Abstract
Purpose
We examined whether eccrine sweat glands ion reabsorption rate declined with age in 35 adults aged 50–84 years. Aerobic fitness (VO2max) and salivary aldosterone were measured to see if they modulated ion reabsorption rates.
Methods
During a passive heating protocol (lower leg 42 °C water submersion) the maximum ion reabsorption rates from the chest, forearm and thigh were measured, alongside other thermophysiological responses. The maximum ion reabsorption rate was defined as the inflection point in the slope of the relation between galvanic skin conductance and sweat rate.
Results
The maximum ion reabsorption rate at the forearm, chest and thigh (0.29 ± 0.16, 0.33 ± 0.15, 0.18 ± 0.16 mg/cm2/min, respectively) were weakly correlated with age (r ≤ − 0.232, P ≥ 0.05) and salivary aldosterone concentrations (r ≤ − 0.180, P ≥ 0.179). A moderate positive correlation was observed between maximum ion reabsorption rate at the thigh and VO2max (r = 0.384, P = 0.015). Salivary aldosterone concentration moderately declined with age (r = − 0.342, P = 0.021). Whole body sweat rate and pilocarpine-induced sudomotor responses to iontophoresis increased with VO2max (r ≥ 0.323, P ≤ 0.027) but only moderate (r = − 0.326, P = 0.032) or no relations (r ≤ − 0.113, P ≥ 0.256) were observed with age.
Conclusion
The eccrine sweat glands’ maximum ion reabsorption rate is not affected by age, spanning 50–84 years. Aldosterone concentration in an aged cohort does not appear to modulate the ion reabsorption rate. We provide further support for maintaining cardiorespiratory fitness to attenuate any decline in sudomotor function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sweat production is an important mechanism of heat loss, but sweat gland atrophy that accompanies age reduces evaporative heat loss and increases the susceptibility of older adults to heat-related illness or injury (Baccini et al. 2008). The age-related decline in sudomotor function reportedly occurs around 60 years of age (Kenney and Fowler 1988; Abdel-Rahman et al. 1992). The evaporative potential of sweat is also influenced by its ion (i.e. sodium chloride) concentration. An elevated concentration of sweated ions on the skin surface reduces the water vapour pressure of the skin surface and thus reduces the water vapour gradient between the skin and ambient air attenuating evaporative heat loss for a given sweat rate. Hall et al. (1990) suggested that sweat sodium concentration increases with age and Inoue et al. (1991, 1995, 1999) have consistently reported attenuated sweat rates in older males, yet similar, if not higher, sweat [Na +] concentrations compared to younger counterparts. If sweat sodium concentration increases with age then this, alongside the age-related decline in sweat gland function may further exacerbate the heat-related illness amongst an older population. Alternatively, could a reduced sweat sodium concentration serve to counteract the effects of a reduced sweat gland output to increase sweating efficiency?
The literature is scant of studies investigating both sweat composition and the associated concentrations in an aged population. More research from this cohort is required, especially with the rapid developments of wearable (sweat) sensors.
The concentration of sodium chloride on the skin surface is influenced by the movement of ions into and out of the gland at two distinct portions of the sweat gland; the secretory coil at the base of the gland and a straight reabsorptive duct that projects towards the skin surface. An essential step in sweat formation is the movement of sodium and chloride ions into the secretory coil following stimulation from adrenergic or cholinergic nerves (Quinton 2007). The accumulation of these ions draws water into the lumen of the gland via osmosis, creating a primary sweat solution that is isotonic relative to plasma. Whilst the movement of these ions into the secretory coil is necessary for sweat formation, an excessive loss has the potential to disrupt homeostasis (Quinton 2007). The straight reabsorptive duct prevents this by reabsorbing sodium and chloride through epithelial sodium channels (ENaC) and cystic fibrosis transmembrane conductance regulator (CFTR) channels, respectively (Reddy and Quinton 2003). The reabsorption rate is limited, which determines the concentration of these ions in surface sweat (Buono et al. 2007, 2008; Amano et al. 2016). The controlling mechanisms are unknown but aldosterone has been reported to promote sodium reabsorption in distal human sweat glands much akin to its function in the kidneys (Hegarty and Harvey 1999; Harvey and Higgins 2000). Decreasing aldosterone concentration reportedly accompanies age (Nanba et al. 2018), which may in turn influence the ion reabsorption of the sweat glands. It is currently unknown whether the maximum ion reabsorption rate of the sweat glands declines with age. Furthermore, the age-related decline in sudomotor function is reportedly body region dependent, with the extremities declining faster than the torso (Inoue and Shibasaki 1996; Coull et al. 2020). We have consistently reported regional differences in the maximum ion reabsorption rates of young healthy adults, with attenuated maximum ion reabsorption rates at the extremities in comparison to torso regions (Amano et al. 2017; Gerrett et al. 2018a). Regional differences in ion reabsorption rates may be associated with the structure and size of the sweat gland or sensitivity to regulating hormones. It is likely that these regional differences are also apparent in older adults but if the eccrine glands’ reabsorption rates decline with age, whether the sweat glands located in extremities show a tendency to deteriorate more quickly than the torso remains unknown.
