Abstract
Purpose
This study aimed to elucidate the impact of anatomic location of residual disease (RD) after initial cholecystectomy on survival following re-resection of incidental gallbladder cancer (IGBC).
Methods
Patients with pT2 or pT3 gallbladder cancer (36 with IGBC and 171 with non-IGBC) who underwent resection were analyzed. Patients with IGBC were classified as follows according to the anatomic location of RD after initial cholecystectomy: no RD (group 1); RD in the gallbladder bed, stump of the cystic duct, and/or regional lymph nodes (group 2); and RD in the extrahepatic bile duct and/or distant sites (group 3).
Results
Timing of resection (IGBC vs. non-IGBC) did not affect survival in either multivariate or propensity score matching analysis. RD was found in 16 (44.4%) of the 36 patients with IGBC; R0 resection following re-resection was achieved in 32 patients (88.9%). Overall survival (OS) following re-resection was worse in group 3 (n = 7; 5-year OS, 14.3%) than in group 2 (n = 9; 5-year OS, 55.6%) (p = 0.035) or in group 1 (n = 20; 5-year OS, 88.7%) (p < 0.001). There was no survival difference between groups 1 and 2 (p = 0.256). Anatomic location of RD was independently associated with OS (group 2, HR 2.425, p = 0.223; group 3, HR 9.627, p = 0.024).
Conclusion
The anatomic location of RD independently predicts survival following re-resection, which is effective for locoregional disease control in IGBC, similar to resection for non-IGBC. Not all patients with RD have poor survival following re-resection for IGBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidental gallbladder cancer (IGBC), which is discovered histologically after simple cholecystectomy for other indications, is a common mode of presentation of gallbladder cancer (GBC) and is well known to have more favorable survival outcomes than non-IGBC, which is usually symptomatic and often presents as an advanced disease [1,2,3,4]. R0 resection provides the only potential cure for patients with GBC and surgical resection is attempted for fit patients without disseminated disease independent of the timing of diagnosis [5]. The goals of re-resection for IGBC after simple cholecystectomy are to improve survival by clearance of possible residual disease (RD) at locoregional sites after initial cholecystectomy and to stage the disease accurately. Indeed, many observational studies have demonstrated that the addition of re-resection is associated with more favorable survival outcomes than simple cholecystectomy alone for patients with pathological T2 (pT2) or pT3 IGBC [6,7,8,9,10,11,12].
Recent studies have demonstrated that RD found at re-resection is the main determinant of the prognosis in patients with IGBC [8,9,10,11, 13,14,15,16]. However, considerable debate persists regarding this issue. Some studies have revealed that RD at locoregional sites is equivalent to stage IV disease clinically [13, 14], whereas others have advocated that the surgical outcome following re-resection depends on the anatomic location of RD [15, 16]. The former claim raises questions as to whether re-resection simply acts as a staging procedure rather than improving patient outcomes by eradicating possible RD and whether IGBC has worse tumor biology than non-IGBC, where the prognosis after resection differs according to tumor spread (local or regional vs. distant). Therefore, the impact of anatomic location of RD after initial cholecystectomy on the outcome in patients with IGBC remains controversial.
To address this issue, this study was designed to investigate differences in tumor biology between IGBC and non-IGBC and to elucidate the impact of anatomic location of RD after initial cholecystectomy on outcomes following re-resection in patients with IGBC. The study goal was to clarify the role of re-resection for IGBC in the management of GBC.
Material and methods
Study population
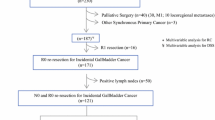
We identified 333 patients with pathologically confirmed GBC who underwent resection with curative intent in the databases of Niigata University Medical and Dental Hospital and Niigata Cancer Center Hospital from May 1982 through August 2019. Patients who underwent only simple cholecystectomy were excluded. To fully assess the effect of re-resection for IGBC on surgical outcomes, patients with pT1 or pT4 GBC were excluded, leaving 207 patients (117 women and 90 men; median age 70 [range, 37 to 90] years) with pT2 or pT3 GBC as the study cohort. IGBC was defined as a tumor first discovered histologically following simple cholecystectomy for presumed benign disease, whereas non-IGBC was defined as a tumor suspected or confirmed on preoperative imaging or intraoperative examination of a frozen section.
