Abstract
Introduction
Sleeve gastrectomy (SG) is currently the most commonly performed bariatric procedure worldwide. The aim of the present study was to evaluate the long-term efficacy of SG as a stand-alone bariatric procedure.
Methods
A single-center retrospective analysis of 104 patients who underwent SG as a stand-alone bariatric procedure between January 2005 and December 2009. Weight loss, weight regain, remission or improvement of comorbidities and the new onset of comorbidities were the main outcomes of the study.
Results
The percent excess body weight loss (%EBWL), percent excess body mass weight (BMI) loss (%EBMIL), and percent total body weight loss (%TBWL) were 59 ± 25, 69 ± 29, and 29 ± 12, respectively, after a mean follow-up of 13.4 years. At the last follow-up, nearly two thirds of patients (67.3%) had an %EBWL greater than 50. The percentage of patients who experienced significant weight regain ranged from 47 to 64%, depending on the definition used for weight regain. The rate of improvement or remission of hypertension, type 2 diabetes, dyslipidemia, obstructive sleep apnea, and degenerative joint disease at a mean follow-up of 13.4 years was 40%, 94.7%, 70%, 100%, and 42.9%, respectively. The new onset of gastroesophageal reflux disease (GERD) symptoms in the same period was 43%.
Conclusion
Our data supports that SG results in long-lasting weight loss in the majority of patients and acceptable rates of remission or improvement of comorbidities. Weight regain and GERD may be issues of particular concern during long-term follow-up after SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleeve gastrectomy (SG) was initially introduced as a component of biliopancreatic diversion with duodenal switch (BPD/DS) [1]. Later, SG was performed as a first step in a two-step approach to BPD/DS or Roux-en-y gastric bypass (RYGB) for high-risk patients with severe obesity (body mass index (BMI) > 50 kg/m2) [2, 3]. Preliminary efficacy and safety results, combined with technical simplicity, made SG acceptable as a stand-alone bariatric procedure. In the last 20 years, SG has gained widespread popularity as a stand-alone procedure and is currently the most commonly performed bariatric procedure worldwide [4].
Despite its widespread use, there is little data on the efficacy of SG after the tenth postoperative year. Two issues of particular concern during long-term follow-up after SG are the occurrence of weight regain and gastroesophageal reflux disease (GERD) [5]. Weight regain limits the potential beneficial effects of SG [6], while GERD affects patients’ quality of life and social functioning and may even cause Barrett’s esophagus and/or esophageal cancer [7]. Both of them increase the rate of revision surgery and may have significant economic burdens.
The aim of the present study was to evaluate the long-term efficacy of SG as a stand-alone bariatric procedure in terms of weight loss, weight regain, remission or improvement of comorbidities, and new onset of comorbidities in a single center series of patients with at least 11 years of follow-up.
Materials and methods
Study design and patients
Between January 2005 and December 2009, 181 consecutive adult patients less than 65 years old with morbid obesity underwent SG as a stand-alone bariatric procedure in the morbid obesity unit of our institution. All patients met the eligibility criteria for bariatric surgery established by the National Institutes of Health (NIH) in 1992 and had not undergone bariatric surgery in the past. Based on the results of the preoperative workup at the time of surgery, all patients had a BMI of less than 52 kg/m2, were not “sweet eaters” and did not suffer from hiatal hernia, severe GERD, or Barrett’s esophagus based on the results of the preoperative workup at the time of surgery. Sweet eating was defined as the consumption at least 3 times a week of more than 300 kcal of sweet foods or beverages. Patients found intraoperatively to have a hiatal hernia were not excluded. They underwent hiatal hernia repair and then SG. Preoperatively, all patients underwent a standard workup (history, physical examination, and laboratory evaluation, including esophagogastroduodenoscopy—EGD). Postoperatively, patients attended the outpatient facilities of our institution at 1, 3, 6, and 12 months after SG and annually thereafter. A high proportion of patients were lost to follow-up after the first postoperative years.
In order to evaluate the long-term efficacy of SG, we conducted a retrospective observational study. The study was approved by our institution’s research ethics committee. From the registry of our unit, we obtained the contact details of 181 eligible patients. One patient died 1 month after surgery for an unspecified reason and was not included in the study. Patients found through the available contact details were invited to participate, and informed consent was requested from them. To be eligible for the study, patients must not (1) have undergone any other surgery that alters the anatomy of the gastrointestinal tract after SG (including conversion, re-sleeve, or endoscopic sleeve gastroplasty); (2) suffer from uncontrolled endocrine disorder, active malignancy, or other disease known to affect appetite and/or intestinal function; and (3) be pregnant. Finally, 104 patients entered the study. The flow chart of the patient selection process is shown in Fig. 1.
