Abstract
Purpose
Data evaluating the risk of lymph node metastasis depending upon the location of the primary tumor are limited in patients with T1 colorectal cancer. We aimed to evaluate the impact of tumor location on lymph node metastasis in T1 colorectal cancer.
Methods
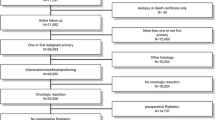
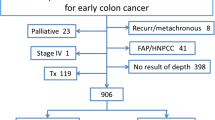
Patients who underwent an oncologic resection with curative intent for T1 adenocarcinoma of the colon and rectum between January 1997 and October 2014 were assessed. Exclusion criteria were distant organ metastases, previous or concurrent cancer, past history of surgical or medical cancer treatment, preoperative chemoradiation, and patients with inflammatory bowel disease or polyposis syndromes.
Results
Out of 232 (56 % male) patients fulfilling the study criteria, 24 (10 %) had lymph node metastasis. Age (65 vs 61 years, p = 0.1), gender (55 vs 63 % male, p = 0.5), tumor size (2 vs 2 cm, p = 0.49), and lymphovascular invasion (5 vs 8 %, p = 0.46) were not associated with lymph node metastasis. While there was no statistical significance (p = 0.2), lymph node positivity was higher in rectal cancer (14 %, n = 11/79) compared to colon cancer (9 %, n = 13/153).
Conclusions
Although it was not statistically significant, lymph node positivity varies based on tumor location of T1 colorectal adenocarcinoma regardless of fundamental tumor characteristics including size, differentiation, and lymphovascular invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early colorectal cancer is defined as carcinoma that invades only into the submucosal layer [1, 2]. Local excision can be performed for T1 colorectal cancer selectively [3]. However, lymph node metastasis in T1 colorectal cancer can be seen up to 10 % [1, 4]. Probability of lymph node involvement is considered in the management since the status of lymph nodes is the main predictive factor survival for colorectal cancer [5]. Lymph node involvement directly changes the Union for International Cancer Control (UICC) stage, treatment strategy including operative approach, chemo, and radiotherapy [6].
Lymph node metastasis is associated with depth of invasion, poor differentiation of tumor, lymphovascular invasion, tumor budding, and low rectal tumor location [1, 6, 7]. Relation between T1 colorectal cancer and lymph node metastasis has not been well-evaluated. Knowledge of lymph node metastasis rates in T1 adenocarcinoma and identification of risk factors associated with lymph node involvement in different parts of the colon and rectum may guide physicians for planning appropriate therapy. This study was planned to evaluate the impact of tumor location on lymph node metastasis in T1 colorectal cancer.
Materials and methods
After obtaining the institutional review board (IRB) approval, patients who underwent an oncologic resection with curative intent for T1 adenocarcinoma of the colon and rectum between January 1997 and October 2014 at a single institution were evaluated. Exclusion criteria were distant organ metastases, previous or concurrent cancer, past history of surgical or medical cancer treatment, preoperative chemoradiation, and patients with inflammatory bowel disease or polyposis syndromes. The data were retrieved from the prospective IRB-approved institutional databases and supplemented by direct chart review if required.
Patients’ demographics (age and gender), tumor characteristics including location, grade, lymphovascular invasion, and differentiation associated with the finding of lymph nodal involvement for patients who underwent radical resection were evaluated.
Association between location of tumor in the colon or rectum and lymph node metastasis was analyzed using the chi square or Fisher exact tests for categorical and the Wilcoxon rank sum test for continuous measures. Quantitative variables were reported as mean ± standard deviation. Categorical variables were reported as numbers and percentages. All tests were performed at a significance level of 0.05.
