Abstract
Background
The purpose of the present study is to assess the value of the LigaSure™ Vessel Sealing System (LVSS) as a means for bowel transection and intestinal anastomosis.
Methods
We compared the LVSS for (1) transecting bowel and (2) creation of an intestinal anastomosis with standard methods such as stapler (S) and hand-sewn (HS) in a porcine model. For each study arm, i.e., bowel transection and anastomosis creation, both the small bowel and colon were examined. In total, ten transections and ten anastomoses were performed for each. Burst and anastomotic leak pressures were compared.
Results
In the study arm 1, LVSS achieved lowest burst pressures in both small bowel (LVSS 39.8 ± 3.6 mmHg, S 81.9 ± 3.9, HS 111.9 ± 14.7 mmHg, p < 0.0001) and colon transections (LVSS 21.5 ± 2.6 mmHg, S 79.5 ± 4.9, HS 91.0 ± 5.2 mmHg, p < 0.0001). There was no difference in burst pressures between S and HS in both small bowel and colon transections. In the study arm 2, LVSS showed the lowest anastomotic leak pressures for small bowel (LVSS 26.4 ± 2.6 mmHg, S 52.1 ± 6.2, HS 87.4 ± 7.0 mmHg, p < 0.0001) and colonic anastomoses (LVSS 16.9 ± 1.3 mmHg, S 55.9 ± 4.3, HS 74.4 ± 4.4 mmHg, p < 0.0001). Furthermore, small bowel and colonic anastomoses using S demonstrated significantly lower leak pressures than HS anastomosis p < 0.001 and p = 0.004, respectively.
Conclusions
The LVSS achieves significantly lower burst pressures and anastomotic leak pressures for bowel transection and intestinal anastomosis than S and HS techniques. However, due to the achieved pressure levels of 39.8 ± 3.6 mmHg, LVSS appears to be a sufficient stand-alone method for bowel transection. Whether it can be used to perform intestinal anastomosis warrants further research in a survival model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal anastomoses are an essential part of many operations. The two standard methods of intestinal anastomoses are the stapler (S) and hand-sewn (HS) technique. Both methods achieve comparable outcomes regarding anastomotic leaks and stenoses [1–5]. The main disadvantage of the HS anastomoses is the required time to perform it, especially in the case of a double-layered anastomosis. In contrast, the S anastomosis has higher expenses due to the price of the cartridges. In both methods, the anastomotic leakage rates range between 3 and 6.4 % for colonic anastomosis and jejunojejunostomy in Roux-Y gastric bypass surgery [6–9].
The LigaSure™ Vessel Sealing System (LVSS) is commonly used for tissue preparation due to its safe and reliable coagulation of tissue resulting in reduced blood loss and has been successfully tested for hepatic, esophageal, and proctologic surgery in recent years [10–13]. Due to its properties regarding reliable tissue coagulation and sealing, the LVSS may have benefits such as a high reproducibility as well as a fast method to seal the bowel endings for creation of intestinal anastomoses compared to the thus far widely used standard methods. Furthermore, since it can also be used for other steps of the operation, it may be more cost-efficient than the standard methods. The purpose of this study was to test the feasibility and stability of bowel transection and creation of an intestinal anastomosis performed by LVSS in a porcine model. This basic research is necessary before introducing a new technique for bowel transection and to create anastomosis in patients.
Materials and methods
Study design
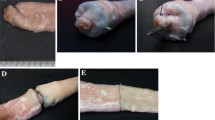
The study consisted of two arms: (1) bowel transection and (2) creation of an intestinal anastomosis. In each study arm, three techniques (LVSS, S, HS) were compared against each other in both small bowel and colon. In study arm 1, ten bowel transections with each technique for small bowel and colon were performed in ten different pigs (Fig. 1a). In study arm 2, end-to-end small bowel and colonic anastomoses were created after transection. Ten anastomoses with each technique for small bowel and colon were performed in ten different pigs (Fig. 1b).