The 104 patients included in the study underwent a structured phone interview process regarding postoperative weight loss outcomes and progression of comorbidities. Additional data from the postoperative follow-up of patients at 1, 5, and 10 years was extracted from the registry of our unit. We chose these time points as representative of short-, mid-, and long-term follow-up. The main outcomes of the present study were (a) weight loss, (b) weight regain, (c) remission or improvement of comorbidities, and (d) the new onset of comorbidities (including GERD symptoms such as heartburn and regurgitation).
Ideal body weight (IBW) was calculated by the Peterson equation as follows [8]:
A target BMI of 22 was used for all calculations.
Excess body weight (EBW) was calculated as the difference between the preoperative total body weight (TBW) and the IBW, while excess BMI (EBMI) was calculated as follows:
Weight loss after SG was expressed as follows:
-
(1)
Percentage of excess body weight loss (% EBWL), calculated as follows:
$$\%\;\mathrm{EBWL}=100\times\frac{\mathrm{Preoperative}\;\mathrm{EBW}-\mathrm{EBW}\;\mathrm{at}\;\mathrm{follow}-\mathrm{up}}{\mathrm{Preoperative}\;\mathrm{EBW}}$$ -
(2)
Percentage of excess BMI loss (% EBMIL), calculated as follows:
$$\%\;\mathrm{EBMIL}=100\times\frac{\mathrm{Preoperative}\;\mathrm{BMI}-\mathrm{BMI}\;\mathrm{at}\;\mathrm{follow}-\mathrm{up}}{\mathrm{Preoperative}\;\mathrm{BMI}-25}$$and
-
(3)
percentage of total body weight loss (% TBWL), calculated as follows:
$$\%\;\mathrm{TBWL}=100\times\frac{\mathrm{Preoperative}\;\mathrm{TBW}-\mathrm{TBW}\;\mathrm{at}\;\mathrm{follow}-\mathrm{up}}{\mathrm{Preoperative}\;\mathrm{TBW}}$$
The overall evaluation of the operation in terms of weight loss was based on the Reinhold classification modified by Christou [9], on Biron et al.’s criteria [10] and on the percentage of patients who maintained %EBWL ≥ 50 at the end of the follow-up [11]. We defined weight nadir as the lowest postoperative recorded weight. Because there is no commonly accepted definition of postoperative weight regain, we used four different definitions to assess it [12]. In particular, weight regain was defined as (1) an increase in TBW of > 10 kg from weight nadir, (2) an increase in TBW of > 15% from weight nadir, (3) an increase in BMI of ≥ 5 kg/m2 from the minimum BMI achieved postoperatively, and (4) greater than 25% EBW regain with respect to weight nadir.
Obesity-related comorbidities evaluated in the present study included hypertension (HTN), type 2 diabetes mellitus (T2DM), dyslipidemia (DLP), obstructive sleep apnea (OSA), degenerative joint disease (DJD), and GERD. Improvement or remission of HTN, T2DM, and DLP was defined as discontinuation of preoperative medical treatment, while improvement or remission of OSA, DJD, and GERD was defined as discontinuation of preoperative specific therapeutic measures (such as continuous positive airway pressure (CPAP) therapy) and improvement/elimination of symptoms. On the other hand, the new onset of HTN, T2DM, and DLP was defined as the new intake of disease-specific medications in patients who did not receive medications preoperatively, while the new onset of OSA, DJD, and GERD was defined as the new onset of symptoms or the application of specific therapeutic measures in asymptomatic patients who did not receive any treatment preoperatively.
Surgical technique
The procedure was performed by the same surgical team, and a laparoscopic or open approach was used in all cases. Regardless of the approach, the greater omentum was divided next to the greater curvature of the stomach using a bipolar vessel sealing system. The gastric fundus was fully mobilized from the left diaphragmatic crus with minimal dissection of the hiatus. In the case of hiatal hernia detection, it was repaired intraoperatively. Stomach resection was performed by dividing the greater curvature of the stomach with continuously applied linear staplers close to a 32F bougie. The resection began approximately 3 cm proximal to the pylorus and continued upwards to the angle of His, while keeping a safe distance of approximately 1 cm from the gastroesophageal junction. In this way, about 85–90% of the stomach was removed and a gastric sleeve was created. In all cases, pericardial strips were used for staple line reinforcement, and a drainage tube was left close to the staple line.
