Abstract
Introduction
It is unknown whether predetermined (un)interrupted sitting within a laboratory setting will induce compensatory changes in human behaviours (energy intake and physical activity) once people return to a free-living environment. The effects of breaking up prolonged sitting on cognition are also unclear.
Methods
Twenty-four (male = 13) healthy participants [age 31 ± 8 y, BMI 22.7 ± 2.3 kg/m2 (mean ± SD)] completed 320 min mixed-feeding trials under prolonged sitting (SIT) or with 2 min walking at 6.4 km/h every 20 min (ACTIVE), in a randomised crossover design. Human behaviours were recorded post-trial under free-living conditions until midnight. Cognitive performance was evaluated before and immediately after SIT and ACTIVE trials. Self-perceived sensations (appetite, energy and mood) and finger prick blood glucose levels were collected at regular intervals throughout the trials.
Results
There were no differences between trials in eating behaviour and spontaneous physical activity (both, p > 0.05) in free-living conditions, resulting in greater overall total step counts [11,680 (10740,12620) versus 6049 (4845,7253) steps] and physical activity energy expenditure (PAEE) over 24-h period in ACTIVE compared to SIT (all, p < 0.05). Greater self-perceived levels of energy and lower blood glucose iAUC were found in ACTIVE trial compared to SIT trial (both, p < 0.05). No differences were found in cognitive performance between trials (all, p > 0.05).
Conclusion
Breaking up sitting does not elicit subsequent behavioural compensation, resulting in greater 24-h step counts and PAEE in healthy adults. Breaking up sitting reduces postprandial glucose concentrations and elicits greater self-perceived energy levels, but these positive effects do not acutely translate into improved cognitive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prolonged sitting and physical inactivity are two predominant risk factors that contribute to obesity, cardiovascular diseases (CVD) (e.g. high blood pressure), type 2 diabetes and impaired cognitive function (Hamilton et al. 2007; Bankoski et al. 2011; Magnon et al. 2018). Due to computer-based work and high reliance of technology, office-based workers spend approximately 65–80% of working hours sitting (Clemes et al. 2014; Waters et al. 2016). Although standing and fidgeting seem to provide applicable approaches to elevate physical activity, the efficacy of using these kinds of low-energy demanding activity are likely to be rather modest (Betts et al. 2019; Koepp et al. 2016). Thus, alternatives and/or more effective strategies to reduce prolonged sitting behaviour and increase physical activity for office workers whilst working are urgently needed.
Replacing sitting time with regular short bouts of walking has been shown as a promising approach to ameliorate detrimental impact of prolonged sitting (e.g. to reduce postprandial glucose and insulin concentrations) and increase physical activity levels in the laboratory (Chen et al. 2018; Loh et al. 2020). However, human behaviours are usually well-controlled within the laboratory setting (i.e. predetermined energy consumption and physical activity) that may impede the application of exercise intervention in the real-world scenario if individuals subsequently show compensatory changes in behaviour (e.g. reduced spontaneous physical activity and/or increases in energy intake) in the (free-living) hours following the trial. In prescribed exercise interventions, the energy deficit elicited can in some cases be less-than-expected, which may be due to the compensatory decreases in physical activity and/or increases in energy intake (Silva et al. 2018; Turner et al. 2010; Melanson et al. 2013; Flack et al. 2018). To date, only two studies have examined breaking prolonged sitting and its influence on human behaviour and only eating behaviour was assessed (Mete et al. 2018; Bailey et al. 2016). These studies found that energy intake was not affected by breaking up sitting (Mete et al. 2018; Bailey et al. 2016), but limited food choices and pre-selected meals in the controlled laboratory period might weaken the ecological relevance to the public. Indeed, studies have demonstrated that built environmental in the free-living condition (public transportation, alternations of work-related activity, high energy density food and more palatable food choices) might lead to the changes in eating and physical activity (Lam et al. 2021). At present, it is still unknown whether predetermined (un)interrupted sitting will induce compensational changes in nutritional intake and physical activity levels once people are in the free-living environment. This has important implications for energy balance and general health.
In addition to the negative effects on the energy balance and metabolic health, prolonged uninterrupted sitting can also impair cognitive function through several mechanisms, including reduced cerebral blood flow (Carter et al. 2018), elevated mental fatigue and lower energy status (Bergouignan et al. 2016; Wennberg et al. 2016), and postprandial hyperglycaemia (Wheeler et al. 2017). Cognitive performance covers several important mental skills, such as working memory, cognitive flexibility and inhibition (i.e. avoiding distractions) that have each been shown to greatly influence work productivity. A recent systematic review has indicated that frequent sitting breaks seems to be able to reverse the negative effects on cognition during prolonged sitting, but the favourable impacts disappeared when relative slow walking speed (e.g. 3.2 km/h) and less frequent sitting breaks (e.g. hourly breaks) were involved (Chueh et al. 2022). Exercise/physical activity intensity plays a moderating role in the regulation cognitive performance (Chang et al. 2012). Therefore, in the present study, walking speed and frequency were both increased, compared to lower intensity and less frequent sitting breaks that have been used previously (Chueh et al. 2022), to examine the effects of breaking up sitting on cognition in the context of an office environment.