Surgical approach
Surgical procedures selected for study patients are shown in Table 1. Re-resection, defined as additional resection with regional lymphadenectomy for IGBC, was performed after simple cholecystectomy for pT2 or higher GBC diagnosed on pathological examination of the initial cholecystectomy specimen. The extent of lymphadenectomy in this study included the lymph nodes in the hepatoduodenal ligament, posterosuperior to the pancreas head, and around the common hepatic artery [17, 18], which were designated as regional nodes based on the Japanese TNM classification [19]. For patients with suspected or confirmed pT2 or higher GBC, these regional lymph nodes were generally dissected en bloc during resection with curative intent. For some patients with advanced age and/or comorbid disease, less extensive lymph node dissection was carried out, in which mainly the pericholedochal and cystic duct lymph nodes were removed. Fifty-eight patients suspected or confirmed metastasis to regional nodes underwent sampling or resection of lymph nodes in the paraaortic (mainly interaortocaval) area. No patients received neoadjuvant chemotherapy.
Histological examination
The pathological findings were generally documented based on the eighth edition of the American Joint Committee on Cancer TNM classification [20]. Depth of tumor penetration (pT classification) was determined according to the histological findings of multiple sections in each gallbladder specimen. Nodal status was classified based on the number of positive lymph nodes as follows: pN0, no regional lymph node metastasis; pN1, metastases to one to three regional lymph nodes; or pN2, metastases to four or more regional lymph nodes. For gallbladder specimens resected for presumed benign disease, an initial histological examination was performed for one or two representative sections of each specimen. When IGBC was found in the specimen, the pT classification was determined based on multiple sections of the whole specimen. The dissected lymph nodes were grouped according to their anatomic location. A representative section cut from each of these nodes was evaluated histologically for nodal metastases. Histologically confirmed distant metastasis (pM1) sites included distant nodes (n = 11), the liver (n = 10), distant nodes plus the liver (n = 4), and the liver plus peritoneal dissemination (n = 1).
Definition of RD
The presence of RD was determined based on findings of microscopic involvement of organs and/or structures on histological examination after re-resection. Some patients had involvement of the cystic duct lymph nodes based on histological examination of the initial cholecystectomy specimen, but no RD was found at re-resection; these patients were classified as pN1 but without RD. On the other hand, patients with regional nodal disease after re-resection were staged as pN1 or pN2 with RD. The patients with IGBC were classified into one of three groups based on the anatomic location of RD: no RD (group 1); RD in the gallbladder bed, stump of the cystic duct, and/or regional lymph nodes (group 2); and RD in the extrahepatic bile duct and/or distant sites (group 3). Tumor invasion to the extrahepatic bile duct is a well-known risk factor for poor survival in patients with GBC [21, 22]; therefore, this feature was classified in the same category as RD at distant sites.
Follow-up after definitive resection
All patients who were discharged were followed up at outpatient clinics for at least 5 years at intervals of 1–6 months; the median follow-up period was 107 (range, 0.5–424) months. Grade IIIb or greater complications [23] developed in 17 (8.2%) of the 207 patients in the study. Five patients (2.4%) died during their hospital stay. Adjuvant chemotherapy was administered to 70 patients (33.8%) at the discretion of the attending surgeons.
Statistical analysis
The chi-squared test or Fisher’s exact test was used to compare categorical variables. Deaths from any cause were defined as failure cases in the analysis of overall survival (OS). Survival time was calculated as the interval from definitive resection to the last follow-up or death. OS curves were generated by the Kaplan–Meier method, and differences in survival between groups were evaluated using the log-rank test. The Cox proportional hazards regression model was used to identify independent prognostic factors. For variables entered into this model, the proportional hazards assumption was assessed and verified graphically using the Kaplan–Meier survival estimates. After the clinicopathological data were compared between patients with IGBC and those with non-IGBC, rigorous adjustments for differences in baseline characteristics between these two groups were performed using propensity score matching [24]. Selection of variables for propensity score calculation was guided by factors that were significantly different between the two groups in our study and by conventional clinically relevant variables. The following 15 variables were selected as covariates in the model using multivariate logistic regression analysis for the propensity score calculation: age; sex; presence of gallstones; preoperative jaundice; extent of resection; pT, pN, and pM classification; histological grade and type; lymphatic, venous, and perineural invasion; residual tumor classification; and adjuvant chemotherapy status. Subsequently, a one-to-one match between the two groups was performed using the nearest-neighbor matching method with a caliper width of 0.01. Finally, OS after resection was compared between the two groups using the Kaplan–Meier method before and after propensity score matching. All statistical calculations were performed using SPSS version 22 (IBM Japan, Tokyo, Japan). A p value < 0.05 was considered statistically significant.