Statistical analysis
All statistical analyses were performed using Jamovi version 1.8.4 for Linux. Categorical variables were expressed as counts (%), while continuous variables were expressed as mean ± standard deviation (SD) unless otherwise specified. Intergroup comparisons of categorical variables were performed using the χ2 test. The evolution of comorbidities over time was assessed with the McNemar test. Intergroup comparisons of continuous variables were performed using a Student’s t test for parametric variables or a Mann–Whitney U test (Wilcoxon rank-sum test) for non-parametric variables. Intragroup-paired comparisons of continuous variables were performed using the paired (dependent) sample t test for parametric variables and the Wilcoxon signed-rank test for non-parametric variables. For all these analyses, p values < 0.05 were considered significant.
Results
Patient characteristics
A subset of 104 patients out of a total of 181 patients who underwent SG as a primary bariatric procedure between January 2005 and December 2009 were included in the present study. Patient characteristics are shown in Table 1. The mean age at surgery was 34.0 ± 10.1 years (range 18.5–60.8 years). Eighty-two (78.8%) of 104 patients were women. The mean preoperative weight was 122.4 ± 16.6 kg (range 87.4–177.0 kg), while the mean preoperative BMI was 43.4 ± 2.9 kg/m2 (range 36.8–51.1 kg/m2). Ninety-nine (95.2%) of 104 patients underwent laparoscopic SG, while the rest underwent open surgery. The conversion rate was 0%. Thirty-nine (37.4%) of 104 patients suffered from at least one comorbidity preoperatively. The most common comorbidities were T2DM (18.3%), HTN (14.4%), OSA (10.6%), and DLP (9.6%). The mean postoperative follow-up was 13.4 ± 1.3 years (range 11.0–15.8 years).
Weight loss
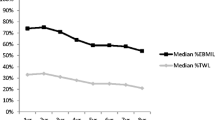
Weight, BMI, %EBWL, %EBMIL, %TBWL, and time course trends during follow-up are shown in Table 2 and Fig. 2. Postoperative weight and BMI were significantly lower at all time points compared to preoperative values (all p < 0.001). Rapid weight loss was observed during the first postoperative year and weight loss peaked 1 year after SG. After the first postoperative year, weight loss began to decrease gradually due to weight regain. At a mean follow-up of 13.4 years, weight loss was significantly lower compared to the first postoperative year (p < 0.001). Weight and BMI increased by 17 ± 20% (an average of 1.2% per year), whereas %EBWL, %EBMIL, and %TBWL decreased by 25 ± 31% (an average of 2.35% per year) between the first postoperative year and the last follow-up. %EBWL, %EBMIL, and %TBWL at the last follow-up were 59 ± 25, 69 ± 29, and 29 ± 12, respectively.
Over two thirds of patients (67.3%) maintained an %EBWL of > 50 at the last follow-up, which is a reliable indicator of long-term weight loss. According to the Reinhold classification modified by Christou and the criteria of Biron et al., 50% of patients showed excellent weight loss outcomes, 32.7% of patients showed good weight loss outcomes, while 17.3% of patients showed suboptimal weight loss outcomes at 11 + years. In addition, successful long-term weight loss at 11 + years (%EBWL > 50) was associated with greater %EBWL at 1 year (p = 0.006) and 5 and 10 years (p < 0.001).
Weight regain
Depending on the definition of weight regain used, the percentage of patients who experienced significant weight regain ranged from 47 to 64% (Table 3). In particular, at a mean follow-up of 13.4 years, 47% of patients experienced > 25% EBW regain with respect to weight nadir, 50% of patients experienced an increase in BMI of ≥ 5 kg/m2 from the minimum BMI achieved postoperatively, 61% of patients experienced an increase in body weight of > 10 kg from weight nadir, and 64% of patients experienced an increase in body weight of > 15% from weight nadir. Overall, the mean weight regain during follow-up was 16.2 ± 12.7 kg, the mean percentage increase in body weight was 25 ± 23%, and the mean increase in BMI was 5.9 ± 4.9 kg/m2.
