressure and increased tension at the anastomotic stoma after intestinal flora imbalance.
BMI, as one of the important indicators of physical fitness, reflects to a certain extent the impact of body size on postoperative complications. In clinical practice, BMI is mostly used to describe a healthy weight and obesity in terms of height and weight; obesity is generally defined as a BMI greater than 30 kg/m2 [42]. In this meta-analysis, BMI and obesity were considered risk factors for the development of AL after colon cancer resection. This finding may be related to the excessive subcutaneous and omental fat accumulation in obese patients, the relative narrowing of the pelvic cavity and poor exposure of the operative field, which increases the difficulty of pelvic surgery and affects the surgical outcome [43] or the impaired metabolic capacity of the body due to dietary habits, thereby impairing postoperative tissue repair to some extent. Furthermore, it may also be related to the fact that obesity may lead to low-grade chronic inflammation, resulting in increased production of pro-inflammatory adipocytokines, downregulation of cytokines and downregulation of anti-inflammatory adipocytokines and cytokines [42].
Based on our results, there was an association between pulmonary disease and AL in patients who underwent colon cancer resection. This may be related to the fact that patients with pulmonary comorbidities are in poorer general condition and have lower pulmonary compensatory capacity, which may predispose them to pulmonary infections and respiratory failure, further reducing pulmonary oxygenation capacity and leading to insufficient oxygenation of the anastomosis; patients with pulmonary comorbidities may also experience frequent and increased coughing after surgery due to pulmonary infections, increasing the tension at the anastomosis and thus increasing the risk of anastomotic fistula. As a result, preoperative lung function assessment should be performed in elective patients, especially in patients with severe combined lung disease. Comprehensive preoperative lung function tests (chest X-ray, lung CT) should also be performed, measures to improve lung function (such as lung function exercises, continuous oxygenation) should be actively implemented, and optimal postoperative care should be given to patients with these conditions to reduce the incidence of anastomotic fistula.
The ASA score classification is a classification by the American Society of Anaesthesiologists based on the patient’s physical condition prior to anaesthesia and surgical risk, classifying patients into six grades. Grade 1 is healthy with normal function of all organs, and grade 6 is the most dangerous with confirmed brain death. Based on the results of this meta-analysis, the anaesthetic ASA score was identified as a risk factor for AL after colon cancer surgery, and an ASA classification ≥ grade III was strongly correlated with the occurrence of AL in patients after surgery. This may be related to the fact that the higher the ASA grade, the poorer the patient’s cardiac, pulmonary, hepatic and renal function, the weaker the tissue healing ability and the prolonged healing time of the anastomosis, which is more consistent with several studies [5, 29, 44] that concluded that the anaesthesia ASA score is significantly correlated with wound healing, prognosis and the occurrence of complications.
Emergency surgery is one of the risk factors for AL after colon cancer surgery. This may be related to the poor general condition of the emergency patient, the fact that the tumour may progress to an intermediate or advanced stage or the combination of intestinal obstruction and intestinal perforation. The intestinal contents that cause the obstruction may interfere with the operation, increase the risks of infection and dilatation and oedema of the obstructed proximal intestine and cause poor blood flow, which may lead to poor healing of the anastomosis. Studies have reported that the incidence of AL was 12.5% in patients who underwent emergency surgery and 3.9% in patients who underwent elective surgery [45], with a higher risk of AL after emergency surgery than elective surgery postoperatively [14]. Another study reported that emergency surgery is not only an independent risk factor for postoperative AL but also an independent risk factor for postoperative fistula-related death [10].
The results of this meta-analysis indicate that open surgery will increase the risk of AL after resection of colon cancer. Studies have shown that patients undergoing open surgery or conversion to open surgery are almost twice as likely to have AL, more than twice as likely to die and have a 1.5-fold higher readmission rate than patients undergoing laparoscopic surgery [5]. In colon cancer surgery, laparoscopic resection was significantly less expensive than open resection, with a 46% reduction in the mortality rate, a 41% reduction in the incidence of serious complications, a 25% reduction in the length of hospital stay and a 65% reduction in the length of ICU stay, a significant difference in the incidence of surgical site infection between laparoscopic and open surgery and an increase in survival rates for both compared to open surgery [49]. This may be related to the fact that compared with laparoscopic surgery, open surgery is associated with larger incisions, more severe intraoperative injuries, more bleeding, more severe postoperative pain, slower recovery, longer hospital stay and worse prognosis; it may also be related to the fact that when patients have contraindications to laparoscopic surgery (e.g. extensive tumour infiltration of surrounding tissues, inability to tolerate CO2 pneumoperitoneum), open surgery is the only option, and these patients often have other underlying conditions. As these patients often have other underlying conditions, the surgery becomes more complex, and the risk of postoperative anastomotic leak increases [46].