Results
For all 207 patients who underwent resection for pT2 or pT3 GBC during the study period, 5-year OS following resection was 51.5% with a median OS of 64.9 months. Of these 207 patients, 36 (17.4%) underwent re-resection for IGBC. Re-resection with regional lymphadenectomy for IGBC included combined resection of the gallbladder bed or the inferior part of Couinaud segment IVb plus segment V and the extrahepatic bile duct in 24 patients, wedge resection of the gallbladder bed in 9, extended right hepatectomy and resection of the extrahepatic duct in 1, regional lymph node dissection alone in 1, and resection of the extrahepatic bile duct in 1 (Table 1). The interval from the initial resection to re-resection ranged from 9 to 172 (median, 59) days. For the remaining 171 patients with non-IGBC, extended cholecystectomy was the most performed surgery as initial resection (Table 1).
Comparison between patients with IGBC and those with non-IGBC
Patients with IGBC were more likely to have gallstones (88.9% vs. 31.6%; p < 0.001) and G1 (well differentiated) tumors (41.7% vs. 23.4%; p = 0.023) and less likely to have preoperative jaundice (5.6% vs. 19.9%; p = 0.026) and pT3 tumors (13.9% vs. 40.9%; p < 0.001) than patients with non-IGBC (Table 2). Patients with IGBC were less likely to undergo extensive resection such as major hepatectomy, pancreaticoduodenectomy, and major hepatectomy combined with pancreaticoduodenectomy (2.8% vs. 17.5%; p < 0.001) (Table 2). There was no significant difference in the incidence of pN1 or pN2 disease (27.8% vs. 36.3 and 8.3% vs. 13.5%, respectively; p = 0.320), pM1 disease (8.3% vs. 19.3%; p = 0.298), or R0 resection (88.9% vs. 95.3%; p = 0.135) between patients with IGBC and those with non-IGBC. Adjuvant chemotherapy was administered in 15 of the 36 patients with IGBC (41.7%) and 78 of the 171 patients with non-IGBC (45.6%) (p = 0.183) (Table 2).
Patients with IGBC had significantly better OS following definitive resection than patients with non-IGBC (5-year OS, 64.3% vs. 48.8%; p = 0.040) (Fig. 1a). For OS, multivariate analysis revealed that age > 70 years (hazard ratio [HR] 2.033, p = 0.001), pT2b tumor (HR 1.978, p = 0.044) and pT3 tumor (HR 3.964, p < 0.001), pN2 disease (HR 10.295, p = 0.002), pM1 disease (HR 3.354, p < 0.001), histological type other than adenocarcinoma (HR 2.313, p = 0.001), and R1 resection (HR 1.971, p = 0.038) were independently associated with adverse outcomes; timing of resection (IGBC vs. non-IGBC) did not remain an independent factor (Table 3). In univariate analysis, OS after resection was significantly better in patients who received adjuvant chemotherapy than in those who did not (5-year OS, 62.0% vs. 45.7%; p = 0.034; Table 3). This effect of adjuvant chemotherapy on OS was also observed for patients with pStage III or higher disease (5-year OS, 68.3% vs. 44.4%; p = 0.028) and for those with pN1/N2 disease (5-year OS, 55.2% vs. 27.5%; p = 0.002).