Taken together, this study aimed to investigate effects of breaking up prolonged sitting with intermittent brisk walking in healthy individuals on (1) post-trial human behaviours including energy intake and physical activity under free-living conditions and (2) cognitive performance in a simulated workplace environment. We hypothesised that breaking up sitting would reduce post-trial physical activity levels due to prescribed intermittent walking in the laboratory and that better cognitive performance would be observed in the breaking up sitting condition.
Methods
Study participants
Studies have shown that office-based workers, with the average age of 37 years, spend 65% of working hours sitting (Clemes et al. 2014). Accordingly, twenty-four young and middle-aged healthy participants (male = 13) age between 20 and 45 years were recruited in the Taipei city via local advertisements in the current study. Participants were confirmed to be without current and any history of brain injury, neurological disorder, cardiometabolic related diseases, diabetes or cancers and were weight stable (not self-reported weight change ± 3 kg at least 3 months) with a body mass index (BMI) ranging between 18.5 and 27 kg/m2. Once consented to take part, a Physical Activity Readiness Questionnaire (PAR-Q) was completed to ensure the participants were able to walk on a treadmill without any safety concerns. Smokers, post-menopausal female, and volunteers who used any medications that could influence metabolic and inflammatory responses, were excluded. The participants’ characteristics are shown in Table 1.
Experimental design
Participants completed two separate main trials (SIT, uninterrupted prolonged sitting; ACTIVE, interrupted prolonged sitting every 20 min with 2 min intermittent walking) at least a 24-h apart within 7 days in a randomised, crossover design (randomisation was performed by YC-Chen using an online programme, randomizer.org). A pedometer (3D TriSport, China) (Walhin et al. 2018) was worn at least 24 h prior to the first main trial for a continuous period of 7 days. Participants were restricted to any vigorous-intensity exercise, alcohol and caffeine consumption 24 h prior to both SIT and ACTIVE trials. Dinner intake was recorded on the day before the first trial and participants were asked to replicate it the day prior to the second trial. Female participants completed two main trials between 3 and 11 days in their menstrual cycle. The study protocol was approved by Tri-Service General Hospital Institute Review Board (IRB reference number: 2-108-05-151) and is registered at ClinicalTrials.gov (ID: NCT05911490). All participants provided written informed consent before taking part.
Studies have shown that prescribed exercise might reduce non-prescribed physical activity (Ridgers et al. 2014; Goran and Poehlman 1992). Therefore, we determined sample size based on the effect of breaking up sitting on energy expenditure in the laboratory. Our previous study demonstrated that breaking up sitting with intermittent walking, compared with prolonged sitting, significantly increased energy expenditure by 216 kcal over a 5.5-h period in 11 participants (Chen et al. 2018). We chose to double the sample size in the present study to increase confidence in observing potential changes in post-trial spontaneous physical activity, along with generating a more representative sample that also enables consideration of the variation of physical activity in different individuals. Twenty-four participants were recruited to allow for expected dropout.
SIT and ACTIVE trials
Participants were required to fast for at least 12-h and minimised their physical activity level before arriving at the exercise physiology laboratory in the National Taiwan Normal University between 0800 and 0900 a.m. on the main trial days. Once anthropometric measurements [including height (Stadiometer JENIX DS-102, Jen An Technology Co., Ltd), weight (InBody 720, Biospace Co. Ltd, Seoul Korea), waist and hip circumference and blood pressure (OMRON HEM-7320, OMRON Co., Ltd., Japan)] completed, participants rested on the chair for 10 min, followed by 10 min expired air sample collections using an automatic gas analysis system (Vmax29, Sensor Medics the Corp., Yorba Linda, CA) to determine resting metabolic rate (RMR) and substrate oxidation (Betts et al. 2011; Compher et al. 2006). The amount of oxygen utilisation (V̇O2) and carbon dioxide production (V̇CO2) were used to calculate carbohydrate and lipid oxidation rates to estimate resting and intermittent walking energy expenditure (Jeukendrup and Wallis 2005; Frayn 1983).