Our analyses suggest that the type of surgical resection was a risk factor for the occurrence of AL after colon cancer surgery. Studies have shown that the rate of AL after right hemicolectomy is surprisingly high (6.4–7.5%) when compared with that for left hemicolectomy (anastomotic leak rate 1.9–6.5%) [29], which differs from the findings of two other studies [30, 34]. Another study showed that the incidence of anastomotic fistula was higher after total colectomy than after left colectomy and right colectomy [26], which may be related to differences in centres, differences in inclusion factors and classification of variables and some bias in the results of the individual studies. It was not possible to determine which specific type of resection had a higher risk of anastomotic fistula in this study, possibly because of the limited amount of literature included in this study and because most studies did not provide sufficient data to allow a combined analysis of resection types or a subgroup analysis to determine which resection type had a higher risk of anastomotic fistula occurrence. Therefore, more multicentre studies with larger samples may be needed in the future to determine the relationship between specific resection types and anastomotic fistula risk.
In addition, age and comorbid cardiovascular disease were not found to be risk factors for AL in this meta-analysis. In addition to the small sample of literature included in this study, the large variation in the sample size of patients between studies, human factors and investigators possibly using different criteria for the diagnosis of anastomotic fistula, such as increased fluid drainage from the drainage tube, drainage fluid containing faecal sludge-like material or pus drainage, some literature may not have explicitly provided diagnostic criteria for anastomotic fistula, so some of the literature included in the meta-analysis may have been biased in the selection of the study population. It may also be related to the fact that the mean age of the patients included the meta-analyses was older than 60 years and that reports of cardiovascular disease are concentrated in European and American countries and are not representative of the relationship between all age groups or all regional populations and AL. Therefore, more multicentre large sample size studies may be needed in the future to demonstrate the association between age and comorbid cardiovascular disease and AL after colon cancer surgery.
Limitations
Although some of the factors presented in the study are known, a systematic literature review approach was employed in this study, incorporating meta-analyses and the use of GRADE, showing a high degree of certainty for many analyses. There are some limitations in this study. In this study, we identified several risk factors for postoperative anastomotic leak in patients, but some have not yet been identified due to the small sample of studies we included, and more evidence cannot be synthesized. The original studies that included people were mainly retrospective or prospective cohort studies, and nonrandomized controlled studies may inevitably have selection bias, implementation bias or outcome measurement bias. Difficulties in data extraction due to the crude nature of the data included in some of the original studies, the different reference ranges chosen for the data and possible measurement errors prevented subgroup analysis of patient age, ASA, type of resection and BMI to compare the effect of different data ranges on the occurrence of postoperative anastomotic fistula in patients, which may have led to some bias in the conclusions of the studies. Although an extensive literature search was carried out in the early stage of this study, there may still be some studies that were not included. Although quality control measures have been implemented, the study sample was still small, which may have affected the reliability of the research results to a certain extent.
Conclusions
In this study, a systematic review and meta-analysis identified male sex, BMI, obesity, comorbid lung disease, anaesthetic ASA score, emergency surgery, open surgery and type of resection as risk factors for AL after colon cancer resection. The identification of such factors can be used to create a more personalized approach to the treatment of patients with colon cancer. Targeted measures such as pulmonary function exercises and aggressive treatment of the underlying fistula indirectly reduce the patient’s risk of anastomotic fistula. However, further research is needed to determine whether age and cardiovascular disease are risk factors for postoperative AL in patients with colon cancer.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA Cancer J Clin 71(3):209–249