Exercise training has been shown to enhance the sweat glands' maximum ion reabsorption rates in young adults (Amano et al. 2017). However, there is some data suggesting that sweat sodium concentration does not differ between young trained and untrained individuals after normalising for sweat rate (Hamouti et al. 2011). It remains unclear the role of fitness status on ion reabsorption rates. However, for older adults, the age-related decline in some parameters of sudomotor function, including sweat sodium concentrations are known to be moderated by maintaining good levels of cardiorespiratory fitness (Inoue et al. 1999).
The aim of this experiment was to determine whether the association between advancing age and the eccrine sweat glands' maximum ion reabsorption rates across different regions of the body. The age-related decline in other sudomotor parameters reportedly occurs around 60 years, so we selected an age group to straddle this (50–84 years). We hypothesised that maximum ion reabsorption rates would decline with advancing age and would show the largest decline at the extremities compared to the torso. We also hypothesised that the decline in ion reabsorption rates would be associated with the age-related decline in aldosterone concentration. In line with other markers of sudomotor function, we hypothesised that there would be a positive relation between maximal oxygen uptake (VO2max) and the maximum ion reabsorption rates. We measured other thermoregulatory responses to a passive heat stress test and sweat response to pilocarpine administrated by a transdermal iontophoresis to observe any concomitant age-related changes.
Methods
Participants were informed about the study purpose and procedures prior to providing verbal and written consent. The Human Subjects Committee of the Graduate School of Human Development and Environment at Kobe University (Japan) approved the study (report no. 259), which conforms to the standards set out by the Declaration of Helsinki. Participants visited the laboratory on two separate occasions. The first visit involved a submaximal assessment of aerobic fitness, skin fold analysis and a sweat sensitivity test using iontophoresis. The second test was a passive heat stress test to assess thermophysiological responses, including the sweat glands' maximum ion reabsorption rates. All testing took place in the morning, starting between 0800-1000 h between the months of October and May (autumn through spring) in Japan. All tests have previously been described as part of another study using a small subset of the recruited participants (n = 10) for a different research question (Gerrett et al. 2020).
Participants
Thirty-five un-acclimated adults spanning the age of 50–84 years were recruited for this study (Table 1). Our previous research has reported no sex-related differences in ion reabsorption rates so males and females were recruited (Amano et al. 2017). Caffeine, alcohol and strenuous exercise 24 h preceding the experimental trials were not permitted and confirmed by all participants. All participants were instructed to consume 10 ml/kg of water 0–3 h prior to all experiments to promote euhydration. Hydration status was measured using a handheld refractometer (Atago Co.Ltd, Tokyo, Japan), with a urine specific gravity value ≤ 1.025 indicating euhydration. All participants met the hydration requirements prior to passive heating. All participants were non-smokers and nine participants were taking Amlodipine for blood pressure regulation, one participant was taking Pitavastatin calcium for high cholesterol and one participant was taking both aforementioned drugs.
Pre-test sessions—fitness, anthropometric and sweat sensitivity tests
Participants' height and body mass were measured and subsequently used to calculate body surface area (Du Bois and Du Bois 1916). Skinfold thicknesses were measured in triplicate (Eiyoken-Type, Meikosha Co., LTd., Tokyo, Japan) at four locations (biceps, triceps, subscapular suprailiac) with median scores recorded and summed. All participants completed the YMCA submaximal fitness test (Golding 2000) on a cycle ergometer (Aerobike 75XLIII, Combi Wellness Corp, Tokyo, Japan) to estimate maximal oxygen uptake (VO2max).
A pilocarpine iontophoresis sweat stimulation test was conducted to assess sweat gland function, sweat gland sensitivity, the number of activated sweat glands (ASG) and the sweat output per gland (SGO). Pilocarpine (1%) dissolved in saline was impregnated into a plastic capsule (6.15cm2) filled gauze (F1515; Osaki Medical, Aichi, Japan). This was attached to the mid-ventral forearm using a spandex rubber band whilst a 1.5 mA iontophoresis current was applied for 5 min (cathode, HV-LLPD, Omron Healthcare, Kyoto, Japan). Immediately after iontophoresis, the capsule was removed and the skin surface wiped clean and then replaced by a ventilated capsule (5.31cm2) to measure sweat rate (SR). A 12-min sample was measured with the initial 2 min were discarded and then a 10 min average obtained. Upon completion, the ventilated sweat capsule was removed, the area wiped dry and then wiped with an iodine-soaked cotton gauze. Residual iodine was removed and then the area was stamped with starch paper for approximately 3 s. The iodine was transferred from the ASG to the paper as indicated by small dots. The paper was scanned and magnified to allow for duplicate visual counting by the same investigator, within a defined area (1cm2). The SGO was calculated by dividing SR by the number of ASG.