Results
Overall evaluation
There were 232 patients (130 (56 %) males) with T1 colon and rectal cancer. Their mean age was 64.7 ± 12.7 years at the time of surgery. Twenty-four (10 %) patients had lymph node metastasis. Age (p = 0.10) and gender (p = 0.50) had no impact on lymph node involvement (Table 1). While risk of lymph node metastasis was similar based on different age groups (p = 0.67), lymph node involvement rate was remarkably low compared to other age groups over age of 80 (Table 2). When we classify the patients as elder (>65 years old; 9 out of 122, 7.4 %) versus younger (≤65 years old; 15 out of 110, 13.6 %), there was no significant difference (p = 0.12). Tumor differentiation, harvested lymph node numbers, lymphovascular invasion, tumor size (Table 1), and tumor location (Table 3) were not associated with lymph node metastasis.
Characteristics of T1 colon cancer
The primary tumor was located in the colon in 153 patients (66 %), who had a mean age of 66.6 ± 12.5 years. Sixty-five (43 %) patients were female with a mean age of 66.1 ± 14.9 years and 88 (57 %) male patients with a mean age of 67.0 ± 10.4 years. The mean tumor size was 2.0 ± 1.7 cm. Colonic cancer was located in the sigmoid colon in 59 (39 %) patients, in the descending colon in 11 (7 %) patients, at the splenic flexure in 3 (2 %) patients, in the transverse colon in 6 (4 %) patients, at the hepatic flexure in 11 (7 %), in the ascending colon in 20 (13 %) patients, and in the cecum in 43 (28 %) patients. The histological type was well-differentiated adenocarcinoma in 35 (23 %) patients, moderately differentiated adenocarcinoma in 104 (68 %) patients, and poorly differentiated adenocarcinoma in 14 (9 %) patients. Thirteen out of 153 (9 %) patients had lymph node metastasis. Age (66.8 ± 12.7 versus 64.3 ± 9.5 years, p = 0.31), gender (f/m (n): 61/79 versus 4/9, p = 0.37), differentiation (moderate/poor/well (n): 95/13/32 versus 9/1/3, p = 0.98), lymphovascular invasion (n = 9 (6 %) versus n = 2 (15 %), p = 0.23), tumor size (2.0 ± 1.7 versus 1.6 ± 1.1 cm, p = 0.67), and number of examined lymph node number (26.3 ± 16.9 versus 23.1 ± 11.8 years, p = 0.58) were not significantly different between the lymph node negative and positive groups, respectively.
Characteristics of T1 rectal cancer
The primary tumor was located in the rectum in 79 (34 %) patients; mean age of these patients was 61.3 ± 12.4 years. There were 37 (47 %) female patients with a mean age of 59.8 ± 12.3 years and 42 (53 %) male patients with a mean age of 62.3 ± 12.6 years. The mean tumor size was 2.5 ± 1.8 cm. Twenty-two (28 %) patients had in the upper rectum (>10 cm from the anal verge), 37 (47 %) patients in the mid rectum (6–10 cm from the anal verge), and 20 (25 %) in the lower rectum (≤5 cm from the anal verge). The histological type was well-differentiated adenocarcinoma in 16 (20 %) patients, moderately differentiated adenocarcinoma in 52 (66 %) patients, and poorly differentiated adenocarcinoma in 11 (14 %) patients. Eleven out of 79 (14 %) patients had lymph node metastasis. Age (61.7 ± 12.04 versus 57.6 ± 14.8 years, p = 0.23), gender (f/m (n): 32/36 versus 5/6, p = 0.92), differentiation (moderate/poor/well (n): 45/9/14 versus 7/2/2, p = 0.91), distance from the anal verge (7.8 ± 4.4 versus 9.1 ± 4.0 cm, p = 0.36), lymphovascular invasion (n = 1 (2 %) versus 0, p > 0.99), tumor size (2.3 ± 1.7 versus 3.1 ± 2.0 cm, p = 0.24), and number of examined lymph node number (24.7 ± 15.5 versus 20.5 ± 7.4, p = 0.65) were similar regardless of lymph node metastasis between the lymph node negative and positive groups, respectively.