Sharma R (2022) A comparative examination of colorectal cancer burden in European Union, 1990–2019: estimates from Global Burden of Disease 2019 study[J]. Int J Clin Oncol 27(8):1309–1320
Siegel RL, Miller KD, Goding Sauer A et al (2020) Colorectal cancer statistics, 2020[J]. CA Cancer J Clin 70(3):145–164
Taieb J (2019) How best to treat older patients with metastatic colorectal cancer?[J]. Lancet Gastroenterol Hepatol 4(5):331–333
Mungo B, Papageorge CM, Stem M et al (2017) The impact of operative approach on postoperative complications following colectomy for colon cancer [J]. World J Surg 41(8):2143–2152
Spinelli A, Anania G, Arezzo A et al (2020) Italian multi-society modified Delphi consensus on the definition and management of AL in colorectal surgery[J]. Updates Surg 72(3):781–792
Krarup PM, Nordholm-Carstensen A, Jorgensen LN et al (2014) Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study[J]. Ann Surg 259(5):930–938
Karim A, Cubas V, Zaman S et al (2020) Anastomotic leak and cancer-specific outcomes after curative rectal cancer surgery: a systematic review and meta-analysis[J]. Tech Coloproctol 24(6):513–525
Kryzauskas M, Bausys A, Degutyte AE et al (2020) Risk factors for AL and its impact on long-term survival in left-sided colorectal cancer surgery[J]. World J Surg Oncol 18(1):205
Zarnescu EC, Zarnescu NO, Costea R (2021) Updates of risk factors for al after colorectal surgery [J]. Diagnostics. 11(12):2382
Lawler J, Choynowski M, Bailey K et al (2020) Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery[J]. BJS Open 4(5):737–747
Stormark K, Krarup PM, Sjövall A et al (2020) Anastomotic leak after surgery for colon cancer and effect on long-term survival. Colorectal Dis 22(9):1108–1118
Arron MNN, Greijdanus NG, Bastiaans S et al (2022) Long-term oncological outcomes after colorectal anastomotic leakage: a retrospective dutch population-based study. Ann Surg 276(5):882–889
Arron MNN, Greijdanus NG, Ten Broek RPG et al (2021) Trends in risk factors of anastomotic leakage after colorectal cancer surgery (2011–2019): a Dutch population-based study. Colorectal Dis 23(12):3251–3261
Kishor S, Singh H, Ghosh A et al (2021) Predictors of leak after colorectal anastomoses: a case series analysis [J]. Indian J Surg 83(1):211–216
Badiani S, Diab J, Woodford E et al (2022) Impact of preoperative smoking on patients undergoing right hemicolectomies for colon cancer [J]. Langenbeck's archives of surgery 407(5):2001–2009
Arron MNN, Greijdanus NG, ten Broek RPG et al (2021) Trends in risk factors of AL after colorectal cancer surgery (2011–2019): a Dutch population-based study[J]. Colorectal Dis 23(12):3251–3261
Frasson M, Granero-Castro P, Ramos Rodriguez JL et al (2016) Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: results from a prospective, multicentric study of 1102 patients[J]. Int J Colorectal Dis 31(1):105–114
Ng CW, Prabhakaran S, Chakraborty J et al (2020) 17 Rate of anastomotic leak following right hemicolectomy by general surgical trainees[J]. Int J Colorectal Dis 35(12):2339–2346
Wada H, Tominaga T, Nonaka T et al (2022) Charlson comorbidity index predicts AL in patients with resected right-sided colon cancer [J]. Surg Today 52(5):804–811
Frasson M, Nick J, Battersby MD, Hervás D (2020) Predictors for anastomotic leak, postoperative complications, and mortality after right colectomy for cancer: results from an international snapshot audit [J]. Dis Colon Rectum 63:606–618
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews[J]. Syst Rev 10(1):89
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group[J]. Jama 283(15):2008–2012
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non randomized studies in meta-analyses [J]. Eur J Epidemiol 25(9):603–605
Ellul P, Acquaviva E, Peyre H et al (2022) Parental autoimmune and autoinflammatory disorders as multiple risk factors for common neurodevelopmental disorders in offspring: a systematic review and meta-analysis[J]. Transl Psychiatry 12(1):112
Marinello FG, Baguena G, Lucas E et al (2016) AL after colon cancer resection: does the individual surgeon matter?[J]. Colorectal Dis 18(6):562–569
Voron T, Bruzzi M, Ragot E et al (2019) Anastomotic location predicts AL after elective colonic resection for cancer [J]. J Gastrointest Surg 23(2):339–347
Layeg H, Meshki VK, Karami MY et al (2022) The Association of Vitamin D3 With Early AL in Patients Undergoing Hemicolectomy Surgery [J]. Surg Innov 29(6):742–746
Jurowieh C, Lichthardt S, Matthes N et al (2019) Effects of anastomotic technique on early postoperative outcome in open right-sided hemicolectomy[J]. Bjs Open 3(2):203–209