a Overall survival (OS) following definitive resection for all 207 patients with pT2/pT3 gallbladder cancer according to timing of diagnosis. OS was better in 36 patients with incidental gallbladder cancer (IGBC) than in 171 patients with non-IGBC (5-year OS, 64.3% vs. 48.8%; p = 0.040). b OS following definitive resection in 30 patients with gallbladder cancer after propensity score matching according to timing of diagnosis. No differences in OS were found between 15 patients with IGBC and 15 patients with non-IGBC (5-year OS, 70.7% vs 77.9%; p = 0.242). c OS after radical resection in 131 patients with pT2a or pT2b disease according to timing of diagnosis. For patients with pT2a tumors, there was no difference in OS between 12 patients with IGBC and 25 patients with non-IGBC (5-year OS, 83.3% vs. 82.9%; p = 0.150). For patients with pT2b tumors, there was no difference in OS between 19 patients with IGBC and 75 patients with non-IGBC (5-year OS, 69.9% vs. 57.5%; p = 0.456). d OS following re-resection in 36 patients with IGBC according to anatomic location of residual disease (RD). OS was worse in group 3 than in Group 1 or group 2 (5-year OS, 14.3% vs 88.7%; p < 0.001 and 5-year OS, 14.3% vs 55.6%; p = 0.035, respectively). OS was comparable between group 1 and group 2 (5-year OS, 88.7% vs. 55.6%; p = 0.256). Group 1, patients without RD; group 2, patients with RD in the gallbladder bed, stump of the cystic duct, and/or regional lymph nodes; group 3, patients with RD in the extrahepatic bile duct and/or distant sites
Propensity score matching was also performed to minimize the risk of selection bias and balance the treatment choice-related characteristics of the two groups. Baseline characteristics were comparable between the two matched groups (Table 4). No significant difference in OS following definitive resection was observed between patients with IGBC and those with non-IGBC (5-year OS, 70.7% vs. 77.9%; p = 0.242; Fig. 1b).
Next, subgroup analyses were performed according to pT classification. For patients with pT2a tumor, there was no difference in OS between 12 patients with IGBC and 25 patients with non-IGBC (5-year OS, 83.3% vs. 82.9%; p = 0.150; Fig. 1c). Furthermore, for patients with pT2b tumor, there was no difference in OS between 19 patients with IGBC and 75 patients with non-IGBC (5-year OS, 69.9% vs. 57.5%; p = 0.456; Fig. 1c). For patients with pT3 tumor, both groups had an unfavorable prognosis after radical resection (5-year OS, 0% vs. 28.6%; p = 0.182).
Impact of anatomic location of RD on OS after re-resection in patients with IGBC
RD after initial cholecystectomy was found in 16 (44.4%) of 36 patients with IGBC who underwent re-resection. These 36 patients were then classified according to the anatomic location of RD as follows: no RD (n = 20; group 1); RD in the gallbladder bed, stump of the cystic duct, and/or regional nodes (n = 9; group 2); and RD in the extrahepatic bile duct and/or distant sites (n = 7; group 3). Sites of RD in group 2 included regional nodes alone in 5 patients, the gallbladder bed alone in 2, the stump of the cystic duct alone in 1, and both regional nodes and the stump of the cystic duct in 1. Sites of RD in group 3 included discontinuous liver involvement and regional nodes in 2 patients; the extrahepatic bile duct, the gallbladder bed, and regional nodes in 2; the extrahepatic bile duct alone in 1; distant nodes alone in 1; and both the extrahepatic bile duct and gallbladder bed in 1.
Comparison of clinicopathological characteristics according to RD status in 36 patients with IGBC is shown in Table 5. Patients in group 3 were more likely to have pT3 tumors (p = 0.005) and preoperative jaundice (p = 0.012) than those in group 1 and group 2 (Table 5). The incidence of pN1/2 disease in groups 1, 2, and 3 was 15.0%, 55.6%, and 71.4%, respectively (p = 0.010); the respective R0 resection rates were 100%, 88.9%, and 57.1% (p = 0.008) (Table 5).
Patients with RD had significantly worse OS following re-resection than those without RD (5-year OS, 37.5% vs. 88.7%; p = 0.014). Notably, OS following re-resection was significantly worse in group 3 than in group 2 (5-year OS, 14.3% vs. 55.6%; p = 0.035) or in group 1 (5-year OS, 14.3% vs. 88.7%; p < 0.001) (Fig. 1d). No statistically significant difference in OS was found between group 1 and group 2 (5-year OS, 88.7% vs. 55.6%; p = 0.256) (Fig. 1d). Multivariate analysis revealed that anatomic location of RD (group 2, HR 2.425, p = 0.223; group 3, HR 9.627, p = 0.024) and pT classification (pT2b, HR 9.309, p = 0.048; pT3, HR 13.762, p = 0.010) were independent prognostic factors for OS (Table 6).