Comparison of patient tumor characteristics in patients with colonic versus rectal T1cancer
The patients with T1 colon cancer were older than the patients with T1 rectal cancer (66.6 ± 12.5 versus 61.1 ± 12.4, p = 0.001). Although it was not significant, lymphovascular invasion was more frequent in colonic T1 cancer (n = 1 (1 %) versus n = 11 (7 %), p = 0.06). Rectal T1 cancers were larger than colonic T1 cancers (2.5 ± 1.8 versus 2 ± 1.7 cm, p = 0.01). Numbers of examined lymph nodes (26.1 ± 16.5 versus 24.1 ± 14.7, p = 0.42), gender (f/m (n): 65/88 versus 37/42, p = 0.53), and tumor differentiation (moderate/poor/well (n): 104/14/35 versus 52/11/16, p = 0.52) were comparable in the colon and rectum. While there was no statistical significance (p = 0.20), lymph node positivity was higher in rectal cancer (14 %, n = 11/79) compared to colon cancer (9 %, n = 13/153). Characteristics of T1 colon and rectal cancers with lymph node metastasis are presented in Table 4.
Impact of lymph node positivity on survival in patients with T1 colorectal cancer
Nine patients (four of them were lymph node positive patients) had recurrent disease and among them only one patient (rectal cancer with no lymph metastasis) had local recurrence in 5.3 years (range 2–17 years) median follow up after surgery. Overall and recurrence free survival was similar regardless of having lymph node metastasis in patients who underwent an oncologic resection with curative intent for T1 adenocarcinoma of the colon and rectum (Figs. 1 and 2).
Discussion
Progression of colorectal cancer is expected to vary upon the tumor location due to anatomical differences. The ability to predict the risk of lymph node metastasis in the particular patient allows for a better selection of the therapeutic approach. Our results confirm that around 10 % of T1 colorectal cancer cases have stage III disease. While lymph node metastases for patients with T1 colorectal cancer are not related to a specific site in the colon and rectum, the distribution of metastatic lymph nodes was remarkable. There was no metastatic lymph node involvement related to tumors of the hepatic flexure, transverse colon, and splenic flexure. It was previously suggested that colon cancer located in the transverse and descending colon is associated with a poor prognosis [8]. A recent Japanese multi-institutional, cross-sectional study including 806 T1 colorectal cancer patients showed metastatic lymph nodes in cancers originating from the transverse colon [9]. However, they performed an overall analysis among all T1 colorectal cancers without applying any exclusion criteria which may affect clinical approach beyond controversy. We intentionally eliminated the patients with distant organ metastases, previous or concurrent cancer, past history of surgical or medical cancer treatment, patients who underwent preoperative chemoradiation, and patients with inflammatory bowel disease or polyposis syndromes. In a larger patient population, identifying patients with lymph node metastasis would be possible. However, it is difficult to estimate impact of large patient population on risk of lymph node metastasis which is also related with other factors including tumor biology, anatomical variability, etc. in each individual. We believe our study methodology is more relevant to clinical practice regarding decision making whether to offer radical surgery or colonoscopic surveillance after local excision. In current clinical practice, detection of lymph node metastasis and local excision potential is more of a realistic possibility for cancers of the rectum compared to colon. While oncologic safety of endoscopic excision for colon tumors is controversial, there are some techniques used for local excision of the colonic lesions. Endoscopic submucosal dissection, endoscopic mucosal resection, combined endoscopic, and laparoscopic surgery are the emerging techniques for local colonic resection. We believe staging of colon cancer will be also more important with the refinement of the local colonic resection techniques. Alterations in arterial, venous, and lymphatic architecture between the segments of the colon and rectum can influence cancer spread depending on tumor location [10]. Our patients with sigmoid colon, upper, and mid rectal tumors had increased risk of lymph node metastasis. A higher frequency of lymph node metastasis was observed with T1 rectal when compared with colon cancer. While several studies have reported a higher risk of nodal metastasis for T1 rectal cancer compared to colon cancer, this did not reach statistical significance in most series [6, 11]. The Mayo clinic study reported a high possibility of lymph node metastasis in T1 rectal cancer located in the lower rectum compared with the other levels of the rectum [1].