The LigaSure Atlas™ device (Medtronic and Covidien, Neustadt/Donau, Germany) was used for the LVSS transection and LVSS anastomosis. For the transection, the bowel was divided using the LVSS while for the LVSS anastomosis, the bowel endings were attached together for an end-to-end anastomosis using the LVSS. For that, the whole bowl walls of both ends were everted and the submucosae of the joining bowl wall endings were placed against each other. The everted bowl walls were then taken with the LigaSure jaws on the serosal side and coagulated. Both the submucosa and the serosa were coagulated with the LVSS. No reinforcement with an additional suture line was performed. The LigaSure was applied using a LigaSure-8 generator (LS-8; Medtronic and Covidien, Neustadt/Donau, Germany) with the automatically set power setting at 2 bars. The voltage output of the LigaSure device depends on the impedance of the tissue which is within the jaws of the instrument connected to the generator. The LS-8 has an instant response technology (IRT) which measures the tissue impedance starting from the closure of the device and 200 times per second over the whole sealing cycle. Based on these measurements, an algorithm within the generator will adjust the output based on the changes of the tissue impedance. The output of the generator can vary within the sealing cycle between 0 and 150 W.
An Endo GIA-45 white cartridge 2.5 mm (Medtronic and Covidien, Neustadt/Donau, Germany) was used both for the bowel transection and for the S anastomosis using standard techniques. Since we used an end-to-end anastomosis for the LVSS arm of the study, we wished to compare it with a stapled end-to-end anastomosis according to Ravitch and Steichen [14]. In short, the two bowel ends are held in place by two holding sutures. First, the backwall of the anastomosis is stapled with a linear stapler. In the next step, the front wall of the anastomosis is stapled. As in the LVSS technique, the suture line is not reinforced.
Regarding the HS arms of the study, a two-layer running HS using PDS 5-0 (Ethicon, Norderstedt, Germany) for small bowel and PDS 4-0 (Ethicon, Norderstedt, Germany) for colon, respectively, was performed for bowel stump closure after transection. For HS anastomoses, a standard two-layer running end-to-end anastomosis was used after bowel transection using a scalpel.
The burst and anastomotic leak pressures of each method were measured using pressure manometry. A purse string suture was placed, and a hole in the bowl wall was made within the preformed suture. The pressure measuring rod was placed in the bowl lumen (double lumen, 8-French rod, type MMS5702) and held in place with the closed purse string suture (Fig. 2). Through the rod, isotonic sodium chloride solution (0.9 %) was pumped into the bowl by the manometry device using a four-roll pump (MMS Solar GI System and pump and infused volume transducer module). The intraluminal pressure was measured simultaneously. The pressures were recorded by a computer with the appropriate software (MMS Database, Version 8.7g, July 12, 2007, Build 1577) and plotted on a graph. The whole pressure manometry set-up including the pump was provided by MMS Germany GmbH. The pressure manometry system is a widely used standard device commonly used in clinical practice. It is used for anal and esophageal manometry as well as urodynamic measurements such as bladder pressure. The device can be used for both urodynamic system and manometry system. Abrupt decrease in pressure indicated leakage.
Animal preparation, anesthesia, and surgical procedure
The study was approved by the German Committee on Animal Care, Regierungspräsidium Karlsruhe, and the medical faculty ethics committee at University of Heidelberg (approval code 35-9185.81/G-91/08).
All animals received humane care in compliance with the national research council’s criteria for humane care, as outlined in the guide for the care and use of laboratory animals prepared by the National Institute of Health (NIH Publication 86-23, revised 1985).
In order to minimize bowel content, the pigs were fed with yogurt 3 days prior to surgery. All pigs were fasted 12 h before surgery with free access to water. The anesthesia was carried out as previously published [15]. After the experiments, the animals were sacrificed with a central venous injection of potassium chloride (2 mmol/kg) in deep anesthesia.
All surgeries were performed by the same surgeons. The pigs were put in supine position. Because of the final character of the experiments, surgeries were not performed under sterile conditions. The abdomen was opened using a midline incision. Adhesions were removed when existent, and the entire intestine was examined.
For study arm 1, a15-cm-long section of small bowel was selected and isolated from the mesentery using a bipolar device. Then, the small bowel was ligated at the oral side and transected at the aboral side according to the above-described techniques (LVSS, S, HS). A purse string suture was created with a 3-0 Prolene thread in the middle of the bowel segment in order to place the manometric tube. The purse string suture was closed using a Kocher’s clamp and the manometry was performed. The colon transection was similarly performed, but a 10-cm bowel segment was used due to the shorter length of the colon instead.
