Abstract
Purpose
This study evaluated the improvement of respiratory function and airway volumes using spirometry and computed tomography (CT) in severely obese Japanese patients undergoing laparoscopic sleeve gastrectomy (LSG). We also evaluated the quality of life (QOL) of enrolled patients using questionnaires.
Methods
A total of 71 patients who underwent LSG at Iwate Medical University Hospital between October 2013 and September 2020 were enrolled. The changes and relationships between respiratory parameters including CT volumetry and weight-loss effects were evaluated. Improvements to QOL and bronchial asthma (BA) were also assessed before LSG and 1 year after LSG.
Results
The mean excess weight loss percentage (%EWL) and total weight loss percentage (%TWL) were measured at 55.1% and 26.1%, respectively. The attack frequency of BA significantly decreased (6.1/month vs. 1.5/month; P < 0.001), and the disease severity decreased according to severity classification (P = 0.032). Almost spirometric parameters, lung volume (LV) (4905.0 mL vs. 5490.3 mL; P < 0.001), and airway volume (AV) (108.6 mL vs. 119.3 mL; P = 0.022) significantly improved. The change of functional residual capacity (FRC) was correlated with both %EWL (ρ = 0.69, P < 0.001) and %TWL (ρ = 0.62, P < 0.001). The increase of LV (ρ = 0.79, P < 0.001) and AV (ρ = 0.69, P < 0.001) were correlated with the increase of FRC. Scores of QOL questionnaires dramatically became better owing to improvements in dyspnea.
Conclusion
Weight loss effects and the reduction of body fat mass correlated significantly with increase in LV and AV. Improvements of respiratory functions after LSG contributes to QOL and BA symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become a worldwide health problem that has reached epidemic levels in not only Western countries but also in Asia [1]. In 2008, it was documented that 1.4 billion adults were considered overweight, as identified by a body mass index (BMI) > 25 kg/m2 [2]. In Japan, the 2019 National Health and Nutrition Survey revealed that 33.0% of male and 22.3% of female adults are overweight; furthermore, 0.5% of all overweight adults are severely obese (BMI > 35.0 kg/m2) [3]. In Japan, this situation has already caused a rapid increase in the prevalence of type 2 diabetes (T2D), non-alcoholic steatohepatitis (NASH), obstructive sleep apnea (OSA), and other obesity-related diseases.
Obesity causes respiratory dysfunction by decreasing expiratory reserve volume (ERV) and functional residual capacity (FRC), while total lung capacity seems to remain normal [4]. On the basis of the pathophysiological explanatory models of obesity, it can be assumed that obese patients have decreased pulmonary volumes, a decrease in the strength of respiratory muscles, and consequently increase in respiratory work and limitation and restriction of diaphragm movement, leading to hypoventilation and hypoxemia manifesting in dyspnea [5]. Dyspnea is a very common and crippling symptom of obesity, and about 80% of people with obesity experience dyspnea in their daily lives; therefore, their quality of life (QOL) significantly decreases [6]. In addition, obesity can induce and progress various respiratory and lung diseases, in particular OSA, obesity hypoventilation syndrome, bronchial asthma (BA), and chronic obstructive pulmonary diseases via multiple mechanisms [7]. However, bariatric procedures can improve airway anatomy, respiratory physiology, and obesity-related inflammation by dramatic weight-loss effects [7, 8].
Currently, high-resolution computed tomography can be applied to various diagnoses and navigations. However, there have been no reports on whole airway and lung volumetry studies to evaluate therapeutic effects of bariatric surgeries; therefore, accuracies and correlations with spirometric parameters of volumetric parameters also have not been investigated yet.
The aim of the present study was to investigate the improvement of respiratory function using spirometric parameters and lung and airway volumetry using CT in Japanese patients with severe obesity undergoing laparoscopic sleeve gastrectomy (LSG). We then analyzed the correlations between weight loss effects and changes of respiratory parameters, including CT volumetry. We also evaluated the improvement in BA and the QOL of enrolled patients using a modified medical research council (mMRC) scale and a modified Borg scale before and after LSG [9, 10].
Patients and methods
Patients and treatments
This study was a single-institution retrospective study involving data collection and analysis. Between October 2013 and September 2020, 71 severely obese Japanese patients underwent LSG at Iwate Medical University Hospital. All patients met the following inclusion criteria for LSG treatment established by Japanese insurance practice: severe obesity with a body mass index (BMI) > 35 kg/m2 and the presence of at least one comorbidity with resistance to medical treatment [11].