Discussion
Although re-resection for IGBC is recommended for selected patients without disseminated disease [1, 2, 5,6,7,8,9,10,11,12], optimal management of IGBC remains a critical issue, purportedly because RD at re-resection for IGBC represents poor outcomes regardless of anatomic location [13]. This multicenter study clearly demonstrates that the anatomic location of RD independently predicts survival following re-resection in patients with IGBC; re-resection may provide survival benefit in patients with RD at locoregional sites excluding the extrahepatic bile duct or distant sites. Our analysis also reveals that timing of diagnosis (IGBC vs. non-IGBC) did not affect survival following definitive resection, indicating that no clear differences in tumor biology exist between IGBC and non-IGBC. Taken together, these findings indicate that re-resection is effective for not only accurate staging but also locoregional disease control in patients with IGBC, like resection in patients with non-IGBC.
Several studies have recently found the presence of RD to be a major determinant of poor survival following re-resection for IGBC and to be associated with higher pT and pN categories [8,9,10,11, 13,14,15,16]. Our results are consistent with those findings. However, this study differs from previous studies in several respects. The present study is the first to classify RD in the extrahepatic bile duct and RD at distant sites in the same category and to identify the clinical significance of classifying the anatomic location of RD in patients with IGBC. Tumor invasion to the extrahepatic bile duct is well recognized as a dismal prognostic factor similar to distant metastasis in patients with GBC [21, 22]. Therefore, our classification of RD based on the anatomic location appears to be clinically plausible and explains the differences in survival observed according to the anatomic location of RD in this study, unlike other studies in which RD in the extrahepatic bile duct was classified as RD at regional sites. Our results indicate that not all patients with RD have poor survival following re-resection for IGBC.
Several clinical studies have focused on clinicopathological features that might contribute to the differences in survival between IGBC and non-IGBC [3, 25,26,27]. Non-IGBC is generally known to have a worse surgical outcome than IGBC, given that patients with non-IGBC tend to be diagnosed in the later stages of the disease [1,2,3,4]. Some studies have reported worse survival in patients with non-IGBC based on subgroup analysis or multivariate analysis and suggested that there may be differences in tumor biology between IGBC and non-IGBC [3, 27]. Our study is consistent with the former hypothesis rendering later diagnosis of non-IGBC, though we found only a univariate association between timing of diagnosis and OS. However, this association did not remain significant in multivariate analysis or on propensity score matching analysis. We believe that a similar treatment strategy is indicated for both IGBC and non-IGBC according to tumor spread.
The latest cancer staging manual published by the American Joint Committee on Cancer subclassifies pT2 disease into pT2a (tumors on the peritoneal side) and pT2b (tumors on the hepatic side) [20]. Several studies have reported that pT2b tumor has a worse prognosis than pT2a tumor because of higher incidences of lymph node metastasis, liver metastasis, vascular invasion, and perineural invasion [18, 28, 29]. Vega et al. [25] reported that patients with T2b IGBC had worse survival after radical resection than their counterparts with T2b non-IGBC and concluded that simple cholecystectomy before radical second resection negatively impacts survival in patients with T2b tumor. Shindoh et al. [28] reported significantly higher incidences of RD in the gallbladder bed, micrometastatic foci in the adjacent hepatic parenchyma, and resected lymph nodes at the time of radical second resection in patients with T2b disease than in those with pT2a disease. This higher incidence of RD at re-resection may partly explain the results reported by Vega et al. [25]. In the present study, however, there was no significant difference in OS between patients with pT2b IGBC and those with pT2b non-IGBC after definitive resection; moreover, the incidence of RD was similar between patients with pT2a IGBC and those with pT2b IGBC (41.7% vs. 31.6%; Table 3). Further study with a greater number of patients with pT2b IGBC is required to investigate the impact of prior cholecystectomy on survival.
With increasing recognition of RD at re-resection as a strong determinant of the prognosis in patients with IGBC, neoadjuvant chemotherapy for patients at high-risk of RD is now a matter of debate [9, 13,14,15]. These patients could be offered neoadjuvant chemotherapy to streamline patient selection for surgery. Some authors have proposed risk stratification for IGBC to predict RD using factors such as pT classification, histologic grade, perineural invasion, and lymphovascular invasion [15]. Although their findings are encouraging, a more robust predictive model for RD would be needed. In the present study, among the pathological factors, pT and pN classifications were associated with having RD. Due to the limitations of currently available preoperative imaging, RD was confirmed by postoperative histological evaluation [13, 15]. Within this context, neoadjuvant chemotherapy currently appears to be a more rational option in patients with IGBC and clinically evident RD on preoperative imaging and in those with several risk factors for RD.