Experimental sessions—passive heat stress test
The passive heat stress test was conducted in an environmental chamber (SR-3000; Nagano Science, Osaka, Japan) maintained at 25 °C, 50% RH with minimal air movement. Participants provided a urine sample to confirm hydration status. Nude body weight was also measured using platform scales (ID1 Mettler-Toledo, Germany; resolution of 1 g). Participants wore shorts (and sports bra for females) and donned a water-perfused suit covering the entire body except the head, neck, hands and below the knee. Participants rested in a semi-supine position whilst measuring instruments were prepared, this included skin (Tsk) and sublingual temperature (Tor), SR, galvanic skin conductance (GSC), skin blood flow, mean arterial pressure and the collection of a salivary aldosterone sample. During this time (approx. 35 min) water at 34 °C was passed through the suit to maintain a uniform and stable resting Tsk. After 5-min baseline measurement, participants submerged their lower legs into a water bath set at 42 °C and the water inside the suit was increased to 38 °C. We aimed for all participants to complete 60 min of passive heating but some found it too uncomfortable and the test was terminated earlier. During the test, once sweating began, the right volar forearm or right anterior thigh was cleaned and a customised sweat patch (Parafilm containing a cotton gauze) was attached for the measurement of sweat NaCl concentration. Prior to the end of the experiment, another salivary aldosterone sample was collected. From the chest, forearm and thigh the number of heat-ASG using the starch iodine technique was measured. After terminating the test, participants towel-dried themselves and were weighed nude body for the assessment of whole-body sweat rate.
Measurement and calculations
Sublingual temperature was used as an index of core body temperature. A sublingual thermocouple (RET-1, Type T, Copper Constantan thermocouple, Physitemp Instruments, Inc., Clifton, NJ, USA) was inserted into the mouth and positioned under the tongue (sulcus). Participants were instructed to keep their mouths closed during the experiment. Skin temperature (Tsk) was measured using copper-constantan thermocouples (Inui Engineering, Higashi Osaka, Japan) from 8 locations (forehead, chest, back, upper arm, forearm, hand, thigh and calf). Mean Tsk was calculated from 8 sites (Stolwijk and Hardy 1966) and mean body temperature (Tb) using 0.9 (Tor) and 0.1 (mean Tsk) weightings (Gisolfi and Wenger 1984).
SR, GSC and skin blood flow were measured from the midventral forearm, mid-chest and thigh (one third the length of the thigh from the knee cap). SR was measured using the ventilated capsule technique, whereby dry nitrogen gas passes through a capsule (3.46 cm2) affixed to the skin at a flow rate of 500 ml/min. The outflow temperature and humidity were measured using a capacitance hygrometer (HMP50; Vaisala, Helsinki, Finland). The apparatus was flushed approximately 1 h prior to each experiment and once the capsule was affixed to the skin and for a further 30 min prior to data collection to ensure stable readings. Capsules were attached to the skin using collodion. Either side of the capsule were two Ag/AgCl electrodes (Vitrode J, Nihon Kohden, Tokyo, Japan) for measuring GSC (MP100 and GSC100C; Biopac, Goleta CA, USA). GSC is expressed as a change from baseline (∆GSC), recorded during the 5-min resting phase prior to heating. Cutaneous vascular conductance was estimated by measuring skin blood flow on each site using laser-Doppler velocimetry (ALF21; Advanced, Tokyo, Japan) adjacent to the sweat capsule and affixed to the skin with tape (3 M Transpore surgical tape, 3 M Health Care, USA). Cutaneous vascular conductance was calculated as a percentage of the baseline value (recorded during the resting phase prior to heating).
Tor, local Tsk, skin blood flow, SR and GSC were recorded every second by a data logger (MX100; Yokogawa, Tokyo, Japan). The core temperature onset thresholds for SR and cutaneous vasodilation at each site were determined using segmental linear regression (Cheuvront et al. 2009). The slopes were defined as the linear portion of the changes in SR and cutaneous vascular conductance after the onset thresholds.
Heart rate and arterial blood pressure were continuously measured on the left middle finger using a Finometer (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands); mean arterial pressure (MAP) was subsequently calculated.
A sweat sample was collected for the measurement of sodium chloride concentration. Despite research suggesting that the thigh sweat samples are most representative of the entire body (Patterson et al. 2000), sweat production from the thigh was often too low for our older adults, so in most cases, the forearm was also sampled and used. After wiping with alcohol, rinsed with distilled water and dried with a sterile towel a 4 × 4 cm custom-made sweat patch (100,601; Askul, Tokyo, Japan) was attached to the skin. Sterile gloves were worn during application and removal to prevent any contamination and sterile tweezers used to remove patches from the skin. The patch application time varied per participant, depending upon visual inspection of the patch and skin surface sweat. The sampling time was recorded. After removal, patches were immediately placed inside an airtight plastic tube (Sarstedt Salivettes), centrifuged at 4000 RPM for 10 min and re-weighed. Extracted sweat was analysed using the Wescor (3120 Sweat-Chek™, Wescor, Logan, UT, USA) which provided a unit of mmol/L (equivalent sodium chloride: NaCl) based on the calculation from the sweat conductivity. The cotton gauze patch (Medical cotton 70,975,000, Suzuran Medical Inc, Japan) was checked for background contamination. The SR (mg/cm2/min) of these samples were determined using the mass (mg) of the patch pre- and post-application, the surface area (cm2) of the patch and the sample time (min). Sweat patches were weighed on scales (AB54 Mettler-Toledo, Germany; resolution of 0.1 mg).