For study arm 2, small bowel and colon were prepared the same way as in study arm 1. An end-to-end small bowel or colonic anastomosis was created using the previously described methods (LVSS, S, HS; Fig. 2). Similar to the first part of the study, pressure manometry was used to measure anastomotic leak pressures.
Statistical analysis
Data are presented using box plots showing mean, median, 25th and 75th percentile, 95 % confidence interval, and outliers. In the text, data are represented with mean ± standard error of the mean (SEM). Groups were compared against each other using an analysis of variance (ANOVA) followed by a Bonferroni correction to adjust for multiple group comparisons. Statistical significance was set at p < 0.05. SPSS PAWS 18.0 statistics package (Chicago, IL, USA) was used for the statistical analysis. Graphs were created using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA).
Results
Study arm 1
Small bowel transection
An overview of the results is shown in Fig. 3a. Small bowel transection using the LVSS resulted in the lowest burst pressures (39.8 ± 3.6 mmHg) whereas the HS group achieved the highest burst pressures (111.9 ± 14.7 mmHg). The S group resulted in a intermediate burst pressure of 81.9 ± 3.9 mmHg which was not only significantly higher than the LVSS alone (p < 0.0001) but also significantly lower than the double-layered HS small bowel transection (p = 0.033). Burst pressures after LVSS bowel transection were significantly lower compared to both other methods (p < 0.001). None of the different methods used for small bowel transection was primarily leaky.
a Burst pressures after small bowel and colon transection for the different surgical techniques. b Anastomotic leak pressures after small bowel and colonic anastomosis for the different surgical techniques. *Compares LVSS against all other methods, †Compares stapler anastomosis with all other methods. LVSS LigaSure™ Vessel Sealing System, S stapler, HS hand-sewn
Colon transection
Figure 3a outlines the results of the burst pressures using the different methods for colon transection. Similarly to the small bowel, LVSS had the lowest burst pressure (21.5 ± 2.6 mmHg) which was significantly lower than all the other methods (p < 0.001) whereas HS demonstrated highest stability (91.0 ± 5.2 mmHg). S transection achieved again not only significantly higher burst pressures than the LVSS alone (79.5 ± 15.4 mmHg, p < 0.0001), but also significantly lower burst pressures than the double-layered hand-sewn colon transection (p = 0.008). None of the different methods used for colon transection resulted in a primary leak.
Study arm 2
Small bowel anastomotic leak pressure
With regard to small bowel anastomosis, LVSS (26.4 ± 2.6 mmHg) demonstrated significantly lower leak pressures than S (52.1 ± 6.2 mmHg, p = 0.01) and HS anastomosis (87.4 ± 7.0 mmHg, p < 0.001), respectively (Fig. 3b). Furthermore, small bowel anastomosis using S demonstrated significantly lower leak pressures than HS anastomosis (p < 0.001). All of the small bowel anastomoses were primarily tight without signs of a leak.
Colonic anastomotic leak pressure
Leak pressure rates for colonic anastomosis yielded similar results as for small bowel (Fig. 3b). Using the LVSS for colonic anastomosis resulted in lowest leak pressures (16.9 ± 1.3 mmHg) compared to S (55.9 ± 4.3 mmHg, p < 0.001) and HS anastomosis (74.4 ± 4.4 mmHg, p < 0.001). In addition, S anastomosis had significantly lower leak pressures than HS (p = 0.004). All of the colonic anastomoses were primarily tight without signs of a leak.