Comorbidities
The evolution of comorbidities is shown in Table 4. The rate of improvement or remission of HTN, T2DM, DLP, OSA, DJD, and GERD at a mean follow-up of 13.4 years was 40%, 94.7%, 70%, 100%, 42.9%, and 25%, respectively. On the other hand, the new onset of HTN, T2DM, DLP, OSA, DJD, and GERD symptoms in the same period was 3.4%, 1.2%, 3.2%, 1.1%, 2.1%, and 43%, respectively. Statistical analysis showed that the incidence of T2DM and OSA at 11 + years after SG was significantly lower (p < 0.001 and p = 0.004, respectively), while the incidence of GERD symptoms at the same time was significantly higher (p < 0.001) compared to preoperatively. In addition, the presence of GERD symptoms postoperatively was not associated with any of the weight loss or weight regain parameters examined.
The great majority of patients with GERD symptoms had undergone at least one EGD at irregular intervals postoperatively, and more than half of them had findings of erosive esophagitis. Almost all of the patients with GERD symptoms were receiving proton pump inhibitors (PPIs) medication. On the other hand, only a few of the patients without GERD symptoms had undergone an EGD. In many patients who underwent EGD, the results were not reported according to the Los Angeles classification system.
Revision or conversion to other bariatric procedures
Of the 107 patients who agreed to participate in the study and were interviewed, only one had undergone a conversion of SG to RYGB. The main indication for conversion in this patient was severe GERD. No other patients had undergone revisional surgery or conversion of the SG to one anastomosis gastric bypass (OAGB)/mini gastric bypass (MGB) or other bariatric procedure due to severe GERD, insufcient weight loss, weight regain, or other reasons (Fig. 1).
Discussion
The current study presents the long-term weight loss outcomes and progression of comorbidities in a series of 104 patients who underwent SG as a stand-alone bariatric procedure at our institution between 2005 and 2009. The vast majority of patients were offered laparoscopic surgery. Five patients from our study group underwent open SG. These were very early SGs at our institution, which were performed in order to familiarize the surgical team with the basic operative technique. Currently, all SGs at our institution are performed laparoscopically. Furthermore, in the last decade or so, we have abandoned the use of pericardial strips. Pericardial strips were widely used in the 00’s. Although pericardial strips may reduce length of stay and bleeding from the staple line, more recent and higher-quality data supports that the use of pericardial strips is associated with an increased leak rate compared with no reinforcement or other reinforcement techniques, as well as increased readmission and reoperation rates compared with other reinforcement techniques [13,14,–, 15].
The last follow-up was at least 11 years after SG. As mentioned in the results, %EBWL and %TBWL at a mean follow-up of 13.4 years were 59 and 29, respectively. Our results are consistent with data from previously published studies presenting long-term (≥ 10 years) weight loss after SG [16,17,18,19,20,21]. According to these studies, %EBWL during long-term follow-up after SG ranges between 50 and 70.5, while %TBWL ranges between 21 and 31.5. However, it should be noted that the weight loss outcomes in these studies are usually overestimated, as they do not include patients undergoing conversion or revision surgery. The conversion or revision surgery rate during long-term follow-up after SG is estimated to be 6–49%. The most common indication for conversion or revision surgery is inadequate weight loss or weight regain [5. ]. In contrast, only one patient in our study was converted to RYGB, and this was due to GERD. The low conversion/revision surgery rate in our study was mainly due to patients’ unwillingness to undergo reoperation. In this respect, our results should be considered more representative of long-term weight loss after SG. On the other hand, patient selection in our study was designed to improve long-term outcomes after SG. For instance, BMI has been shown to be a negative predictor of weight loss after SG [22]. However, patients with a BMI ≥ 52 kg/m2 were not eligible for SG at our institution. In this regard, weight loss outcomes may have been overestimated in our study as well.