To confirm the acute thermal load of the passive heating test on aldosterone concentration, a saliva sample was collected prior to, and after the passive heat tests. Saliva was collected from the participants by chewing a plain cotton swab for 60 s. The cotton swab was placed into a Salivette™ (Sarstedt, Newton, NC, USA) tube and spun at 4000RPM for 10 min. Salivary samples were frozen at −30 °C until later analysis. After thawing, aldosterone (pg/ml) levels were quantified by competitive ELISA (LDN, GmbH & Co.KG, Germany).
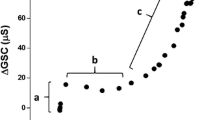
The determination of the sweat glands’ maximum reabsorption rate of the sweat glands was obtained by plotting ΔGSC against ∆SR (Amano et al. 2016, 2017; Gerrett et al. 2018a). By plotting this relation, it is possible to identify three distinct phases; representing different stages of sweat production. In the first phase, there is an increase in ∆GSC but no change in ∆SR, which represents the isosmotic precursor sweat production in the proximal secretory coil. Such changes in ∆GSC and no changes in ∆SR are frequently utilised to identify pre-secretory sweat gland activity (Machado-Moreira et al. 2009; Gerrett et al. 2018b). In the second phase, an increased ∆SR without an increase in ΔGSC can be observed. As ∆GSC is influenced by both the amount of sweat produced as well as the electrolyte concentration the fact that ∆SR increases but there is no change in ∆GSC represents reabsorption of sweated ions in the sweat duct. Once the rate of sweat ion secretion exceeds its reabsorption limit in the duct then the third phase occurs where there is a proportional increase in ΔGSC with increasing ∆SR. The point at which the 2nd and 3rd phases intersect is used to identify the maximum rate of sweat glands’ ion reabsorption. In the present study, the thresholds were determined using segmented regression analysis.
Data analysis
Statistical analyses were carried out using SPSS (Version 25). Figures were produced using GraphPad Prism (version 7). Significance was set at P < 0.05 and data are presented as mean and standard deviations (± SD), unless otherwise stated. To assess the normality of the data, frequency histograms and Q–Q plots were visually inspected and Kolmogorov–Smirnov test was conducted. Data that violated normality were transformed using either natural-log or square root prior to further statistical tests. All transformed data are illustrated as raw data for clarity. To determine the relation between the sweat glands' maximum ion reabsorption rates and age, VO2max or aldosterone concentration, Pearson’s product-moment correlation was conducted. The relation between thermoregulatory responses to the passive heat test with age or VO2max was also explored using Pearson’s product-moment correlation. Pearson’s correlation coefficient was classifieds as being weak (0.10 ≤ r < 0.30), moderate (0.30 ≤ r < 0.50), and strong (r ≥ 0.50). The 95% confidence interval (95% CI) of the Pearson’s coefficient was included. To determine any age-related regional differences, the slopes were compared between the three locations with univariate analysis.
One-way repeated measure ANOVAs were conducted to determine any regional differences in the following sudomotor and vasomotor responses: sweat glands’ maximum ion reabsorption rates, local SR, the Tor threshold and slope for sweating and vasodilation. Bonferroni adjusted Students t tests were used post hoc for analysis of the main effect of regional location. All regional data were compared to each other (i.e., forearm, chest and thigh). All data were adjusted using Greenhouse–Geisser method when the sphericity assumption was violated. Non-normal data were log transformed prior to analysis, this applied to the maximum ion reabsorption data. A student paired t test was used to compare pre- and post-passive heating salivary aldosterone concentrations.
Results
Prior to passive heating, the mean resting heart rate, mean arterial pressure, mean Tsk and Tor for all participants were 64 ± 10 bpm, 97.7 ± 10.2 mmHg, 33.76 ± 0.56 °C, 36.76 ± 0.25 °C, respectively. We aimed for all participants to complete 60 min of passive heating and the average test duration was 54 ± 7 min. Two participants requested for the test to be terminated early after 35 min due to thermal discomfort. The thermophysiological, sudomotor and hormonal responses to the passive heating test are shown in Table 2, whilst the cardiovascular responses are shown in Table 3. Due to the different passive heating duration, relevant data are presented as the mean of the last 5 min of passive heating. Regardless of their passive heating exposure time, the thresholds and slopes for sweating and vasodilation could be identified in all participants. The ion reabsorption rates at the chest and forearm were identified in all participants, but in three participants, the thigh maximum ion reabsorption was not achieved.