In both SIT and ACTIVE trials, breakfast was consumed after RMR collection, while lunch was provided 3 h afterwards. The test meal was prescribed according to participants’ total body mass (contained 8.8 kcal energy per kilogramme body mass) and the percentage of energy from macronutrients was 77% carbohydrate, 16% fat and 7% protein. The composition of the meal was selected to reflect typical breakfasts in the morning (O'Neil et al. 2014). To avoid the possible interactive effects of energy intake on the major outcomes in this study, the same meal was consumed for lunch. The test meal consisted of oatmeal (Quaker; Taiwan) and fructose (Fong Leng, Taiwan), guava, mango and orange mixed fruit juice (Kung Chuan, Taiwan) and digestive biscuits (McVitie’s, Great Britain). The oatmeal with water and fructose were provided firstly, while juice and biscuits were provided thereafter. Both breakfast and lunch (identical) were consumed within a 10-min period and equal amount of energy was consumed during the SIT and the ACTIVE trials (breakfast and lunch energy intake were 1142 ± 67 kcal, 95% CI [1075, 1209]).
Trial days: laboratory data collection
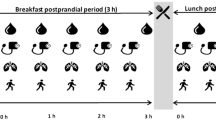
The experimental protocol was based on our previous publication (Chen et al. 2018) and is illustrated in Fig. 1. In the SIT trial, participants were required to remain seated throughout the whole period. In the ACTIVE trial, after breakfast consumption, participants walked 2 min at 6.4 km/h speed every 20 min on a treadmill (h/p/cosmos mercury® med, Germany) for the following 3 h. After finishing lunch, participants continued to walk in the same pattern for the following 2 h. Apart from walking, participants sat on a chair for the remaining period. In total, participants performed 15 two-min bouts of walking (i.e. totally 30 min over 320 min). Heart rate (Polar M430, Polar Electro Oy, Kempele, Finland) and RPE (Borg 6–20) were collected in the last 30 s of each 2-min bout of walking during the ACTIVE trial. Moreover, two 2-min of expired air samples were collected to estimate walking energy expenditure and substrate utilisation in the ACTIVE trial. In both SIT and ACTIVE trials, cognitive performance was assessed using computerised cognitive tests at the beginning and the end of the trials. Visual analogue scales (VAS) were recorded hourly and blood pressure was measured 3 times at baseline, before lunch and at the end of the trials. Finger pricking whole blood glucose concentrations (Bayer Contour Plus, Germany) were measured in a regular pattern (7 times) during the trials.
Participants were only allowed to read, use a laptop, or watch television but were otherwise asked to keep as still as possible (e.g. instructions to avoid fidgeting) while sitting in both SIT and ACTIVE trials. Temperature and humidity were recorded at 0-, 190- and 320-min during trials and there were no differences between different times and trials (both, p > 0.05, data not shown).
Trial days—free-living environment data collection
After data collection was completed in the laboratory, in both trials participants were asked to keep wearing the same pedometer and to record any food and liquid consumption using a weighed food diary for the rest of the day (until midnight, approximately 8.5 h duration) under free-living conditions. No restrictions were placed on free-living energy intake and physical activity outside the laboratory, and participants did not know this was the one of main research outcomes. Participants were required to weigh all the amount of food and liquid consumed with a given digital weight scale up to 0.1 g (I-2000, China).
Cognitive tests
Cognitive performance was determined using Task Switching and Flanker tasks employing Unity software (SF, USA) and familiarisation tests were provided prior to the main trials. Both tasks took approximately 5 min, for the total duration was about 10 min. Task switching test was based on the design of Hung et al. (Hung et al. 2016). In the Task Switching task, a white numeric digit (digits 1–9, excluding 5) was presented in the centre of a computer screen on a black background. Participants had to identify whether the number surrounded by a solid-line rectangle, was smaller (1, 2, 3, 4) or bigger (6, 7, 8, 9) than 5 (i.e. all tasks were categorised as “A” type) and the number surrounded by a dashed square was odd (1, 3, 7, 9) or even (2, 4, 6, 8) (i.e. all tasks were categorised as “B” type) to press the left (number is smaller 5 or odd) or the right (number is larger than 5 or even) button using the index fingers of their left or right hand as fast and as accurately as possible. While the task was combined with an alternative run switching paradigm (i.e. ABBBA…) and repeated twice. The following task was the modified Eriksen Flanker task (Eriksen and Eriksen 1974). Participants were instructed to identify the direction of the middle arrow in either congruent (> > > > >) or incongruent (> > < > >) stimuli by pressing the left (left-pointing arrow) or the right (right-pointing arrow) button using the index fingers of their left or right hand as fast and as accurately as possible, and repeated the same paradigm for the second round.
Self-perceived sensations assessment
Subjective sensations were measured hourly (6 times in total) using 100-mm VAS for 6 variables including appetite (hunger and satiety), fatigue (fatigue and energy) or mood (tension and relaxation) paired with the opposing sensation (e.g. ‘extremely hungry’ and ‘not hungry at all’). Participants were asked to draw a vertical dash on each line that best matched their feeling at that time. Each score was determined by measuring the distance from the left side of the line (0-mm) to the right (100-mm).