This study has some limitations worth mentioning. First, it had a retrospective design and included a relatively small number of patients with IGBC who were diagnosed and treated over a long period of time. Second, the surgical procedures performed were not consistent between the patients with IGBC and those with non-IGBC, although the indications and choice of surgical procedures were uniform throughout the study period at both institutions. Nevertheless, we believe that the differences in OS between the groups were large enough to suggest that these shortcomings did not have a significant effect on the results. Given that survival remains unsatisfactory in patients with IGBC who have RD, especially those with RD in the extrahepatic bile duct and/or distant sites, these patients appear to be good candidates for adjuvant chemotherapy or chemoradiotherapy [30, 31].
Conclusion
The anatomic location of RD after initial cholecystectomy independently predicts survival following re-resection in patients with IGBC; re-resection may provide survival benefit in patients with RD at locoregional sites excluding the extrahepatic bile duct or distant sites. Not all patients with RD have poor survival following re-resection for IGBC. Timing of diagnosis (IGBC vs. non-IGBC) did not affect survival after definitive resection, indicating that there are no clear differences in tumor biology between IGBC and non-IGBC. Our study underscores the finding that re-resection is effective for not only accurate staging but also locoregional disease control in patients with IGBC.
References
Shirai Y, Yoshida K, Tsukada K, Muto T (1992) Inapparent carcinoma of the gallbladder: an appraisal of a radical second operation after simple cholecystectomy. Ann Surg 215:326–331
Søreide K, Guest RV, Harrison EM , Kendall TJ, Garden OJ, Wigmore SJ (2019) Systemic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg 106:32–45
Ethun CG, Le N, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick BA, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Russell MC, Maithel SK (2017) Pathologic and prognostic implications of incidental versus nonincidental gallbladder cancer: a 10-institution study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg 83:679–686
Cherkassky L, D’Angelica M (2019) Gallbladder cancer: managing the incidental diagnosis. Surg Oncol Clin N Am 28:619–630
Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJF, Jarnagin WR (2015) Gallbladder: expert consensus statement. HPB 17:681–690
Wakai T, Shirai Y, Hatakeyama K (2002) Radical second resection provides survival benefit for patients with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg 26:867–871
Wakai T, Shirai Y, Yokoyama N, Ajioka Y, Watanabe H, Hatakeyama K (2003) Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol 10:447–454
Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Andersson B, Sandström P (2019) Management of incidental gallbladder cancer in a national cohort. Br J Surg 106:1216–1227
de Savornin Lohman EAJ, van der Geest LG, de Bitter TJJ, Nagtegaal ID, van Laarhoven CJHM, van den Boezem P, van der Post CS, de Reuver PR (2020) Re-resection in incidental gallbladder cancer: survival and the incidence of residual disease. Ann Surg Oncol 27:1132–1142
Fuks D, Regimbeau JM, Le Treut YP, Bachellier P, Raventos A, Pruvot FR, Chiche L, Farges O (2011) Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg 35:1887–1897
Butte JM, Waugh E, Meneses M, Parada H, de la Fuente HA (2010) Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol 102:620–625
Goetze TO, Paolucci V (2010) Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 24:2156–2164
Butte JM, Kingham TP, Gönen M, D’Angelica MI, Allen PJ, Fong Y, DeMatteo RP, Jarnagin WR (2014) Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg 219:416–429
Gil L, de Aretxabala X, Lendoire J, Duek F, Hepp J, Imventarza O (2019) Incidental gallbladder cancer: how residual disease affects outcome in two referral HPB centers from South America. World J Surg 43:214–220
Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Jin LX, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Merchant N, Cardona K, Maithel SK (2017) A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol 24:1343–1350
Ramos E, Lluis N, Llado L, Torras J, Busquets J, Rafecas A, Serrano T, Mils K, Leiva D, Fabregat J (2020) Prognostic value and risk stratification of residual disease in patients with incidental gallbladder cancer. World J Surg Oncol 18:18