The group average ∆SR threshold for an increasing ∆GSC was 0.29 ± 0.16 mg/cm2/min for the forearm, 0.33 ± 0.15 mg/cm2/min for the chest and 0.18 ± 0.16 mg/cm2/min for the thigh. There was a main effect of location (P = 0.001) and post hoc analysis indicated that the forearm and chest were both higher than the thigh (P = 0.007 and P = 0.001, respectively). The forearm and chest were not different (P = 0.999). There were no differences in the slopes of the regression lines between the three locations (P = 0.777). For the three measured locations, the relation between the ∆SR threshold for an increasing ∆GSC with age, VO2max and aldosterone concentration is illustrated in Fig. 1. The ∆SR threshold for an increasing ∆GSC, across the three measured locations, were weakly correlated with age (r ≤ − 0.232, P ≥ 0.05). There was also no correlation between the ∆SR threshold for an increasing ∆GSC and VO2max at the forearm (r = 0.012, P = 0.472) or chest (r = − 0.134, P = 0.222) but a moderate positive correlation for the thigh (r = 0.384, P = 0.015). Weak correlations were observed between the ∆SR threshold for an increasing ∆GSC and pre-passive heating salivary aldosterone concentrations for all locations (r = 0.013 to − 0.180, P ≥ 0.179).
The relation between age (left-side panels), VO2max (middle panels), pre-passive heating saliva aldosterone concentration (right-side panels) and the ∆SR threshold for an increasing ∆GSC at the forearm (n = 35), chest (n = 35) and thigh (n = 32). The middle line is the regression line and the outer lines are the borders of the 95% confidence intervals for the regression lines. Black circles indicate individual data points for males, whilst open circles are female participants
Sweat samples, from the forearm, were collected and analysed for the equivalent sweat NaCl concentration. Due to low forearm SR in our cohort, it was only possible to collect enough sweat for NaCl analysis from 21 of our 35 participants. The group average sweat NaCl concentration after passive heating was 50 ± 13.5 mmol/L. After being corrected for local SR the average was 33.4 ± 20.4 nmol/cm2/min. The relation between sweat NaCl concentration and age and VO2max are displayed in Fig. 2. There was no relation between sweat NaCl and age (r = 0.06, P = 0.796) and a weak positive relation between sweat NaCl and VO2max (r = 0.104, P = 0.653). Whilst a weak positive relation was observed between sweat NaCl corrected for SR with age (r = 0.124, P = 0.593), a strong relation was observed with VO2max (r = 0.527, P = 0.014).
The relation between age (top panel) and VO2max (bottom panel) and the equivalent sweat NaCl concentration (n = 21). For 2 participants the thigh was the sample site, for the remaining participants the forearm was the sample site. The midlle line is the regression line and the outer lines are the borders of the 95% confidence intervals for the regression lines. Black circles indicate individual data points for males, whilst open circles are female participants
The group average whole body sweat rate after passive heating was 0.28 ± 0.17 L/m2/hr. There was a moderate negative relation between whole body sweat rate and age (r = − 0.326, P = 0.032) and a moderate positive relation between whole body sweat rate and VO2max (r = 0.484, P = 0.002, Fig. 3). When Tor increased by + 0.8 °C, the local SR at the forearm, chest and thigh were 0.46 ± 0.20, 0.54 ± 0.29 and 0.26 ± 0.17 mg/cm2/min, respectively. There was a main effect of location (P = 0.001) and post hoc analysis indicated that SR was greater at the chest than both the forearm (P = 0.016) and thigh (P = 0.001). Forearm SR was also greater than the thigh (P = 0.001). Local SR at all locations was weakly correlated with age (r ≤ − 0.286, P ≥ 0.045) and there were no differences in the slopes of the regression lines between the three locations (P = 0.829). Forearm SR was strongly correlated with VO2max (r = 0.560, P = 0.001) whilst weak correlations with VO2max were observed with the chest (r = − 0.134, P = 0.222) and thigh (r = 0.219, P = 0.100).
The relation between age (top panel) and whole body sweat rate (WBSR) and VO2max (bottom panel) and WBSR (n = 35). The middle line is the regression line and the outer lines are the borders of the 95% confidence intervals for the regression lines. Black circles indicate individual data points for males, whilst open circles are female participants
Pilocarpine was administered via iontophoresis and the density of ASG from this test was 107 ± 25 glands/cm2. The SR was 0.58 ± 0.24 mg/cm2/min and the SGO was 5.4 ± 1.9 µg/gland/min. There were weak correlations between these parameters and age (r ≤ -0.113, P ≥ 0.256). However, moderate-to-strong correlations were observed between VO2max with the number of ASG (r = 0.323, P = 0.027), the SGO (r = 0.482, P = 0.001) and local SR (r = 0.562, P < 0.001) (see Fig. 4).
The relation between age (left-sided panels) or VO2max (right-sided panels) with (A) the activated sweat gland density, (B) the sweat gland output per gland and (C) the sweat rate following pilocarpine stimulation via iontophoresis (n = 35). The middle line is the regression line and the outer lines are the borders of the 95% confidence intervals for the regression lines. Black circles indicate individual data points for males, whilst open circles are female participants
The group average Tor onset for sweating was 37.06 ± 0.29 °C for the forearm, 37.12 ± 0.30 °C for the chest and 37.05 ± 0.32 °C for the thigh. There was a main effect of location (P = 0.040) but location differences were not identified post hoc (P ≥ 0.098). For the three measured locations, the relation between Tor onset for sweating and age is illustrated in Fig. 5. Moderate correlations were observed between the Tor onset for sweating on the forearm r = 0.351, P = 0.018) and thigh (r = 0.377, P = 0.012) but not the chest (r = 0.276, P = 0.052). There were no differences in the slopes of the regression lines between the three locations (P = 0.855). There was also no correlation between VO2max and the Tor onset for sweating at any location (r ≤ 0.172, P ≥ 0.158). There was a main effect of location for the slopes for sweating (P = 0.001), with all locations being significantly different from each other (P ≤ 0.040). The Tor slopes for sweating (i.e., sweat) were weakly correlated with age for all locations (r ≤ 0.173, P ≤ 0.294) but no differences in the slopes of these regression lines between the three locations were found (P = 0.942). The Tor slopes for sweating (i.e. sweat) were weakly correlated with VO2max (r ≤ 0.168, P ≥ 0.164, see Table 2).