Patients began a very low-calorie, preoperative diet after their initial visit to our clinic. The LSG was set to occur at the time that the patients’ preoperative total weight loss percentage (%TWL) was 5%. Regarding the surgical procedures, the LSGs influenced a gastric-volume reduction of 70–80% by resecting the stomach alongside a 36-French bougie beginning 4 cm from the pylorus and ending at the angle of His.
Data collection
For all enrolled patients, clinical data and weight loss effects were evaluated at the baseline and 1 year after LSG. Body weight, BMI, excess weight loss percentage (%EWL), %TWL, visceral fat area (VFA), and subcutaneous fat area (SFA) were collected as weight loss parameters. VFA and SFA were measured using a 64-row CT (AquilionTM, Toshiba Medical Systems Corporation, Tokyo, Japan) at the umbilicus-level single slice. We expressed the SFA and VFA in cm2, and these parameters were measured simultaneously. Vital capacity (VC), vital capacity percentage (%VC), forced expiratory volume percentage in one second (%FEV1.0), ERV, FRC, residual volume (RV), carbon monoxide diffusing capacity (DLco), and DLco/alveolar volume (DLco/AV) were measured using spirometers (FUDAC-7; Fukuda Denshi, Tokyo, Japan) after broncholysis using bronchodilators. Lung volume (LV) and airway volume (AV) were also measured at the same time using a 64-row CT and a computer workstation (SYNAPSE VINCENT v6.1; Fujifilm, Tokyo, Japan) (Fig. 1). Regarding diagnosis, practical management of BA and treatments for its severity were administered using Japanese guidelines for adult asthma published in 2020 [12]. Subjective BA symptoms were also evaluated by Asthma Control Test (ACT) recognized by the National Institutes of Health in its 2007 asthma guideline [13, 14]. The changes in BA severity and ACT were evaluated by hearing frequency and intensity of BA symptoms during the past 4 weeks at each point. With regard to the period of preoperative evaluation, all examinations were performed within 1–2 weeks before LSG. In this study, all patients have been followed until 1-year post-LSG evaluation.
Questionnaire for QOL improvements
Dyspnea in daily living was evaluated using the mMRC scale (Supplementary Fig. 1) [9]. The mMRC scale consists of five statements that almost entirely describe the range of dyspnea from none (grade 0) to almost complete incapacity (grade 4). We also used a modified Borg scale for dyspnea in each activity (at rest, at work, and during strong exercise) (Supplementary Fig. 2) [10]. These questionnaires were recorded at baseline and 1-year post-LSG.
Statistical analysis
Data are presented as numbers for categorical variables, and as means ± standard deviations for continuous variables. Statistical analysis was performed using chi-square tests for categorical variables and Student’s t-tests or Mann–Whitney U tests for continuous variables. We used paired t-tests and Wilcoxon tests for continuous variables to enable the comparison of all parameters between pre-and postoperative measures. Additionally, we utilized Spearman’s rank correlation coefficient to investigate the relationships between weight-loss effects, changes in respiratory parameters, and CT volumetry parameters. All statistical analyses were performed using JMP statistical software, version 15 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and weight loss effects
Table 1 summarizes the clinical characteristics at baseline and therapeutic effects at 1-year post-LSG. The mean %EWL and %TWL were 55.1% and 26.1%, respectively. The mean VFA (266.1 cm2 vs. 145.1 cm2; P < 0.001) and SFA (525.9 cm2 vs. 333.4 cm2; P < 0.001) significantly decreased. For the 12 patients with BA, attack frequency significantly decreased (6.1/month vs. 1.5/month; P < 0.001), and the distribution of severity also significantly improved (P = 0.032). In addition, ACT significantly increased and the number of ACT ≥ 20 at 1-year post-LSG increased 6 to 10. VC (3.5 L vs. 3.9 L; P < 0.001), %VC (95.2% vs. 105.0%; P < 0.001), ERV (0.8 L vs. 1.5 L; P < 0.001), FRC (2.8 L vs. 3.0 L; P < 0.001), and DLco/VA (6.7 mL/min/mmHg vs. 6.3 mL/min/mmHg; P < 0.001) saw significant decrease alongside the mean LV (4905.0 mL vs. 5490.3 mL; P < 0.001) and AV (108.6 mL vs. 119.3 mL; P = 0.022) as well. In this cohort, there was a patient with chronic obstructive pulmonary disease at both baseline and 1-year post-LSG. However, there were 2 patients with %FEV1.0 < 70% due to BA at baseline.