Sakata J, Kobayashi T, Tajima Y, Ohashi T, Hirose Y, Takano K, Takizawa K, Miura K, Wakai T (2017) Relevance of dissection of the posterior superior pancreaticoduodenal lymph nodes in gallbladder carcinoma. Ann Surg Oncol 24:2474–2481
Toge K, Sakata J, Hirose Y, Yuza K, Ando T, Soma D, Katada T, Miura K, Takizawa K, Kobayashi T, Wakai T (2019) Lymphatic spread of T2 gallbladder carcinoma: regional lymphadenectomy is required independent of tumor location. Eur J Surg Oncol 45:1446–1452
Japanese Society of Hepato-Biliary-Pancreatic Surgery (2013) General rules for clinical and pathological studies on cancer of the biliary tract, 6th edn. Kanehara & Co., Ltd, Tokyo
Zhu AX, Pawlik TM, Kooby DA, Schefter TE, Vauthey JN (2017) Gallbladder. In: AJCC cancer staging manual, 8th edn. Springer, New York, pp 303–309
Ishihara S, Horiguchi A, Miyakawa S, Endo I, Miyazaki M, Takada T (2016) Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci 23:149–157
Birnbaum DJ, Viganò L, Ferrero A, Langella S, Russolillo N, Capussotti L (2014) Locally advanced gallbladder cancer: which patients benefit from resection? Eur J Surg Oncol 40:1008–1015
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, Berry MF, Schrag D, Pang HH (2017) Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 109:djw323
Vega EA, Vinuela E, Okuno M, Joechle K, Sanhueza M, Diaz C, Jarufe N, Martinez J, Troncoso A, Diaz A, Chun YS, Tzeng CWD, Lee JE, Vauthey JN, Conrad C (2019) Incidental versus non-incidental gallbladder cancer: index cholecystectomy before oncologic re-resection negatively impacts survival in T2b tumors. HPB 21:1046–1056
Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L (2007) Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 11:1478–1487
Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA (2007) Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 245:893–901
Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, Javle M, Conrad C, Maru DM, Aoki T, Vigano L, Ribero D, Kokudo N, Capussotti L, Vauthey JN (2015) Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg 261:733–739
Lee W, Jeong CY, Jang JY, Kim YH, Roh YH, Kim KW, Kang SH, Yoon MH, Seo HI, Yun SP, Park JI, Jung BH, Shin DH, Choi YI, Moon HH, Chu CW, Ryu JH, Yang K, Park YM, Hong SC (2017) Do hepatic-sided tumors require more extensive resection than peritoneal-sided tumors in patients with T2 gallbladder cancer? Results of a retrospective multicenter study. Surgery 162:515–524
Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J, Primrose JN, Fox RP, Morement H, Chan O, Rees C, Ma YT, Hickish T, Falk S, Finch-Jones M, Pope I, Corrie P, Crosby T, Sothi S, Sharkland K, Adamson D, Wall L, Evans J, Dent J, Hombaiah U, Iwuji C, Anthoney A, Bridgewater J, Cunningham D, Gillmore R, Ross P, Slater S, Wasan H, Waters J, Valle JW, Palmer D, Malik H, Neoptolemos J, Faluyi O, Sumpter K, Dernedde U, Maduhusudan S, Cogill G, Archer C, Iveson T, Wadsley J, Darby S, Peterson M, Mukhtar AA, Thorpe JG, Bateman A, Tsang D, Cummins S, Nolan L, Beaumont E, Prasad R, Mirza D, Stocken D, Praseedom R, Davidson B, Raftery J, Zhu S, Garden J, Stubbs C, Coxon F (2019) Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 20:663–673
Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, el-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK (2019) Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol 37:1015–1027
Author information
Authors and Affiliations
Contributions
Study conception and design: Ando T and Sakata J; acquisition of data: Ando T, Sakata J, Hirose Y, Takano K, Takizawa K, and Miura K; analysis and interpretation of data: Ando T, Sakata J, Kobayashi T, Ichikawa H, Hanyu T, Shimada Y, and Nagahashi M; drafting of manuscript: Ando T and Sakata J; critical revision of manuscript: Nomura T, Miura K, Takizawa K, Kobayashi T, Ichikawa H, Shimada Y, Nagahashi M, Kosugi SI, and Wakai T
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the institutional review boards of Niigata University and Niigata Cancer Center Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration.
Informed consent
The need for informed consent was waived due to the retrospective observational nature of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ando, T., Sakata, J., Nomura, T. et al. Anatomic location of residual disease after initial cholecystectomy independently determines outcomes after re-resection for incidental gallbladder cancer. Langenbecks Arch Surg 406, 1521–1532 (2021). https://doi.org/10.1007/s00423-021-02165-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02165-1