The relation between age or VO2max with the Tor threshold for sweat onset (left-sided panels) and the Tor threshold for vasodilation (right-sided panels) (n = 35). The middle line is the regression line and the outer lines are the borders of the 95% confidence intervals for the regression lines. Black circles indicate individual data points for males, whilst open circles are female participants
The group average Tor onset for vasodilation was 37.10 ± 0.38 °C for the forearm, 37.14 ± 0.38 °C for the chest and 37.25 ± 0.36 °C for the thigh. There was a main effect of location (P = 0.002) with a lower onset Tor for vasodilation occurring at the forearm compared to the thigh (P = 0.039) but no differences were found between the chest- and forearm (P = 0.052), or the chest and thigh (P = 0.937). For the three measured locations, the relation between Tor onset for vasodilation and age is illustrated in Fig. 5. Moderate correlations were observed between the Tor onset for vasodilation on the forearm r = 0.403, P = 0.007) and chest (r = 0.379, P = 0.011) and the thigh (r = 0.357, P = 0.016). There were no differences in the slopes of the regression lines between the three locations (P = 0.958). There was also no correlation between VO2max and the Tor onset for vasodilation at any location (r ≤ 0.179, P ≥ 0.147). There was a main effect of location for the slopes for cutaneous vascular conductance (P = 0.003), but location differences were not identified post hoc (P ≥ 0.076). The Tor slopes for cutaneous vascular conductance were weakly correlated with age for all locations (r ≤ −0.137, P ≥ 0.301) and there were no differences in the slopes of the regression lines between the three locations (P = 0.828). The Tor slopes for cutaneous vascular conductance were weakly correlated with VO2max (r ≤ 0.142, P ≥ 0.449, Table 3).
Pre- and post-passive heating salivary aldosterone concentrations were not different (84.5 ± 44.7 pg/ml and 98.1 ± 34.9 pg/ml, P = 0.124). There were weak correlations between pre and post-passive heating salivary aldosterone concentration and age (r = 0.198–0.225, P ≥ 0.112) and no relation with urine aldosterone concentration and age (r = 0.196, P = 0.137).
There was a moderate negative correlation between VO2max and age (0.342, P = 0.021).
Discussion
The primary aim of this study was to investigate the relation between advancing age (50–84 years) and the maximum ion reabsorption rates of eccrine sweat glands. In contrast to our hypothesis, we found that, unlike other sudomotor responses, the maximum ion reabsorption rate of eccrine glands is not affected by age (spanning 50–84 years). We found no relation between ion reabsorption rate and aldosterone concentration, which has previously been proposed as a reason for reduced sweat sodium output in older individuals. Our data do not support this hypothesis. We found some evidence providing further support for the importance of maintaining cardiorespiratory fitness to attenuate any decline in sudomotor function, but for the ion reabsorption rate, this was not homogenous across the body.
Ion reabsorption rates with age
Normative data on sweat sodium and chloride concentration in older adults are sparse, with findings suggesting either similar or greater sweat sodium concentration in older compared to younger individuals (Hall et al. 1990; Inoue et al. 1991, 1995, 1999). In contrast to previous studies, we did not compare groups of distinctly different ages. We assessed the relation between sweat NaCl and age in healthy adults spanning an age range of 50–84 years and found no relation. The mean sweat sodium chloride concentration in the present study (50 ± 13.5 mmol/L) is similar to previously reported concentrations in younger athletic adults (age 34 ± 4 years, midventral forearm sodium: 51.3 ± 21.5 mmol/L and chloride: 36.9 ± 23.2 mmol/L) (Baker et al. 2020). However, it is important to note that these values are not corrected for sweat rate, which is typically lower in older adults. The lower sweat rates in older individuals would suggest a higher sweat sodium concentration for a given sweat rate, indicating an impaired ion reabsorption capacity. However, we found no relation between the sweat glands’ maximum ion reabsorption rate and age, ranging from 50 to 84 years. Previously, we have conducted similar passive heating protocols with young adults (21.7 ± 3.0 years, n = 12) and observed local maximum reabsorption rates (chest: 0.39 ± 0.27 and forearm: 0.36 ± 0.40 mg/cm2/min (Gerrett et al. 2018a) that are comparable to the average of the older adults in the present study (chest and forearm; 33 ± 0.15 and 29 ± 0.16 mg/cm2/min, respectively). The slightly lower maximum ion reabsorption rates in our older participants are most likely associated with small methodological differences (i.e. the use of a water perfused suit) between the two studies. For exploratory purposes, we included this data into the correlation analysis and we still observed no relations between ion reabsorption rate and age at the chest (r = − 0.071, P = 0.633) or forearm (r = 0.094, P = 0.528). Collectively, the data does not indicate any effect of advancing age (> 50 years) on the sweat glands' capacity for ion reabsorption.