Human behaviours (energy intake and spontaneous physical activity)
Total calorie and macronutrient consumption (carbohydrate, fat and protein) in the free-living environment were carefully analysed and calculated through MyFitnessPal (Teixeira et al. 2018). The number of steps were recorded (from home to laboratory, in the laboratory and in free-living environment) over 24-h period and physical activity energy expenditure (PAEE) was assumed as step counts with stride length of 0.825 m for male and 0.745 m for female (Auvinet et al. 2002) based on an oxygen cost of 0.125 ml/kg/min (Dill 1965).
Statistical analysis
Participant characteristics were presented as means ± standard deviation (SD). Outcomes of variables and variance bars on figures were presented as means with 95% confidence interval (CI). Time series data were analysed using a two-way repeated measures analysis of variance ANOVA (trial [SIT versus ACTIVE] × time) irrespective of minor deviations from a normality of distribution (Maxwell and Delaney 1990) but with Greenhouse–Geisser corrections utilised for intra-individual contrasts where ε < 0.75, and the Huynh–Feldt correction for less severe asphericity (Atkinson 2002) using SPSS version 23 (IBM, Armonk, NY). Where a trial × time interaction was found, post-hoc Holm–Bonferroni corrections were used to characterise differences between trials (Atkinson 2002). Incremental area under the curve (iAUC) for glucose was calculated using the trapezoid method (Narang et al. 2020) and analysed using a paired t-test. Cognitive tests both in accuracy and reaction time were calculated using rate of change ([post-trial—baseline/ baseline] × 100%) and analysed using paired t-test. Statistical significance was set at p ≤ 0.05.
Results
Physiological responses during the ACTIVE trials in the laboratory
In the ACTIVE trial, during the accumulated 30 min of walking, average heart rate reached 112 (95% CI 107, 118) bpm with an RPE (Borg 6–20 scale) of 10 (95% CI 9.7, 10.7). Estimated 30 min walking energy expenditure was 149 (95% CI 138, 160) kcal and averaged RER was 0.87 (95% CI 0.85, 0.9) via expired air collection.
Steps counts and estimated physical activity energy expenditure on trial days
No differences between trials were shown in step counts and estimated PAEE from home to laboratory (p = 0.892 and p = 0.914, respectively) and in the free-living environment between the end of the trial and midnight (p = 0.416 and p = 0.393, respectively) (Table 2). The step counts and estimated PAEE in the laboratory were higher in ACTIVE compared with SIT (both, p < 0.001), resulting in greater total steps [11,680 (95% CI 10740, 12620) versus 6049 (95% CI 4845, 7253) steps] and estimated PAEE [364 (95% CI 326, 402) versus 186 (95% CI 148, 225) kcal] in the ACTIVE trial compared to the SIT trial (both, p < 0.001) (Table 2).
Post-trial eating behaviour in the SIT and ACTIVE trials
No differences between trials were found in the total energy intake (SIT: 1021, 95% CI [860, 1182] kcal versus ACTIVE: 1132, 95% CI [953, 1311] kcal) and macronutrient consumption (45% versus 46% in carbohydrate, 20% versus 19% in protein and 35% versus 35% in fat between SIT and ACTIVE, respectively) in the post-trial free-living environment (all, p > 0.05) (n = 21, due to 3 participants not completing records).
Blood glucose concentrations
Blood glucose concentrations did not differ at baseline between trials (p = 0.561). There was an interaction effect (time x trial) in blood glucose concentrations between trials (p = 0.038, Fig. 2A). The glucose iAUC value was lower in the ACTIVE trial compared to the SIT trial [7389 (95% CI 5801, 8978) mg/320 min/dL versus 9703 (95% CI 7790,11616) mg/320 min/dL, p = 0.007, Fig. 2B].
Visual analogue scale (VAS) for appetite and mood status
All VAS values did not differ at baseline between trials (all, p > 0.063). There was no interaction effect (trial x time) in any of VAS values (all, p > 0.05, Fig. 3A–F). Time effects were showed in both hunger and satiety (both, p < 0.001, Fig. 3A, B). Trial effects were observed indicating greater energy and lower fatigue in the ACTIVE trial compared to the SIT trial (p < 0.001 & p = 0.039, respectively, Fig. 3C, D).