Discussion
We found in this study that both bowel transection and intestinal anastomosis using the LigaSure™ Vessel Sealing System (LVSS) can be performed with no primary leaks. The potential benefits of using the LVSS are likely a reduction in operative time due to its fast-sealing technique as it has been shown for other procedures [16, 17]. Furthermore, the use of the LVSS may be cost-effective due to the reduction in use of staplers and reduced operative time [18–20]. However, LVSS achieved the lowest burst and leakage rates whereas S and HS demonstrated significantly higher burst and leak pressures. Although these differences were all statistically highly significant, the burst and leak pressures should be related to the actual intraluminal pressure that such a transection must withstand. Resting intraluminal pressures in small bowel and colon have been reported around 0 to 7 mmHg [21–23]. During intestinal pressure waves, the pressure may rise up to maximum levels of 50 mmHg in the physiological setting [21, 23]. However, after surgery, bowel movements are highly reduced and it is unlikely that such high pressures as in physiological situations will be achieved. Since the LVSS device alone achieved burst pressures around 40 mmHg after small bowel transection and 22 mmHg after colon transection, the LVSS may be sufficient as a stand-alone method for bowel transection, especially for small bowel in a postoperative patient with reduced bowel movements. In fact, previous studies found that the LVSS can be used for bowel stump closure or for closure of the appendectomy stump [24–26].
With regard to bowel anastomosis, the LVSS as stand-alone method does not appear to achieve sufficient stability to withstand physiologic intraluminal pressures during intestinal pressure waves. Leaks occurred around 26 mmHg after small bowel anastomosis and 17 mmHg after colonic anastomosis whereas the double-layered HS anastomosis achieved leak pressures around 87 and 74 mmHg, respectively. S anastomosis, however, demonstrated leak pressure around 52 and 56 mmHg which are both just slightly above physiologic intraluminal pressures. Since there are no differences regarding anastomotic leakage rates between S and HS anastomoses [1, 3, 4], it is unlikely that the intraluminal bowel pressures after intestinal surgery are comparable to the pressures seen in the healthy, physiologic state. It has been shown that the bowel motility is strongly reduced for at least 2–3 days after intestinal surgery [27–29]. Nonetheless, further investigations are necessary whether the LVSS can be used for intestinal anastomoses. The currently available data are conflicting. Three studies reported that the LVSS can be safely used to perform intestinal anastomoses [30–32] whereas other results indicate that the LVSS may not be similarly safe as S or HS techniques [33]. An explanation for the differences in the study results may be different techniques used to perform the intestinal anastomoses with the LVSS. Further studies should test different methods of intestinal anastomoses with the LVSS as well as should investigate whether reinforcement with a single-layered hand-sewn suture can increase the stability of LVSS intestinal anastomosis.
Some limitations must be considered when the results of this study are interpreted. First and foremost is the use of an animal model. Second, the burst and leak pressures for this study were directly determined after performing the transection or anastomosis and the influence of healing, and therefore, increased stability of the stump or anastomosis could not be accounted for. Furthermore, the burst and leak pressures were measured and defined by a highly non-physiological method using a pressure measuring device. The pressure changes of normal, postoperative bowel movements are likely different. Lastly, due to postoperative gut paralysis and gradual increase in diet after gastrointestinal resections, it is unclear if patients develop intestinal pressure waves of a magnitude that actually may disrupt the stump or anastomosis. In order to address these limitations of the current study, further experiments performing intestinal anastomosis in a survival model and histological evaluation of the healing of the anastomosis several days after the procedure are necessary.
Conclusion
The LVSS achieves significantly lower burst pressures and anastomotic leak pressures for bowel transection and intestinal anastomosis than S and HS techniques. Nonetheless, the burst pressures after LVSS appear to be a sufficient as a stand-alone method for bowel transection. Whether it can be used to perform intestinal anastomosis warrants further research in a survival model.
References
Neutzling CB, Lustova SA, Proenca M, da Silva EM, Matos D (2012) Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev. Feb 15;2:CD003144. doi: 10.1002/14651858.CD003144.pub2.
Sajid MS, Siddiqui MR, Baig MK (2012) Single layer versus double layer suture anastomosis of the gastrointestinal tract. Cochrane Database Syst Rev. Jan 18;1:CD005477. doi: 10.1002/14651858.CD005477.pub4.
Gong J, Guo Z, Li Y, Gu L, Zhu W, Li J, Li N (2013) Stapled vs hand suture closure of loop ileostomy: a meta-analysis. Colorectal Dis 15(10):e561–8. doi:10.1111/codi.12388
Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie A, Fitzgerald A (2011) Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev. Sep 7;(9):CD004320. doi: 10.1002/14651858.CD004320.pub3.