Alongside assessing sweat glands' ion reabsorption rate, we also measured other sudomotor responses to passive heat stress and pilocarpine stimulation via iontophoresis. As expected, we did observe a moderate negative relation between age and whole body sweat rate. However, local SR at all locations was weakly correlated with age. This relation was assessed using local SR data that corresponded to an increased ∆Tor of 0.8 °C at all locations, and as such removed the influence of the age-related difference in body core temperature. Previous studies have reported a delay or higher body temperatures for the onset of sweating (Inoue and Shibasaki 1996; Smith et al. 2013) and we did observe moderate correlations with the Tor threshold and the slope for sweating with age. The age-related decline in sudomotor function has been associated with attenuated sudomotor innervation (Vilches et al. 2002) sweat gland atrophy and/or decreases in cholinergic sensitivity (Kenney and Fowler 1988; Inoue et al. 1999). The latter has recently been disputed by Smith et al. (2013) and our data supports this, as we observed no relation between age and sweat rate responses to pilocarpine stimulation via iontophoresis. Collectively our data suggest that the decline in sudomotor function, which reportedly occurs around 60 years of age, is specific to certain parameters of sudomotor function, of which the sweat glands’ ion reabsorption rate and cholinergic sensitivity is not one of them.
Cardiorespiratory fitness
It is well known that sweat glands adapt to repeated heat exposure (i.e., heat acclimation) by increasing the sweat output per gland and enhancing the ion reabsorption capacity to prevent an excess sweated ion loss. Most of the evidence for this is on young adults (Buono et al. 2007; Amano et al. 2016) but we have recently reported this adaptation can also occur in healthy older adults if the stimulus for sweating during heat acclimation is strong enough (Gerrett et al. 2020). Habitual exercise training shares common heat acclimation phenotypes (Ravanelli et al. 2020), but it is not fully clear if enhanced sodium reabsorption is one of them. Amano et al. (2017) previously investigated the effect of exercise training on maximum ion reabsorption rates and found that sedentary young adults had a lower ion reabsorption rate compared to trained sprinters and endurance athletes. However, Hamouti et al. (2011) reported no differences between training status and Na+ reabsorption rate. They categorised continuous data (VO2max) and compared groups, whilst we have used linear regression to assess if any association exists. Furthermore, they tested young adults, whilst we sampled an older population (> 50 years) and research suggests that maintaining good cardiorespiratory fitness attenuates the deterioration in numerous thermoregulatory responses to heat stress. In older adults, we observed moderate correlations between maximum ion reabsorption rates at the thigh and VO2max but not the chest or forearm. It is not clear why this differed regionally across the body but one possibility could reside in the aforementioned hypothesis proposed by Inoue et al. (2004) regarding regional decline in sweat gland function. If sweat gland function does decline at the lower extremities first with advancing age but exercise training attenuates the decline in sweat gland function, maybe those with a higher VO2max can preserve sudomotor function, especially at the thigh.
Whilst the estimated VO2max of our older cohort is much lower than the aforementioned studies with young adults, they are representative of the fitness status of our sampled age group (Kaminsky et al. 2015). In response to the pilocarpine stimulation via iontophoresis, there was no association with advancing age but moderate correlations with VO2max. In line with previous studies, we also observed positive relations between whole body sweat rate and VO2max (Greenhaff 1989; Ichinose-Kuwahara et al. 2010). Inoue et al. (1999) suggested that that regular aerobic training and a high VO2max (e.g., 47.5 ± 4.1 ml/kg/min) can delay the age-related decline in sweat gland output and ion reabsorption. Whilst we did observe positive relation between some sudomotor responses and VO2max, only one participant had an estimated VO2max that exceeded 40 ml/kg/min. It may be that higher levels of aerobic fitness are required in older adults for cardiorespiratory fitness to have an effect on ion reabsorption rates.
Aldosterone and ion reabsorption rates with advancing age
The previously reported higher sweat sodium concentrations in older adults have been linked to the reduced circulating aldosterone concentration that accompanies ageing (Nanba et al. 2018). However, we observed no correlation between aldosterone concentration and advancing age and no relation between the maximum ion reabsorption rate and aldosterone concentration. The role of aldosterone on sodium reabsorption is better understood in kidneys, with sodium reabsorption occurring through genomic (slow) and nongenomic (fast response) mechanisms (Harvey and Higgins 2000). The nongenomic response of aldosterone has been shown to target the basolateral K + pump and Na + /H + exchanger via the activation of protein kinase C and calcium signalling, which stimulate Na+ reabsorption by increasing ENaC activity. This mechanism is well known to occur in the kidneys and despite structural differences, researchers have suggested that this occurs within the sweat gland due to similar functionality (i.e., ion perseveration). There is some evidence indicating that this also occurs in distal human sweat glands (Hegarty and Harvey 1999) but we are yet to find a relation between aldosterone and ion reabsorption rates in an ageing population. It is also feasible that end-organ sensitivity may affect ion reabsorption rate but there is no evidence to suggest that this declines with ageing. More research is required to determine the humoral mechanism that regulates ion reabsorption in the straight duct of eccrine sweat glands. Ten (of 35) participants were taking Amlodipine, which may have lowered circulating aldosterone concentration in these individuals.