Most studies with long-term follow-up agree that weight loss failure (weight regain and/or insufficient weight loss) is the most common cause of reoperation after SG [13, 15,16,17,18], although some studies suggest that GERD may be a more common cause of reoperation [14, 20,23]. It has been shown that during the medium-term follow-up (5–7 years) after SG, up to 40% of patients exhibit an increase in body weight of > 10 kg from their weight nadir [24, 25], while up to 30% of patients exhibit > 25% EBW regain with respect to their weight nadir [26, 27]. Weight regain during long-term follow-up after SG cannot be precisely estimated, as some patients undergo conversion or revision surgery. Our study showed that 61% of patients experienced an increase in body weight of > 10 kg from their weight nadir and 47% of patients experienced > 25% EBW regain with respect to their weight nadir at a mean follow-up of 13.4 years. In line with previous observations [6], this indicates an increasing trend in the proportion of patients experiencing weight regain after SG as the time after surgery increases. Although weight regain may be a major late complication of SG, it is a common phenomenon after all types of bariatric surgery, and it does not necessarily imply weight loss failure or the need for reoperation. The mean annual increase in body weight and the decrease in percent EBWL in our study group during the 10 + years after the first postoperative year were only 1.2% and 2.5%, respectively. This suggests acceptable to good long-term weight loss maintenance for the majority of patients. Patient selection and surgical technique are crucial for long-term weight loss maintenance after SG, as large residual gastric volume, large bougie size, removed gastric volume of < 500 cc, limited antral resection, stress, anxiety, high serotonin levels, eating disorders, pregnancy, lack of exercise, and poor nutrition habits (such as high fat intake and increased consumption of sweets) have been identified as predictors for weight regain [28]. Surgical options for the treatment of weight regain after SG include repeat sleeve gastrectomy (re-sleeve), endoscopic sleeve gastroplasty (ESG), and conversion to RYGB, BPD-DS, OAGB/MGB, or single anastomosis duodeno-ileal bypass (SADI-S) [29].
According to the results of our study, SG resulted in an extremely high rate of remission or improvement in T2DM and OSA, a fairly high rate of remission or improvement in DLP, and a modest remission or improvement in HTN and DJD. Comparison with other studies was not possible due to the heterogeneity of the definitions used in the literature. Nevertheless, most published studies with long-term follow-up agree that SG is more effective in remission or improvement of T2DM and OSA than HTN and DLP [16, 18, 20]. Preoperative duration of T2DM has been shown to be a predictor of remission or improvement after SG. A preoperative duration of T2DM > 10 years has been associated with a lower postoperative remission rate [25]. Therefore, SG is a reasonable option for patients with morbid obesity, recent onset of T2DM, and good preservation of β cells [30]. In other cases, bariatric surgery with duodenal exclusion may be more appropriate [31]. Preoperative weight has also been shown to be a negative predictor of remission of T2DM after SG. In particular, higher preoperative weight has been associated with lower postoperative remission rates of T2DM [20]. Therefore, even in this case, patient selection seems to play an important role in the remission or improvement of T2DM after SG. The rate of new onset of HTN, T2DM, DLP, OSA, and DJD was very low. This indicates a possible role for SG in preventing the onset of these comorbidities. In summary, despite high weight regain rates, SG resulted in acceptable to high rates of remission or improvement and low rates of new onset of HTN, T2DM, DLP, OSA, and DJD during long-term follow-up.
Due to patient selection criteria, the remission or improvement of preexisting GERD after SG cannot be reliably evaluated by the present study. The new onset of GERD symptoms during long-term follow-up was 43%. The incidence of de novo GERD during long-term follow-up after SG in the literature is as high as 58.4% [16,17,18, 20, 21], and this may be the Achilles’ heel of the procedure. The pathogenesis of GERD after SG is attributed to a complex interaction of anatomical, physiological, and physical factors. The shape of the sleeve, the extent of injury to the lower esophageal sphincter, and the presence of hiatal hernia are factors of particular importance for the postoperative occurrence of GERD [7]. So, pitfalls in surgical technique may increase the incidence of GERD after SG. SG is now considered a low-compliance, high-pressure system, responsible for