With regard to improvements of major obesity-related diseases, 67.4% (31/46) of T2D patients achieved drug withdrawal of antidiabetic agents including insulin use with Hemoglobin A1c < 6.5%, 50.1% (35/69) of OSA patients could discontinue continuous positive airway pressure owing to reduction of apnea–hypopnea index < 15 by full-night polysomnography, and 85.5% (59/69) of NASH also improved evaluated by total NAFLD activity score by liver biopsy at 6 months or 1-year post-LSG [15].
Correlation between weight loss effects and respiratory parameters
Table 2 shows the correlation analyses between weight loss effects and respiratory parameters. %EWL (ρ = 0.42, P = 0.002) and %TWL (ρ = 0.34, P = 0.013) were found to be correlated with changes in ERV. The change in the FRC was closely correlated with both %EWL (ρ = 0.69, P < 0.001) and %TWL (ρ = 0.62, P < 0.001). The change in SFA was correlated with an increase in FRC (ρ = 0.46, P = 0.046). On the other hand, the change in VFA was correlated with an increase in VC (ρ = 0.46, P < 0.001), %VC (ρ = 0.30, P = 0.037), and ERV (ρ = 0.52, P < 0.001). The increases in LV (ρ = 0.79, P < 0.001) and AW (ρ = 0.69, P < 0.001) were correlated with the increase in FRC.
QOL improvement after LSG
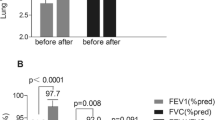
The mean scores of each questionnaire at baseline and 1-year post-LSG are shown in Fig. 2. The mean mMRC scale significantly improved at 1-year post-LSG (1.83 points vs. 0.25 points; P < 0.001) (Fig. 2A). The modified Borg scales for every exercise intensity also significantly improved (Fig. 2B–D).
Discussion
There have been reports about relationships between weight loss effects and improvement of respiratory function [6, 11,12,13]. However, this is the first study to specifically assess these relationships by employing CT volumetry and QOL questionnaires. The changes in ERV and FRC were closely correlated with both weight loss effects and changes in body fat mass. In addition, we successfully demonstrated the usefulness of CT volumetry in the evaluation of LV and AV expansion after LSG. Furthermore, dyspnea scales and the symptoms of BA also significantly improved after LSG.
Previous studies have reported an improvement in overall respiratory functions, BA control, and dyspnea. One reason for this improvement is related to a reduction in intraabdominal pressure [2, 6, 16, 17]. Spirometric parameters such as VC, %VC, ERV, and FRC dramatically improved due to weight loss effects [17]. Furthermore, we successfully demonstrated a slight but significant decrease in DLco/VA. This phenomenon may be explained by the following physiological changes: (1) preoperative DLco in severely obese patients is estimated to be higher because of an increase in intrathoracic blood volume, (2) an increase in VC and FRC expands alveolar volume, (3) a reduction in thoracic wall weight also releases restriction, and (4) the changes in VA are higher than those of DLco; therefore, DLco/VA decreases [18].
In this study, we could not find a significant change of DLco at 1-year post-LSG. This phenomenon may be also explained by the physiological backgrounds in obese patients: (1) obese patients have latent cardiac load due to OSA, hypertension, and chronic kidney disease; therefore, pulmonary capillary bed increases; (2) angiogenesis may be promoted in obese patients with BA by upregulating vascular endothelial growth factor and matrix metalloproteinase-9 [19].