Cognitive function
Neither Task Switching, nor Flanker Task differed at baseline between trials (all, p > 0.308). The rate of percentage changes (%) in accuracy for Task Switching [1.9 (95% CI −2.4, 6.2) versus 1.7 (95% CI −0.8, 4.1)] and Flanker [2.5 (95% CI −1.4, 6.5) versus 1.3 (95% CI −0.3, 2.9)] did not differ between SIT and ACTIVE (all, p > 0.05, Fig. 4A). The rate of percentage changes (%) in reaction time between SIT and ACTIVE trials showed no difference for Task Switching [−7.3 [95% CI −14, −1.1) versus −12 (95% CI −18, −6.5)] and Flanker Task [−1.1 (95% CI −3.7, 1.5) versus 0.6 (95 CI −1.5, 2.7)] (all, p > 0.05, Fig. 4B).
Blood pressure
There were no differences observed in systolic and diastolic pressure between the SIT and the ACTIVE trials at baseline, 190 min [99 (95% CI 94, 104) versus 99 (95% CI 93, 104) mmHg; 65 (95% CI 62, 68) versus 68 (95% CI 65, 72) mmHg] and 320 min [101 (95% CI 95, 106) versus 101 (95% CI 96, 106) mmHg; 64 (95% CI 61, 67) versus 66 (95% CI 63, 69) mmHg] (all, p > 0.05).
Discussions
In the present study, we investigated the effects of breaking up sitting on human behaviours (energy intake and spontaneous physical activity) and cognitive performance. In the post-trial free-living environment, we found that there were no differences in eating and physical activity behaviours between the SIT and the ACTIVE trials. Therefore, the predetermined breaking up of prolonged sitting with intermittent walking in the ACTIVE trial, elicited greater physical activity levels and created negative energy balance over the 24-h monitoring period, compared to the SIT trial. In the context of office-based conditions, breaking up sitting attenuated postprandial glycaemic iAUC and induced greater self-perceived level of energy, but there were no differences in cognitive performance (accuracy or reaction time) between trials.
In prescribed exercise interventions, the energy deficit (i.e. weight management) elicited in some occasions can be less-than-expected, which may be due to the compensatory behaviours, such as a decrease in physical activity or an increase in energy intake, or the combination of both (Silva et al. 2018; Turner et al. 2010; Melanson et al. 2013; Flack et al. 2018). In the present study, a total of 30 min short bouts of brisk walking did not lead to obvious post-trial behavioural compensation in either energy intake or in physical activity, suggesting that breaking sitting can be an applicable and practical approach to enhance physical activity levels and create negative energy balance for office-based workers over a 24-h period.
The volumes and/or the intensity of previous bouts of exercise and/or physical activity plays a vital role in regulation of the subsequent volitional physical activity (Rowland 1998; Garland et al. 2011). Indeed, studies have shown that prescribed exercise and/or physical activity might spontaneously reduce non-prescribed physical activity (Ridgers et al. 2014; Goran and Poehlman 1992). In the current study, approximately 150 kcal energy was expended accompanied by relatively low self-rated walking intensity (RPE rated at 10 in Borg 6–20 scale) in the ACTIVE trial. Therefore, the volumes and/or the intensity of our breaking sitting protocol may not reach the threshold of inducing behavioural compensation (i.e. decrease in physical activity). In addition, office-based workers do not appear to be more physically active outside the office to compensate for high durations of sedentary behaviour at work (Clemes et al. 2014; Waters et al. 2016). Importantly, our research design (2 min walking every 20 min over 5.5 h) increased step counts by approximately 5,000 steps, resulting in greater physical activity over the 24-h period in the ACTIVE trial. There is a dose–response effect of daily walking steps for health (Jayedi et al. 2022; Saint-Maurice et al. 2020) and each 1000 daily step count increase from baseline has been shown to reduce the risk all-cause mortality (6–36%) and CVD (5–21%) (Hall et al. 2020). Therefore, breaking up prolonged sitting with intermittent walking might have the potential to reduce the risks of CVD related diseases if individuals apply this strategy repeatedly. One limitation that is important to mention is that we do not know the intensity of physical activity and non-exercise activity thermogenesis such as fidgeting, standing and posture changes in all activities of daily living can substantially influence energy expenditure. More sophisticated tools such as accelerometers to precisely capture the intensity of physical activity as well as non-exercise activity thermogenesis are warranted in future investigations. On the other hand, physical activity assessed via pedometer is an easy metric of measurement that help generally quantifying daily activity and which has been well-recognised to be strongly associated with the risk of obesity, CVD and type 2 diabetes in the clinical research (Jayedi et al. 2022; Saint-Maurice et al. 2020).