the development of GERD symptoms and/or endoscopic findings of GERD, erosive esophagitis, and Barret’s esophagus [32, 33]. Although GERD symptoms are effectively treated with PPIs in most patients, the occurrence of Barrett’s esophagus is a potentially threatening condition. The incidence of Barrett’s esophagus during medium- and long-term follow-up after SG has been estimated at 14–17.2% [18, 33]. It is worth noting that several studies have shown no correlation between clinical and endoscopic findings after SG [33, 34]. Taking into account the relatively high incidence of Barrett’s esophagus and the mismatch of clinical and endoscopic findings, endoscopic surveillance seems to be necessary for all patients after SG [35, 36]. Unfortunately, EGD after SG was not routinely performed at our institution until recently. Patients with mild GERD symptoms after SG were treated with continuous, intermittent, or on-demand PPI administration, while patients with severe symptoms, patients with persistent symptoms unresponsive to PPIs, and those with suspected GERD complications (e.g., dysphagia, anemia) were advised to undergo EGD and barium swallow tests. Patients with non-erosive reflux (NERD) on EGD were also treated with continuous, intermittent, or on-demand PPI administration. Patients with mild erosive esophagitis on EGD were treated with PPIs for at least 8–12 weeks, and then, treatment was individualized. Patients with severe erosive esophagitis were treated with PPIs indefinitely and were offered a surgical consultation for revisional surgery. The only proven surgical option for treating intractable GERD after SG is conversion to RYGB [36]. Hiatal hernia repair with gastropexy and the LINX® Reflux Management System (Torax Medical, St. Paul, MN) has also been used in some cases [17
Limitations
Our study has several limitations. First, the retrospective nature of the study, the lack of a control group, and lack of randomization are important limitations. Second, the single-center characteristic of our study limits the generalizability of the results. Third, there was significant selection bias in our study, as patients with BMI ≥ 52 kg/m2, patients who were “sweet eaters” and patients who suffered from hiatal hernia, severe GERD or Barrett’s esophagus at the time of surgery were not eligible for SG. Fourth, only 59% of patients eligible for follow-up were included in the present study. According to the Surgical Review Corporation “Centers of Excellence” program, a 75% or greater follow-up at 5 years is mandated [37]. Fifth, the weight loss data extracted from the structured phone interview was self-reported, in contrast to the weight loss data during the scheduled follow-up that emerged from the physical examination. Sixth, the lack of preoperative data regarding patients’ quality of life (QoL) did not allow evaluation of long-term effects of SG on QoL. Seventh, the cohort we studied includes some of the first SGs ever performed at our institution and in which the surgical technique may not have been sufficiently standardized. Eight, the comorbidities were only indirectly evaluated according to their symptoms and/or treatment (which is mainly determined by primary care physicians), rather than objective findings, such as biochemical laboratory tests, polysomnography, endoscopy, pH-metry, and high-resolution manometry. Therefore, the efficacy of LSG in comorbidities cannot be reliably assessed. Finally, οnly some of the patients we studied had undergone EGD, and from the patients who underwent EGD we could not draw firm conclusions because of methodological issues mentioned earlier. Thus, the effect of SG on the esophagus cannot be accurately assessed and this may conceal a higher rate of failure and need for conversion.
Conclusions
Our study evaluated the long-term (11 + years) efficacy of SG (as performed at our institution in the 00’s) as a stand-alone procedure for the treatment of morbid obesity in a group of patients with specific preoperative characteristics. SG remains (with some modifications) along with OAGB, one of the most frequently performed bariatric procedures at our institution. During long-term follow-up, SG resulted in good or excellent weight loss outcomes (BMI ≤ 35 kg/m2) in > 80% of patients, in successful long-term weight loss maintenance (%EBWL > 50) in approximately two thirds of them and in an extremely high rate of remission or improvement in T2DM and OSA. On the other hand, moderate or high rates of weight regain and the de novo development of GERD symptoms were the main drawbacks of the procedure. Proper patient selection and surgical technique may be of particular importance for the long-term success of SG.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00423-023-02824-5
References