Identification of lymph node metastasis is crucial, since the management strategy and life expectancy differs between the stage I and stage III T1 colorectal cancer. The 5-year postoperative overall survival rate of patients with T1 colon cancer was more than 90 %, even if nodes were involved, and the prognosis after radical resection for T1 colon cancer did not differ between patients with and without lymph node metastasis in some published series [12]. Therefore, some authors offer surgery with lymph node dissection for patients with T1 colorectal cancer with a risk of lymph node metastasis [12]. A recent study from the Surveillance, Epidemiology, and End Results (SEER) database showed that local excision for early colorectal cancer seems oncologically equivalent to major surgery for carcinoma in situ and T1 rectal cancer, while it is inferior for T1-2 colon and T2 rectal cancer [13]. Local excisions are preferred in special cases due to their recovery and quality of life benefits over radical resections [14]. Current surgical technology allows us to remove colorectal tumors endoscopically [15, 16]. Based on our study data, T1 colon cancer affects older patients and has smaller sizes compared to T1 rectal cancer and overall risk of lymph node metastasis is lowest in patients older than 80 years. Elderly patients who cannot tolerate major abdominal surgery are the potential candidates for a local excision [17]. The T1 cancers with lymph node metastasis in the colon and rectum had similar characteristics including tumor size, tumor differentiation, and lymphovascular invasion. Lymphovascular invasion has been suggested as an independent predictor of poor survival in patients with colorectal cancer [18]. Although this value did not reach statistical significance in our patients, a type II error may exist due to the small numbers of patients in the subgroups. While lymphovascular invasion, tumor differentiation, tumor budding, and low rectal tumors have been suggested as predictors of lymph node metastasis in early colorectal cancer by various authors [1, 12, 19, 20]. Kikuchi et al. reported that lymphovascular invasion, diameter and histologic grade of adenocarcinoma are not predictors of lymph node metastasis in T1 colorectal carcinoma [21].
This study has some drawbacks which are mostly related its retrospective nature. Higher patient population would clarify the relations between tumor localization and lymph node metastasis. Since recruiting larger patient population may take years, multi-institutional or nationwide studies with similar design may increase power of further studies. We did not have data regarding invasion depth in sub-mucosa. Deep submucosal invasion and tumor budding are associated with increased risk of lymph node metastasis in early colorectal cancer [9, 21, 22]. Matrilysin and DcR3 expression are the other markers that predict nodal metastasis of colorectal cancer [23]. We did not evaluate these biomarkers since our study data was recruited from routinely used pathological reports. Another ongoing debate especially for T1-T2 rectal cancer is other therapeutic strategies including chemoradiotherapy and watchful waiting versus chemoradiotherapy and surgery. Current setting of the study limits us to mention about this topic, since we excluded the patients with preoperative chemoradiation from the study population.
In series including all stages of colorectal cancer, old age and male gender were considered as adverse factors for survival [24]. However, lymph node metastasis did not effect survival after radical oncologic surgery in patients with T1 colorectal cancer in our and some other studies [12]. While male gender is predominant in patients with lymph node metastasis in our series, the risk of positive lymph nodes for each gender was equal. It was nine out of 102 (11.3 %) for females and 15 out of 130 (8.7 %) patients in males.
Our series is one of the largest series, from a single institution. While there was no statistically significant association between tumor location and lymph node metastasis in T1 colorectal cancer, our study shows that lymph node positivity differs based on tumor location of T1 colorectal adenocarcinoma regardless of fundamental tumor characteristics including size, differentiation, and lymphovascular invasion. Absence of metastatic lymph nodes among the tumors located in the hepatic flexure, transverse colon, and splenic flexure in more than 200 patients is a notable finding. Individualized decision making by considering tumor- and patient-related characteristics may be required whether to perform local excision or radical surgery for T1 colorectal cancer. Radical oncologic resection for T1 colorectal cancer may provide acceptable long-term control in presence of lymph node involvement. Routinely used pathological and clinical parameters seem not to be sufficient to predict the presence of lymph node metastasis and therefore, new diagnostic approaches (e.g., evaluation of invasion depth and immunohistochemistry) need to be involved in to the routine clinical practice extensively.