In terms of eating behaviour, interestingly, similar to ad libitum energy intake assessed in the laboratory (Bailey et al. 2016; Mete et al. 2018), we did not find a significant difference in the eating behaviour in the post-trial free-living environment between the SIT and the ACTIVE trials. Exercise and/or physical activity may also substantially affect subsequent energy intake, in part due to exercise induced energy deficit and/or glycogen depletion and/or changes in appetite (Gonzalez et al. 2019). Given that our participants had breakfast prior to the first bout of walking (i.e. walking in the fed state), alongside the very low energy cost during each bout of walking, insufficient energy storage and glycogen depletion are unlikely to happen. Moreover, in line with previous results (Mete et al. 2018; Bailey et al. 2016), there were no differences in subjective feelings of hunger and satiety between SIT and ACTIVE trials in our study. Taken together, those might be the most likely potential reasons why we did not observe differences of total energy intake in the current study in free-living conditions. One of the strengths in our study was the use of weighed food diaries which allowed participants to choose their food intake without limitations compared with a controlled eating behaviour assessment in the laboratory.
Previous studies have shown that sitting breaks did not acutely improve cognition, which may in part be due to the low intensity and/or infrequent physical activity (Chueh et al. 2022). In this study, we had increased both walking speed and frequency compared to previous studies, but we still did not find an acute effect of breaking sitting on cognition. The underlying properties of study population may influence the effects of breaking up prolonged sitting on cognitive function. One study revealed that cognitive performance could be improved via 3 min walking every 30 min at speed of ~ 6 km/h (ranged between 5.0 and 8.3 km/h) with a total of 27 min physical activity in young sedentary females over 5-h trial (Chrismas et al. 2019). However, another investigation which applied 5 min cycling every 30 min (30 min cycling in total) in young overweight and obese people suggested there was no significant benefit on the cognitive performance (Wanders et al. 2021). Our study design was very similar (2 min walking every 20 min at 6.4 km/h with 30 min walking over 5.5 h period) and we did not find positive effects on cognition in the healthy active young adults. Considering active individuals generally have better cognitive function than inactive people (Falck et al. 2017; Erickson et al. 2019), it is maybe not surprising that breaking sitting with walking had limited ability to further enhance their cognitive function as observed in our data. Moreover, a wide range of walking speed between 5.0 and 8.3 km/h was used (Chrismas et al. 2019), which could be a confounding factor impacting the results. Different cognitive tests used between studies might also lead to conflicting findings despite the overall similar experimental design between studies.
Mood status (Boksem et al. 2005) and glycaemic control have been suggested to influence cognitive function (Wheeler et al. 2017). Consistent with previous studies (Wennberg et al. 2016; Bergouignan et al. 2016; Wanders et al. 2021), we also found that greater self-perceived level of energy and lower postprandial glucose concentrations were present in the ACTIVE trial, but these beneficial effects did not acutely translate into better cognitive performance. It is also possible that this approach (regular breaking sitting) would have to be continuously adhered to over several weeks or months for any positive effects to be observed and this requires further long-term investigation. Notably, interventions with exercise programmes sometimes might be detrimental for the cognition. Breaking up sitting via intermittent fidgeting (Stoner et al. 2019) and acute bouts of high-intensity exercise (Chang et al. 2012) has been observed to impair cognitive functions. Although we did not observe any favourable effects on cognition via intermittent brisk walking, it is important to note that cognitive performance during demanding the tasks were not compromised either.
Finally, postprandial hyperglycaemia is associated with increased potential of developing CVD even for the normoglycaemic individuals (Levitan et al. 2004), and replacing sitting with physical activity may reduce all-cause and CVD mortality risk (Stamatakis et al. 2019). In accordance with previous findings (Chen et al. 2018), we also showed that regular short bouts of intermittent walking reduced postprandial glucose iAUC by approximately 31%. Therefore, our results suggest that breaking prolonged sitting with walking can be a useful strategy to reduce the likelihood for developing to CVD for the office-based workers.
There are potential limitations in the current study. The frequent short bouts of intermittent walking is one of the strengths in our study; however, oxygen uptake might not reach a steady state during a 2-min walking bout. Nevertheless, this would not impact our conclusions on cognition or post-laboratory free-living energy intake and spontaneous physical activity. Moreover, due to safety considerations for the participants, speed of the treadmill was accelerated slowly from 0 to 6.4 km/h (followed by a full 2-min walking at 6.4 km/h); this inevitably means that participants accumulated a small number of additional steps in the laboratory.
Conclusions
Predetermined sitting breaks did not lead to post-trial compensatory changes of human behaviours (i.e. decrease in physical activity and/or increase in energy intake) in the free-living environment. This suggests that breaking up prolonged sitting with short bouts of intermittent walking can be an applicable and effective strategy to enhance 24-h physical activity levels for office-based workers. Moreover, breaking prolonged sitting reduced postprandial glycaemic concentrations and enhanced self-rated energy status but these positive effects did not acutely translate into the improvements in cognitive function in young and middle-aged healthy individuals.
Data availability
Data generated or analysed during this study are available from the corresponding author upon reasonable request.