Hess DS, Hess DW (1998) Biliopancreatic diversion with a duodenal switch. Obes Surg 8(3):267–282. https://doi.org/10.1381/096089298765554476
Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, Ramanathan R, Schauer P (2006) Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc 20(6):859–863. https://doi.org/10.1007/s00464-005-0134-5
Silecchia G, Rizzello M, Casella G, Fioriti M, Soricelli E, Basso N (2009) Two-stage laparoscopic biliopancreatic diversion with duodenal switch as treatment of high-risk super-obese patients: analysis of complications. Surg Endosc 23(5):1032–1037. https://doi.org/10.1007/s00464-008-0113-8
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N (2017) Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 27(9):2279–2289. https://doi.org/10.1007/s11695-017-2666-x
Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A (2018) Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 14(6):741–747. https://doi.org/10.1016/j.soard.2018.02.027
Lauti M, Kularatna M, Hill AG, MacCormick AD (2016) Weight regain following sleeve gastrectomy-a systematic review. Obes Surg 26(6):1326–1334. https://doi.org/10.1007/s11695-016-2152-x
Felinska E, Billeter A, Nickel F, Contin P, Berlth F, Chand B, Grimminger P, Mikami D, Schoppmann SF, Müller-Stich B (2020) Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann N Y Acad Sci 1482(1):26–35. https://doi.org/10.1111/nyas.14467
Peterson CM, Thomas DM, Blackburn GL, Heymsfield SB (2016) Universal equation for estimating ideal body weight and body weight at any BMI. Am J Clin Nutr 103(5):1197–1203. https://doi.org/10.3945/ajcn.115.121178
Christou NV, Look D, Maclean LD (2006) Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 244(5):734–740. https://doi.org/10.1097/01.sla.0000217592.04061.d5
Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Simard S, Marceau P (2004) Twenty years of biliopancreatic diversion: what is the goal of the surgery? Obes Surg 14(2):160–164. https://doi.org/10.1381/096089204322857492
Brolin RE, Kenler HA, Gorman RC, Cody RP (1989) The dilemma of outcome assessment after operations for morbid obesity. Surgery 105(3):337–346
Nedelcu M, Khwaja HA, Rogula TG (2016) Weight regain after bariatric surgery-how should it be defined? Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 12(5):1129–1130. https://doi.org/10.1016/j.soard.2016.04.028
Gagner M, Buchwald JN (2014) Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 10(4):713–723. https://doi.org/10.1016/j.soard.2014.01.016
Barreto TW, Kemmeter PR, Paletta MP, Davis AT (2015) A comparison of a single center’s experience with three staple line reinforcement techniques in 1,502 laparoscopic sleeve gastrectomy patients. Obes Surg 25(3):418–422. https://doi.org/10.1007/s11695-014-1432-6
Gagner M, Kemmeter P (2020) Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc 34(1):396–407. https://doi.org/10.1007/s00464-019-06782-2
Arman GA, Himpens J, Dhaenens J, Ballet T, Vilallonga R, Leman G (2016) Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy Surgery for obesity and related diseases : official. J American Soc Bariatric Surg 12(10):1778–1786. https://doi.org/10.1016/j.soard.2016.01.013
Chang DM, Lee WJ, Chen JC, Ser KH, Tsai PL, Lee YC (2018) Thirteen-year experience of laparoscopic sleeve gastrectomy: surgical risk, weight loss, and revision procedures. Obes Surg 28(10):2991–2997. https://doi.org/10.1007/s11695-018-3344-3
Felsenreich DM, Ladinig LM, Beckerhinn P, Sperker C, Schwameis K, Krebs M, Jedamzik J, Eilenberg M, Bichler C, Prager G, Langer FB (2018) Update: 10 years of sleeve gastrectomy-the first 103 patients. Obes Surg 28(11):3586–3594. https://doi.org/10.1007/s11695-018-3399-1
Felsenreich DM, Artemiou E, Steinlechner K, Vock N, Jedamzik J, Eichelter J, Gensthaler L, Bichler C, Sperker C, Beckerhinn P, Kristo I, Langer FB, Prager G (2021) Fifteen years after sleeve gastrectomy: weight loss, remission of associated medical problems, quality of life, and conversions to roux-en-y gastric bypass-long-term follow-up in a multicenter study. Obes Surg 31(8):3453–3461. https://doi.org/10.1007/s11695-021-05475-x
Castagneto Gissey L, Casella Mariolo JR, Genco A, Troisi A, Basso N, Casella G (2018) 10-year follow-up after laparoscopic sleeve gastrectomy: outcomes in a monocentric series Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 14(10):1480–1487. https://doi.org/10.1016/j.soard.2018.06.021
Hauters P, Dubart JW, Desmet J, Degolla R, Roumain M, Malvaux P (2021) Ten-year outcomes after primary vertical sleeve gastrectomy for morbid obesity: a monocentric cohort study. Surg Endosc 35(12):6466–6471. https://doi.org/10.1007/s00464-020-08137-8
Cottam S, Cottam D, Cottam A (2019) Sleeve gastrectomy weight loss and the preoperative and postoperative predictors: a systematic review. Obes Surg 29(4):1388–1396. https://doi.org/10.1007/s11695-018-03666-7