References
Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR (2002) Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 45:200–206
Morson BC, Bussey HJ (1970) Predisposing causes of intestinal cancer. Curr Probl Surg 1–46
Tanaka S, Haruma K, Teixeira CR et al (1995) Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol 30:710–717
Sakuragi M, Togashi K, Konishi F et al (2003) Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum 46:1626–1632
Wong SL (2009) Lymph node counts and survival rates after resection for colon and rectal cancer. Gastrointest Cancer Res 3:S33–S35
Okuyama T, Oya M, Ishikawa H (2002) Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis Colon Rectum 45:628–634
Rasheed S, Bowley DM, Aziz O et al (2008) Can depth of tumour invasion predict lymph node positivity in patients undergoing resection for early rectal cancer? A comparative study between T1 and T2 cancers. Color Dis 10:231–238
Sjo OH, Lunde OC, Nygaard K et al (2008) Tumour location is a prognostic factor for survival in colonic cancer patients. Color Dis 10:33–40
Kawachi H, Eishi Y, Ueno H et al (2015) A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol 28:872–879
Khan S, Goh V, Tam E et al (2012) Perfusion CT assessment of the colon and rectum: feasibility of quantification of bowel wall perfusion and vascularization. Eur J Radiol 81:821–824
Kitajima K, Fujimori T, Fujii S et al (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39:534–543
Kobayashi H, Mochizuki H, Morita T et al (2011) Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol 46:203–211
Bhangu A, Brown G, Nicholls RJ et al (2013) Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg 258:563–565
Doornebosch PG, Gosselink MP, Neijenhuis PA et al (2008) Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Color Dis 23:709–713
Lee SW, Garrett KA, Shin JH et al (2013) Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum 56:869–873
Gorgun IE, Aytac E, Costedio MM et al (2014) Transanal endoscopic surgery using a single access port: a practical tool in the surgeon’s toybox. Surg Endosc 28:1034–1038
van den Boezem PB, Kruyt PM, Stommel MW et al (2011) Transanal single-port surgery for the resection of large polyps. Dig Surg 28:412–416
Lim SB, Yu CS, Jang SJ et al (2010) Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum 53:377–384
Minsky BD, Mies C, Rich TA et al (1989) Lymphatic vessel invasion is an independent prognostic factor for survival in colorectal cancer. Int J Radiat Oncol Biol Phys 17:311–318
Wada H, Shiozawa M, Katayama K et al (2015) Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol 2015 [Epub ahead of print]
Kikuchi R, Takano M, Takagi K et al (1995) Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 38:1286–1295
Akagi Y, Adachi Y, Kinugasa T et al (2013) Lymph node evaluation and survival in colorectal cancer: review of population-based, prospective studies. Anticancer Res 33:2839–2847
Zong L, Chen P, Wang DX (2014) Death decoy receptor overexpression and increased malignancy risk in colorectal cancer. World J Gastroenterol 21(20):4440–4445
Storli KE, Søndenaa K, Bukholm IR, Nesvik I, Bru T, Furnes B, Hjelmeland B, Iversen KB, Eide GE (2011) Overall survival after resection for colon cancer in a national cohort study was adversely affected by TNM stage, lymph node ratio, gender, and old age. Int J Color Dis 26:1299–1307
Acknowledgments
The study was a poster presentation at the American Society of Colon and Rectal Surgeons Annual Meeting, May 30–June 3, 2015, Boston, MA. This study was supported by the Ed and Joey Story Endowed Chair in Colorectal Surgery. Erman Aytac is an assistant professor of surgery at the Acibadem University School of Medicine in Istanbul, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aytac, E., Gorgun, E., Costedio, M.M. et al. Impact of tumor location on lymph node metastasis in T1 colorectal cancer. Langenbecks Arch Surg 401, 627–632 (2016). https://doi.org/10.1007/s00423-016-1452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1452-x