Shikata S, Yamagishi H, Taji Y, Shimada T, Noguchi Y (2006) Single- versus two- layer intestinal anastomosis: a meta-analysis of randomized controlled trials. BMC Surg 6:2
Herron D, Roohipour R (2012) Complications of Roux-en-Y gastric bypass and sleeve gastrectomy. Abdom Imaging 37:712–718. doi:10.1007/s00261-012-9866-6
Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U, Gastinger I, Lippert H, Study group Qualitätssicherung Kolon/Rektum-Karzinome (Primärtumor) (Quality assurance in primary colorectal carcinoma) (2010) Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 36:120–124. doi:10.1016/j.ejso.2009.08.011
Krarup P-M, Jorgensen LN, Andreasen AH, Harling H, Danish Colorectal Cancer Group (2012) A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis Off J Assoc Coloproctology G B Irel 14:e661–667. doi:10.1111/j.1463-1318.2012.03079.x
Leichtle SW, Mouawad NJ, Welch KB, Lampman RM, Cleary RK (2012) Risk factors for anastomotic leakage after colectomy. Dis Colon Rectum 55:569–575. doi:10.1097/DCR.0b013e3182423c0d
Kössi J, Luostarinen M, Kontula I, Laato M (2007) Laparoscopic sigmoid and rectal resection using an electrothermal bipolar vessel sealing device. J Laparoendosc Adv Surg Tech A 17:719–722. doi:10.1089/lap.2006.0238
Saiura A, Yamamoto J, Koga R, Seki M, Yamaguchi T (2008) Liver transection using the LigaSure sealing system. HPB 10:239–243. doi:10.1080/13651820802167714
Nielsen HUK, Trolle W, Rubek N, Homøe P (2013) New technique using LigaSure for endoscopic mucomyotomy of Zenker’s diverticulum: diverticulotomy made easier. The Laryngoscope. doi:10.1002/lary.24558
Chen H-L, Woo X-B, Cui J, Chen C-Q, Peng J-S (2014) Ligasure versus stapled hemorrhoidectomy in the treatment of hemorrhoids: a meta-analysis of randomized control trials. Surg Laparosc Endosc Percutan Tech. doi:10.1097/SLE.0000000000000009
Ravitch MM, Steichen FM (1972) Technics of staple suturing in the gastrointestinal tract. Ann Surg 175(6):815–37
Gehrig T, Manzini G, Fonouni H, Golriz M, Hafezi R, Rahbari N, Brand K, Hinz U, Müller-Stich BP, Gutt CN, Mehrabi A (2013) Comparison of two different transection techniques in liver surgery—an experimental study in a porcine model. Langenbecks Arch Surg 398(6):909–15. doi:10.1007/s00423-013-1094-1
Macario A, Dexter F, Sypal J, Cosgriff N, Heniford BT (2008) Operative time and other outcomes of the electrothermal bipolar vessel sealing system (LigaSure) versus other methods for surgical hemostasis: a meta-analysis. Surg Innov 15(4):284–91. doi:10.1177/1553350608324933
Saint Marc O, Cogliandolo A, Piquard A, Famà F, Pidoto RR (2007) LigaSure vs clamp-and-tie technique to achieve hemostasis in total thyroidectomy for benign multinodular goiter: a prospective randomized study. Arch Surg 142(2):150–6, discussion 157
Janssen PF, Brölmann HA, Huirne JA (2012) Effectiveness of electrothermal bipolar vessel-sealing devices versus other electrothermal and ultrasonic devices for abdominal surgical hemostasis: a systematic review. Surg Endosc 26(10):2892–901
Contin P, Gooßen K, Grummich K, Jensen K, Schmitz-Winnenthal H, Büchler MW, Diener MK (2013) ENERgized vessel sealing systems versus CONventional hemostasis techniques in thyroid surgery—the ENERCON systematic review and network meta-analysis. Langenbecks Arch Surg 398(8):1039–56. doi:10.1007/s00423-013-1137-7
Piccinni G, Pasculli A, D’Ambrosio E, Gurrado A, Lissidini G, Testini M (2013) Retrospective comparison of Traditional vs. LigaSure impact dissection during pancreatoduodenectomy: how to save money by using an expensive device. Surg Technol Int 23:88–93
Imam H, Sanmiguel C, Larive B, Bhat Y, Soffer E (2004) Study of intestinal flow by combined videofluoroscopy, manometry, and multiple intraluminal impedance. Am J Physiol Gastrointest Liver Physiol 286(2):G263–70