Boru CE, Greco F, Giustacchini P, Raffaelli M, Silecchia G (2018) Short-term outcomes of sleeve gastrectomy conversion to R-Y gastric bypass: multi-center retrospective study. Langenbeck’s Archives of Surg 403(4):473–479. https://doi.org/10.1007/s00423-018-1675-0
Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC (2012) Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surgical laparoscopy, endoscopy & percutaneous techniques 22(6):479–486. https://doi.org/10.1097/SLE.0b013e318262dc29
Casella G, Soricelli E, Giannotti D, Collalti M, Maselli R, Genco A, Redler A, Basso N (2016) Long-term results after laparoscopic sleeve gastrectomy in a large monocentric series Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 12(4):757–762. https://doi.org/10.1016/j.soard.2015.09.028
Liu SY, Wong SK, Lam CC, Yung MY, Kong AP, Ng EK (2015) Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for a morbidly obese Chinese population. Obes Surg 25(10):1901–1908. https://doi.org/10.1007/s11695-015-1628-4
Iossa A, Coluzzi I, Giannetta IB, Silecchia G (2020) Weight loss and eating pattern 7 years after sleeve gastrectomy: experience of a bariatric center of excellence. Obes Surg 30(10):3747–3752. https://doi.org/10.1007/s11695-020-04699-7
Yu Y, Klem ML, Kalarchian MA, Ji M, Burke LE (2019) Predictors of weight regain after sleeve gastrectomy: an integrative review Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 15(6):995–1005. https://doi.org/10.1016/j.soard.2019.02.009
de Moura D, Barrichello S Jr, de Moura E, de Souza TF, Neto DPG, M., Grecco, E., Sander, B., Hoff, A. C., Matz, F., Ramos, F., de Lima, J., Teixeira, L., Dib, V., Falcão, M., Potti, H., Baretta, G., Jirapinyo, P., & Thompson, C. C. (2020) Endoscopic sleeve gastroplasty in the management of weight regain after sleeve gastrectomy. Endoscopy 52(3):202–210. https://doi.org/10.1055/a-1086-0627
Lee WJ, Almulaifi A, Tsou JJ, Ser KH, Lee YC, Chen SC (2015) Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 11(5):991–996. https://doi.org/10.1016/j.soard.2014.12.027
Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD (2011) Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 7(6):683–690. https://doi.org/10.1016/j.soard.2011.07.009
Tolone S, Cristiano S, Savarino E, Lucido FS, Fico DI, Docimo L (2016) Effects of omega-loop bypass on esophagogastric junction function Surgery for obesity and related diseases: official. J American Soc Bariatric Surg 12(1):62–69. https://doi.org/10.1016/j.soard.2015.03.011
Genco A, Soricelli E, Casella G, Maselli R, Castagneto-Gissey L, Di Lorenzo N, Basso N (2017) Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication Surgery for obesity and related diseases : official. JAmerican Soc Bariatric Surg 13(4):568–574. https://doi.org/10.1016/j.soard.2016.11.029
Yeung K, Penney N, Ashrafian L, Darzi A, Ashrafian H (2020) Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg 271(2):257–265. https://doi.org/10.1097/SLA.0000000000003275
Qumseya BJ, Qumsiyeh Y, Ponniah SA, Estores D, Yang D, Johnson-Mann CN, Friedman J, Ayzengart A, Draganov PV (2021) Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc 93(2):343-352.e2. https://doi.org/10.1016/j.gie.2020.08.008
Crawford C, Gibbens K, Lomelin D, Krause C, Simorov A, Oleynikov D (2017) Sleeve gastrectomy and anti-reflux procedures. Surg Endosc 31(3):1012–1021. https://doi.org/10.1007/s00464-016-5092-6
American Society for Bariatric Surgery Standards Committee, 2004-2005, Oria HE, Carrasquilla C, Cunningham P, Hess DS, Johnell P, Kligman MD, Moorehead MK, Papadia F S, Renquist KE, Rosenthal R & Stellato TA (2005) Guidelines for weight calculations and follow-up in bariatric surgery. Surgery for obesity and related diseases: Off J Am Soc for Bariatric Surg 1(1):67–68
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
Study conception and design: I. Kehagias and Ch. Lampropoulos; acquisition of the data: A. Bellou and D. Kehagias; analysis and interpretation of the data: G. Markopoulos and Ch. Lampropoulos; drafting of the manuscript: Th. Amanatidis and Ch. Lampropoulos; critical revision of the manuscript: I. Kehagias, A. Alexandrou, and K. Albanopoulos. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted in compliance with the current version of the Declaration of Helsinki and was approved by research ethics committee of University Hospital of Patras.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kehagias, I., Bellou, A., Kehagias, D. et al. Long-term (11 + years) efficacy of sleeve gastrectomy as a stand-alone bariatric procedure: a single-center retrospective observational study. Langenbecks Arch Surg 408, 4 (2023). https://doi.org/10.1007/s00423-022-02734-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-022-02734-y