We first demonstrated changes in LV and AV before and after LSG using CT volumetry. The correlation coefficients between VC and LV at baseline and 1-year post-LSG were 0.687 and 0.702, respectively. There were some discrepancies between these results, which were mainly caused by the following factors: (1) physiological dead space was not considered in LV, (2) the positions of the respiratory function test and CT examination differed, and (3) the same deep respiration was not made during spirometry and CT examination. However, LV analysis by CT volumetry may be an alternative method of representing respiratory function. We initially expected that AV would not change before and after LSG because the airway might maintain the lumen owing to the coverage provided by tracheal cartilages. Contrary to our expectations, AV also significantly increased at 1-year post-LSG. Previous studies have clarified that, in addition to medical treatment, bariatric procedures also dramatically reduce pericardiac fat [20, 21]. Chen et al. also reported that mediastinal fat accumulation is associated with the severity of metabolic syndrome [22]. The compartment pressure of the mediastinum may be higher in patients with severe obesity; therefore, the trachea and main bronchi may be compressed, and AV may also decrease. LSG contributes to the reduction of fat accumulation in the pericardium and mediastinum; then, the compression of the trachea and main bronchi is released by a decrease in mediastinal pressure. We hypothesize that this phenomenon may affect the improvement of BA.
There are a few main mechanisms relating to the relationship between BA and obesity. The primary mechanical defect was reported to be a high percentage of excess body fat that compresses the lungs, which reduces lung capacity and limits the free movement of air [23]. This mechanism is linked to spasms of the peripheral bronchi and a decrease in bronchial elastance. Furthermore, an increase in AV brought about by mediastinal fat reduction is the secondary mechanism for the elastance recovery of the trachea and main bronchi. Third, increased adiposity causes increased airway inflammation due to low-grade systemic inflammation resulting from adipose tissue breakdown, which results in macrophage activation and the generation of cytokines and adipokines that impact bronchial sensitivity [23]. We have previously reported that visceral adipocytes in severely obese patients produce significantly higher levels of leptin (P < 0.001), plasminogen activator inhibitor type-1 (P = 0.020), and tumor necrosis factor-α (P = 0.003) compared to a non-obese control group [24]. Furthermore, airway epithelial cells express leptin receptors, and this is suggested as an alternative route of BA pathogenesis separate from systemic inflammation [25]. Bariatric procedures, including LSG, improve every mechanism mentioned above through weight loss effects and attack frequency. Finally, the severity of the BA dramatically improves.
With regard to accumulated fat reduction, the percentage of fat reduction for VFA and SFA was 45.5% and 36.6% at 1 year after LSG, respectively, and this is the reason behind the assertion that visceral fat reduction contributes to metabolic effects; however, we consider that subcutaneous fat reduction plays an important role in improving respiratory function after bariatric surgery. Subcutaneous fat also surrounds the thoracic cavity, and the thickening of the thoracic wall due to subcutaneous fat accumulation may restrict the contraction of the thoracic cavity due to its weight and traction against respiratory movements. Therefore, the increase in FRC was correlated with the changes in SFA. On the other hand, VFA represents the volume of fat in the abdominal cavity. Abdominal fat accumulation compresses the diaphragm due to an increase in abdominal pressure and restricts expansion of the thoracic cavity; therefore, the increase in VC and %VC was correlated with the change of VFA. The correlation with systemic weight loss effects, such as %EWL and %TWL, is only observed in the increase of ERV and FRC, as previous reports have shown [2, 17]. We have demonstrated that LV and AV are useful parameters for evaluating the improvements in VC and FRC after LSG because the changes in LV and AV are closely correlated with %TWL (LV; ρ = 0.532, P = 0.023, AV; ρ = 0.639, P = 0.004) and %EWL (LV; ρ = 0.671, P = 0.002, AV; ρ = 0.629, P = 0.005).
There have been many reports of QOL improvements after bariatric procedures, including meta-analysis [26,27,28]. However, there are also many questionnaires used to evaluate QOL, such as the 36-item short-form health survey (SF-36) and the gastrointestinal quality of life index (GQOLI). Seki et al. have demonstrated that metabolic surgery improves some categories of SF-36 1 year after surgery, including physical functioning (P < 0.001), bodily pain, general health (P < 0.001), vitality (P = 0.001), and mental health (P = 0.02) [26]. Major et al. also reported that similar parameters of SF-36 were well maintained for 10 years after surgery [27], and Małczak et al. reported that there were no significant differences in QOL improvement between LSG and laparoscopic Roux-en Y gastric bypass (LRYGB) groups using the GIQOLI [28]. In this study, we focused on the improvement of dyspnea, given that most daily activities require some amount of exercise; therefore, improvement in dyspnea is directly linked to improvement in QOL.