References
Atkinson G (2002) Analysis of repeated measurements in physical therapy research: multiple comparisons amongst level means and multi-factorial designs. Phys Ther Sport 3:191–203
Auvinet B, Berrut G, Touzard C, Moutel L, Collet N, Chaleil D, Barrey E (2002) Reference data for normal subjects obtained with an accelerometric device. Gait Posture 16:124–134
Bailey DP, Broom DR, Chrismas BC, Taylor L, Flynn E, Hough J (2016) Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl Physiol Nutr Metab 41:324–331
Bankoski A, Harris TB, Mcclain JJ, Brychta RJ, Caserotti P, Chen KY, Berrigan D, Troiano RP, Koster A (2011) Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 34:497–503
Bergouignan A, Legget KT, de Jong N, Kealey E, Nikolovski J, Groppel JL, Jordan C, O’day R, Hill JO, Bessesen DH (2016) Effect of frequent interruptions of prolonged sitting on self-perceived levels of energy, mood, food cravings and cognitive function. Int J Behav Nutr Phys Act 13:113
Betts JA, Thompson D, Richardson JD, Chowdhury EA, Jeans M, Holman GD, Tsintzas K (2011) Bath Breakfast Project (BBP)–examining the role of extended daily fasting in human energy balance and associated health outcomes: study protocol for a randomised controlled trial [ISRCTN31521726]. Trials 12:172
Betts JA, Smith HA, Johnson-bonson DA, Ellis TI, Dagnall J, Hengist A, Carroll H, Thompson D, Gonzalez JT, Afman GH (2019) The energy cost of sitting versus standing naturally in man. Med Sci Sports Exerc 51:726–733
Boksem MA, Meijman TF, Lorist MM (2005) Effects of mental fatigue on attention: an ERP study. Brain Res Cogn Brain Res 25:107–116
Carter SE, Draijer R, Holder SM, Brown L, Thijssen DHJ, Hopkins ND (2018) Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol 1985(125):790–798
Chang YK, Labban JD, Gapin JI, Etnier JL (2012) The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res 1453:87–101
Chen YC, Betts JA, Walhin JP, Thompson D (2018) Adipose tissue responses to breaking sitting in men and women with central adiposity. Med Sci Sports Exerc 50:2049–2057
Chrismas BCR, Taylor L, Cherif A, Sayegh S, Bailey DP (2019) Breaking up prolonged sitting with moderate-intensity walking improves attention and executive function in Qatari females. PLoS ONE 14:e0219565
Chueh TY, Chen YC, Hung TM (2022) Acute effect of breaking up prolonged sitting on cognition: a systematic review. BMJ Open 12:e050458
Clemes SA, Patel R, Mahon C, Griffiths PL (2014) Sitting time and step counts in office workers. Occup Med (lond) 64:188–192
Compher C, Frankenfield D, Keim N, Roth-Yousey L, Evidence Analysis Working, G (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106:881–903
Dill DB (1965) Oxygen used in horizontal and grade walking and running on the treadmill. J Appl Physiol 20:19–22
Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, For Physical Activity Guidelines Advisory, C (2019) Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 51: 1242-1251
Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16:143–149
Falck RS, Davis JC, Liu-Ambrose T (2017) What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med 51:800–811
Flack KD, Ufholz K, Johnson L, Fitzgerald JS, Roemmich JN (2018) Energy compensation in response to aerobic exercise training in overweight adults. Am J Physiol Regul Integr Comp Physiol 315:R619–R626
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55:628–634
Garland T, JR Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, Van Dijk G, Kotz CM, Eisenmann JC (2011) The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206-29
Gonzalez JT, Betts JA, Thompson D (2019) Carbohydrate availability as a regulator of energy balance with exercise. Exerc Sport Sci Rev 47:215–222
Goran MI, Poehlman ET (1992) Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol 263:E950–E957
Hall KS, Hyde ET, Bassett DR, Carlson SA, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Kraus WE, Lee IM, Matthews CE, Omura JD, Paluch AE, Thomas WI, Fulton JE (2020) Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act 17:78
Hamilton MT, Hamilton DG, Zderic TW (2007) Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56:2655–2667
Hung CL, Huang CJ, Tsai YJ, Chang YK, Hung TM (2016) Neuroelectric and behavioral effects of acute exercise on task switching in children with attention-deficit/hyperactivity disorder. Front Psychol 7:1589
Jayedi A, Gohari A, Shab-Bidar S (2022) Daily step count and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Sports Med 52:89–99
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26(Suppl 1):S28-37
Koepp GA, Moore GK, Levine JA (2016) Chair-based fidgeting and energy expenditure. BMJ Open Sport Exerc Med 2:e000152
Lam TM, Vaartjes I, Grobbee DE, Karssenberg D, Lakerveld J (2021) Associations between the built environment and obesity: an umbrella review. Int J Health Geogr 20:7
Levitan EB, Song Y, Ford ES, Liu S (2004) Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 164:2147–2155
Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ (2020) Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sports Med 50:295–330
Magnon V, Vallet GT, Auxiette C (2018) Sedentary behavior at work and cognitive functioning: a systematic review. Front Public Health 6:239
Maxwell SE, Delaney HD (1990) designing experiments and analyzing data: a model comparison perspective, Belmont, CA. Wadsworth, USA
Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA (2013) Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc 45:1600–1609
Mete EM, Perry TL, Haszard JJ, Homer AR, Fenemor SP, Rehrer NJ, Skeaff CM, Peddie MC (2018) Interrupting prolonged sitting with regular activity breaks does not acutely influence appetite: a randomised controlled trial. Nutrients, 10.