Paral J, Lochman P, Blazej S, Pavlik M (2014) Glued versus stapled anastomosis of the colon: an experimental study to determine comparative resistance to intraluminal pressure. Asian J Surg Jul 37(3):154–61. doi:10.1016/j.asjsur.2014.01.007
Chaikomin R, Wu KL, Doran S, Jones KL, Smout AJ, Renooij W, Holloway RH, Meyer JH, Horowitz M, Rayner CK (2007) Concurrent duodenal m6anometric and impedance recording to evaluate the effects of hyoscine on motility and flow events, glucose absorption, and incretin release. Am J Physiol Gastrointest Liver Physiol 292(4):G1099–104
Moreno-Sanz C, Picazo-Yeste J, Seoane-Gonzáles J, Manzanera-Díaz M, Tadeo-Ruiz G (2008) Division of the small bowel with the LigaSure Atlas device during the right laparoscopic colectomy. J Laparoendosc Adv Surg Tech A 18(1):99–101. doi:10.1089/lap.2007.0014
Santini M, Fiorelli A, Messina G, Laperuta P, Mazzella A, Accardo M (2013) Use of the LigaSure device and the Stapler for closure of the small bowel: a comparative ex vivo study. Surg Today43(7):787-93. doi: 10.1007/s00595-012-0336-0
Elemen L, Yazir Y, Tugay M, Akay A, Aydin S, Yanar K, Ceylan S (2010) LigaSure compared with ligatures and endoclips in experimental appendectomy: how safe is it? Pediatr Surg Int 26(5):539–45. doi:10.1007/s00383-010-2557-x
Wilson JP (1975) Postoperative motility of the large intestine in man. Gut 16(9):689–92
Kemen M, Bein N, Homann HH, Bauer KH, Zumtobel V (1991) Postoperative small intestinal motility after abdominal surgery. Infusionstherapie 18(5):233–5
Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE (1992) Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg 163(1):144–8, discussion 148-9
Santini M, Fiorelli A, Messina G, Mazzella A, Accardo M (2015) The feasibility of LigaSure to create intestinal anastomosis: results of ex vivo study. Surg Innov 22(3):266–73. doi:10.1177/1553350614547771
Smulders JF, de Hingh IH, Stavast J, Jackimowicz JJ (2007) Exploring new technologies to facilitate laparoscopic surgery: creating intestinal anastomoses without sutures or staples, using a radio-frequency-energy-driven bipolar fusion device. Surg Endosc 21(11):2105–9
Rumbaugh ML, Burba DJ, Natalini C, Hosgood G, Moore RM (2003) Evaluation of a vessel-sealing device for small intestinal resection and anastomosis in normal horses. Vet Surg 32(6):574–9
Sánchez-De Pedro F, Moreno-Sanz C, Morandeira-Rivas A, Tenías-Burillo JM, Alhambra-Rodríguez De Guzmán C (2014) Colorectal anastomosis facilitated by the use of the LigaSure(®) sealing device: comparative study in an animal model. Surg Endosc 28(2):508–14. doi:10.1007/s00464-013-3194-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the German Committee on Animal Care, Regierungspräsidium Karlsruhe, and the medical faculty ethics committee at University of Heidelberg (approval code 35-9185.81/G-91/08). All animals received humane care in compliance with the national research council’s criteria for humane care, as outlined in the guide for the care and use of laboratory animals prepared by the National Institute of Health (NIH Publication 86-23, revised 1985).
Disclosures and freedom of investigation
The authors have no financial (interests) or other disclosures relating to state. No external funds were used to perform the evaluation, and all of the tested technology was separately purchased to complete the study. In addition, the authors had full control of the design of the study, methods used, outcome measurements, analysis of data, and production of the written report.
Rights and permissions
About this article
Cite this article
Gehrig, T., Billeter, A.T., Wekerle, A.L. et al. Evaluation of the LigaSure™ Vessel Sealing System for bowel transection and intestinal anastomosis—an experimental study in a porcine model. Langenbecks Arch Surg 401, 381–387 (2016). https://doi.org/10.1007/s00423-016-1406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1406-3