The limitations of this study include its single-center nature, small sample size, and the relatively short duration of follow-up. It is known that a relevant number of patients gain weight several years after LSG; therefore, long-term outcomes and analyses same as this study should be provided in near future. Additionally, our study population included only patients who underwent LSG, as LRYGB and laparoscopic sleeve gastrectomy with duodenal-jejunal bypass were not covered by the national health insurance system [29]. Finally, we did not use any objective parameters, such as blood gas analysis, 6-min walk tests [30], and cardiopulmonary exercise testing. Furthermore, whole-body plethysmography may be an alternative to evaluate FRC on awake and nonrestricted conditions; therefore, employing whole-body plethysmography will bring another interesting result. For these reasons, the results of this study should be interpreted cautiously. Furthermore, large, multicentric, and multidimensional studies are warranted to confirm these results and clarify the systemic improvements in respiratory functions and remission of BA after bariatric procedures.
In conclusion, LSG is a promising procedure for improving respiratory function, BA control, and QOL specific to dyspnea due to its weight loss effects and the resulting reduction in systemic body fat for patients with severe obesity. Furthermore, we have demonstrated that LV and AV measured by CT volumetry are useful parameters to evaluate the improvements of VC and FRC after LSG, as the changes of LV and AV are closely correlated with %TWL. Dyspnea is one of the symptoms most responsible for worsening the respondents’ QOL, and this study has demonstrated that LSG could dramatically improve dyspnea at rest, at work, and during strenuous exercise.
References
Oh TJ, Lee HJ, Cho YM (2022) East Asian perspectives in metabolic and bariatric surgery. J Diabetes Investig. https://doi.org/10.1111/jdi.13748
Alsumali A, Al-Hawag A, Bairdain S, Eguale T (2018) The impact of bariatric surgery on pulmonary function: a meta-analysis. Surg Obes Relat Dis 14:225–236
Nikai H, Sasaki A, Umemura A, Takahashi N, Nitta H, Akasaka R, Kakisaka K, Kuroda H, Ishida K, Takikawa Y (2021) Predictive scoring system for advanced liver fibrosis in Japanese patients with severe obesity. Surg Today 51:1513–1520
Littleton SW (2012) Impact of obesity on respiratory function. Respirology 17:43–49
Behazin N, Jones SB, Cohen RI (1985) Loring SH (2010) Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 108:212–218
Boissière L, Perotin-Collard JM, Bertin E, Gaubil I, Diaz Cives A, Barbe C, Dury S, Nardi J, Lebargy F, Deslée G, Launois C (2017) Improvement of dyspnea after bariatric surgery is associated with increased Expiratory Reserve Volume: a prospective follow-up study of 45 patients. PLoS ONE 12:e0185058
Brock JM, Billeter A, Müller-Stich BP, Herth F (2020) Obesity and the lung: what we know today. Respiration 99:856–866
Yanari S, Sasaki A, Umemura A, Ishigaki Y, Nikai H, Nishijima T, Sakurai S (2022) Therapeutic effect of laparoscopic sleeve gastrectomy on obstructive sleep apnea and relationship of type 2 diabetes in Japanese patients with severe obesity. J Diabetes Investig. https://doi.org/10.1111/jdi.13755
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, Lebargy F, Deslee G (2012) The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med 12:61
Sasaki A, Yokote K, Naitoh T et al (2021) Metabolic surgery in treatment of obese Japanese patients with type 2 diabetes: a joint consensus statement from the Japanese Society for Treatment of Obesity, the Japan Diabetes Society, and the Japan Society for the Study of Obesity. Diabetol Int 13:1–30
Nakamura Y, Tamaoki J, Nagase H, Yamaguchi M, Horiguchi T, Hozawa S, Ichinose M, Iwanaga T, Kondo R, Nagata M, Yokoyama A, Tohda Y, Japanese Society of Allergology (2020) Japanese guidelines for adult asthma 2020. Allergol Int 69:519–548
Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB (2004) Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 113:59–65
Nelsen LM, Kosinski M, Rizio AA, Jacques L, Schatz M, Stanford RH, Svedsater H (2022) A structured review evaluating content validity of the Asthma Control Test, and its consistency with U.S. guidelines and patient expectations for asthma control. J Asthma 59:628–637
Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN) (2011) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53:810–20
Upala S, Thavaraputta S, Sanguankeo A (2019) Improvement in pulmonary function in asthmatic patients after bariatric surgery: a systematic review and meta-analysis. Surg Obes Relat Dis 15:794–803
Duymaz T, Karabay O, Ural IH (2020) The effect of chest physiotherapy after bariatric surgery on pulmonary functions, functional capacity, and quality of life. Obes Surg 30:189–194
Saydain G, Beck KC, Decker PA, Cowl CT, Scanlon PD (2004) Clinical significance of elevated diffusing capacity. Chest 125:446–452
Ding Z, Yu F, Sun Y, Jiao N, Shi L, Wan J, Liu Q (2022) ORMDL3 promotes angiogenesis in chronic asthma through the ERK1/2/VEGF/MMP-9 pathway. Front Pediatr 9:708555
Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clément K, Bernard M, Dutour A (2012) Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol 60:1381–1389
Sarmiento-Cobos M, Valera R, Botero Fonnegra C, Alonso M, Rivera C, Montorfano L, Wasser E, Lo Menzo E, Szomstein S, Rosenthal RJ (2022) Ventricular conduction improvement after pericardial fat reduction triggered by rapid weight loss in subjects with obesity undergoing bariatric surgery. Surg Obes Relat Dis 18:288–294
Chen O, Sharma A, Ahmad I, Bourji N, Nestoiter K, Hua P, Hua B, Ivanov A, Yossef J, Klem I, Briggs WM, Sacchi TJ, Heitner JF (2015) Correlation between pericardial, mediastinal, and intrathoracic fat volumes with the presence and severity of coronary artery disease, metabolic syndrome, and cardiac risk factors. Eur Heart J Cardiovasc Imaging 16:37–46
Hossain N, Arhi C, Borg CM (2021) Is bariatric surgery better than nonsurgical weight loss for improving asthma control? A systematic review. Obes Surg 31:1810–1832
Umemura A, Sasaki A, Nitta H, Otsuka K, Suto T, Wakabayashi G (2014) Effects of changes in adipocyte hormones and visceral adipose tissue and the reduction of obesity-related comorbidities after laparoscopic sleeve gastrectomy in Japanese patients with severe obesity. Endocr J 61:381–391
Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M (2009) Leptin and leptin receptor expression in asthma. J Allergy Clin Immunol 124:230–237
Seki Y, Pantanakul S, Kasama K, Kikkawa E, Nakazato T, Porciuncula JP (2019) Impact of metabolic surgery on health-related quality of life and quality of alimentation. Surg Obes Relat Dis 15:488–496
Major P, Stefura T, Dziurowicz B, Radwan J, Wysocki M, Małczak P, Pędziwiatr M (2020) Quality of life 10 years after bariatric surgery. Obes Surg 30:3675–3684
Małczak P, Mizera M, Lee Y, Pisarska-Adamczyk M, Wysocki M, Bała MM, Witowski J, Rubinkiewicz M, Dudek A, Stefura T, Torbicz G, Tylec P, Gajewska N, Vongsurbchart T, Su M, Major P, Pędziwiatr M (2021) Quality of life after bariatric surgery-a systematic review with Bayesian network meta-analysis. Obes Surg 31:5213–5223
Ohta M, Kasama K, Sasaki A et al (2021) Current status of laparoscopic bariatric/metabolic surgery in Japan: the sixth nationwide survey by the Japan Consortium of Obesity and Metabolic Surgery. Asian J Endosc Surg 14:170–177
Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM (1982) Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 284:1607–1608
Author information
Authors and Affiliations
Contributions
Study conception and design: Akira Umemura. Acquisition of data: Hiroyuki Nitta, Hirokatsu Katagiri, Shoji Kanno, Daiki Takeda, Satoshi Amano, Toshifumi Morishita, Makoto Eizuka, and Tomofumi Oizumi. Analysis and interpretation of data: Haruka Nikai, Shingo Yanarai, Hideki Ishioka, and Naoto Takahashi. Drafting of the manuscript: Akira Umemura. Critical revision of the manuscript: Hiroyuki Nitta and Akira Sasaki.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the institutional ethics committee (approval number: H27-47) and conducted according to the ethical principles in the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards.
Informed consent
We obtained informed consent from each patient before enrollment, and patient anonymity was strictly protected.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Umemura, A., Sasaki, A., Nikai, H. et al. Improvements of lung volumes and respiratory symptoms after weight loss through laparoscopic sleeve gastrectomy. Langenbecks Arch Surg 407, 2747–2754 (2022). https://doi.org/10.1007/s00423-022-02549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02549-x