Narang BJ, Atkinson G, Gonzalez JT, Betts JA (2020) A tool to explore discrete-time data: the time series response analyser. Int J Sport Nutr Exerc Metab 30:374–381
O'neil CE, Byrd-Bredbenner C, Hayes D, Jana L, Klinger SE, Stephenson-Martin S (2014) The role of breakfast in health: definition and criteria for a quality breakfast J Acad Nutr Diet 114:S8–S26
Ridgers ND, Timperio A, Cerin E, Salmon J (2014) Compensation of physical activity and sedentary time in primary school children. Med Sci Sports Exerc 46:1564–1569
Rowland TW (1998) The biological basis of physical activity. Med Sci Sports Exerc 30:392–399
Saint-maurice PF, Troiano RP, Bassett DR, JR, Graubard BI, Carlson SA, Shiroma EJ, Fulton JE, Matthews CE (2020) Association of daily step count and step intensity with mortality among US adults. JAMA, 323: 1151–1160
Silva AM, Judice PB, Carraca EV, King N, Teixeira PJ, Sardinha LB (2018) What is the effect of diet and/or exercise interventions on behavioural compensation in non-exercise physical activity and related energy expenditure of free-living adults? A systematic review. Br J Nutr 119:1327–1345
Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D (2019) Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol 73:2062–2072
Stoner L, Willey Q, Evans WS, Burnet K, Credeur DP, Fryer S, Hanson ED (2019) Effects of acute prolonged sitting on cerebral perfusion and executive function in young adults: a randomized cross-over trial. Psychophysiology 56:e13457
Teixeira V, Voci SM, Mendes-Netto RS, da Silva DG (2018) The relative validity of a food record using the smartphone application MyFitnessPal. Nutr Diet 75:219–225
Turner JE, Markovitch D, Betts JA, Thompson D (2010) Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. Am J Clin Nutr 92:1009–1016
Walhin JP, Chen YC, Hengist A, Bilzon J, Betts JA, Thompson D (2018) The effects of different forms of daily exercise on metabolic function following short-term overfeeding and reduced physical activity in healthy young men: study protocol for a randomised controlled trial. Trials 19:199
Wanders L, Cuijpers I, Kessels RPC, Van de Rest O, Hopman MTE, Thijssen DHJ (2021) Impact of prolonged sitting and physical activity breaks on cognitive performance, perceivable benefits, and cardiometabolic health in overweight/obese adults: the role of meal composition. Clin Nutr 40: 2259-2269
Waters CN, Ling EP, Chu AH, Ng SH, Chia A, Lim YW, Muller-Riemenschneider F (2016) Assessing and understanding sedentary behaviour in office-based working adults: a mixed-method approach. BMC Public Health 16:360
Wennberg P, Boraxbekk CJ, Wheeler M, Howard B, Dempsey PC, Lambert G, Eikelis N, Larsen R, Sethi P, Occleston J, Hernestal-Boman J, Ellis KA, Owen N, Dunstan DW (2016) Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study. BMJ Open 6:e009630
Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW (2017) Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (n Y) 3:291–300
Acknowledgements
The authors thank all the participants for their time and effort in participating in this project.
Author information
Authors and Affiliations
Contributions
F-CK was responsible for study design, funding, data collection and manuscript revision. Y-TL was responsible for study conduct, data collection and manuscript revision. T-YC, Y-KC and T-MH were responsible for the design of cognitive tests and manuscript revision. Y-CC was responsible for the study design, funding, data and statistical analysis, manuscript revision and wrote the initial draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Klaas R Westerterp.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuo, FC., Lin, YT., Chueh, TY. et al. Breaking prolonged sitting increases 24-h physical activity and self-perceived energy levels but does not acutely affect cognition in healthy adults. Eur J Appl Physiol 124, 445–455 (2024). https://doi.org/10.1007/s00421-023-05278-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05278-1