Masoomi H, Buchberg B, Dang P et al (2011) Outcomes of right vs. left colectomy for colon cancer[J]. J Gastrointest Surg 15(11):2023–2028
Pellino G, Frasson M, Garcia-Granero A et al (2018) Predictors of complications and mortality following left colectomy with primary stapled anastomosis for cancer: results of a multicentric study with 1111 patients[J]. Colorectal Dis 20(11):986–995
Kwak HD, Kim S-H, Kang DW et al (2017) Risk factors and oncologic outcomes of anastomosis leakage after laparoscopic right colectomy [J]. Surg Laparosc Endosc Percutaneous Tech 27(6):440–444
Frasson M, Ramos JL, Flor B et al (2015) Risk factors for anastomotic leak after colon resection for cancer multivariate analysis and nomogram from a multicentric, prospective, national studywith 3193 patients [J]. Colorectal Dis 3:7
Bakker IS, Grossmann I, Henneman D et al (2014) Risk factors for AL and leak-related mortality after colonic cancer surgery in a nationwide audit[J]. Br J Surg 101(4):424–432 (discussion 432)
Verduin WM, Warps A-LK, van den Helder R et al (2021) Visceral fat and AL after colon cancer resection [J]. Dis Colon Rectum 64(2):163–170
Cakir H, Heus C, Verduin WM et al (2015) Visceral obesity, body mass index and risk of complications after colon cancer resection: a retrospective cohort study[J]. Surgery 157(5):909–915
Siegel RL, Miller KD, Fuchs HE et al (2022) Cancer statistics, 2022[J]. CA Cancer J Clin 72(1):7-33.A
Arron MNN, Greijdanus NG, Ten Broek RPG et al (2021) Trends in risk factors of AL after colorectal cancer surgery (2011–2019): a Dutch population-based study[J]. Colorectal Dis 23(12):3251–3261
Kjaer M, Kristjánsdóttir H, Andersen L et al (2018) The effect of gender on early colonic anastomotic wound healing[J]. Int J Colorectal Dis 33(9):1269–1276
Damanakis AI, Toader J, Wahler I et al (2022) Influence of patient sex on outcomes after pancreatic surgery: multicentre study[J]. Br J Surg 109(8):746–753
Nikolenko VN, Oganesyan MV, Sankova MV et al (2021) Paneth cells: maintaining dynamic microbiome-host homeostasis, protecting against inflammation and cancer[J]. Bioessays 43(3):e2000180
Simillis C, Taylor B, Ahmad A et al (2022) A systematic review and meta-analysis assessing the impact of body mass index on long-term survival outcomes after surgery for colorectal cancer[J]. Eur J Cancer 172:237–251
Polednak AP (2006) Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: a population-based study[J]. Cancer Detect Prev 30(5):466–472
Voeten DM, van der Werf LR, Wijnhoven BPL et al (2020) Failure to cure in patients undergoing surgery for esophageal carcinoma: hospital of surgery influences prospects for cure: a nation-wide cohort study [J]. Ann Surg 272(5):744–750
Wako G, Teshome H, Abebe E (2019) Colorectal anastomosis leak: rate, risk factors, and outcome in a tertiary teaching hospital, Addis Ababa Ethiopia, a Five Year Retrospective Study[J]. Ethiop J Health Sci 29(6):767–774
Zhang Z, Wang D, Xu C et al (2019) Laparoscopy adjuvant total colorectal resection for the treatment of familial adenomatous polyposis (FAP)[J]. Clin Transl Oncol 21(6):753–759
Govaert JA, Fiocco M, van Dijk WA et al (2017) Multicenter stratified comparison of hospital costs between laparoscopic and open colorectal cancer resections: influence of tumor location and operative risk [J]. Ann Surg 266(6):1021–1028
Reeves N, Cuff S, Boyce K et al (2021) Diagnosis of colorectal and emergency surgical site infections in the era of enhanced recovery: an all-Wales prospective study[J]. Colorectal Dis 23(5):1239–1247
Pucher PH, Mackenzie H, Tucker V et al (2021) A national propensity score-matched analysis of emergency laparoscopic versus open abdominal surgery[J]. Br J Surg 108(8):934–940
Funding
The work was supported by Chengdu Medical College and Mianyang Central Hospital.
Author information
Authors and Affiliations
Contributions
Juan He is the first author, Ji-Hong Tang is co-first author, and Mei He is the corresponding author. Juan He and Mei He conceived and designed the study; Juan He and Ji-Hong Tang designed the draft search strategy; Juan He, Ji-Hong Tang and Xian-Hua Wang extracted, analysed and interpreted the data; Juan He and Mei He drafted the initial and final plan; Mei He revised the manuscript. All authors approved the final version of the scheme.
Corresponding author
Ethics declarations
Consent for publication
Not required.
Provenance and peer review
Not commissioned; externally peer-reviewed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, J., He, M., Tang, JH. et al. Anastomotic leak risk factors following colon cancer resection: a systematic review and meta-analysis. Langenbecks Arch Surg 408, 252 (2023). https://doi.org/10.1007/s00423-023-02989-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02989-z