Thermoeffector function and regional variations
Inoue and Shibasaki (1996) suggested that the decline in sudomotor function with ageing occurs sequentially across the body with the lower limbs declining first, then the upper body, upper limbs and the head last. In healthy older adults we did observe a lower maximum ion reabsorption rate at the thigh, but no differences between the chest and forearm. Ion reabsorption rates in young healthy adults have consistently been reported as heterogeneous across body sites (Amano et al. 2017; Gerrett et al. 2018a, 2020); with the torso area having a higher maximum ion reabsorption rate than the extremities. Whilst regional differences were apparent in our older adults, the information to date would suggest that this is an inherent physiological response as opposed to an age-related regional decline. We have previously suggested that these regional differences may be associated with structural differences in the sweat gland, particularly the length of the reabsorptive duct. Sato and Sato (1983) reported that persons with greater sweat gland output have larger glands. Although not reported in the results, we did observe a moderate relation between sweat gland output and the maximum ion reabsorption rate at the forearm (r = 0.439, P = 0.05) and chest (r = 0.277, P = 0.06) but not the thigh (r = − 0.047, P = 0.404). This provides some initial evidence that maximum ion reabsorption rates could be influenced by sweat gland size, but further exploration is warranted.
Regional differences in heat loss effector functions (i.e., sweating and cutaneous vasodilation) are frequently reported in the literature and our findings agree with these general observations: chest > forearm > thigh. The age-related decline in heat loss effector functions has been reported to occur at a non-uniform rate over the body (Inoue and Shibasaki 1996; Coull et al. 2020) but our data does not support this. We did not observe any differences between the slopes of the regressions lines for advancing age and sweat rate, sweat onset, or the sensitivity of the sweat glands to changes in Tor (i.e., the slope). This contrasts to the findings of Inoue and Shibasaki (1996) and Coull et al. (2020) but is in agreement with Smith et al. (2013) who also did not observe the aforementioned age-related sequential decline in regional sweat rate or sweat onset. The disparities between studies may be associated with the different heating methods (passive heating or exercise). Furthermore, the influence of age on some sudomotor functions has been reported to occur by the 5th decade (Larose et al. 2013) and thus any noticeable influence of age may have already taken place in our cohort.
We observed stronger relations between age and the onset and sensitivity for vasodilation than for sweating. This supports the findings of Inoue (1996) and Inoue et al. (2004) who proposed that the reductions in cutaneous vasodilation with age occur prior to sudomotor function loss. The onset for vasodilation occurred at a lower oral temperature at the forearm than the thigh. The onset for vasodilation was moderately correlated with age, but the slopes of the regressions lines for age and the Tor threshold for vasodilation did not differ significantly. The regional differences we observed in heat loss effector functions, in the present study, appear to be a result of natural heterogeneity across the body, as opposed to any age-related regional decline. Smith et al. (2013) noted no regional differences in cutaneous vasodilatation thresholds when data were expressed relative to a maximum CVC. The discrepancy amongst studies may be associated with normalisation procedures. We acknowledge that standardising to a baseline is dependent on a true baseline and our results may be confounded by this approach, especially the regional variations reported. We also analysed the absolute values (laser doppler flow, data not shown) and our results remain unchanged, so to our interpretation.
Limitations
The presented data are inclusive of male and female participants and the number of each sex was not balanced in each age group (Table 1). We included both sexes based on the assumption of no sex-related differences from our previous findings (Amano et al. 2017). Furthermore, D’Souza et al. (2020) reported that whilst age and sex are determinants of whole body heat exchange, the effects of increasing age on whole body heat loss are not different between men and women. Both of these findings are based on exercise heat stress, but we employed a passive heating protocol to remove any potential differences in water regulatory hormones released during exercise. Although these aforementioned findings were confirmed during exercising protocols, we have no reason to believe that sex would modulate ion reabsorption rates during passive heating protocols.
It must be noted that 10 (of 35) participants were taking Amlodipine, to treat high blood pressure. This calcium channel blocker may inhibit the renin–angiotensin–aldosterone system, increase nitric oxide bioavailability, improve endothelial activity and vascular function. We do not know the impact of habitual medication use on our results. Whilst exploring the data, the 10 medicated users were removed and this had no impact on our reported findings or interpretation. However, we cannot rule out that our medicated user may have influenced the data, and indeed more research is needed to investigate the effect of medications on thermoregulatory response with age. However, taking medication is increasingly common in an aged population and whilst it is important to understand the true ageing effect on heat loss effector function, excluding habitual prescribed drug users limits the generalisability of the findings and bears little resemblance to real-world situations. The rising number of medicated adults, especially in a globally advancing population will warrant more research comparing non- versus medicated groups in the future. However, we acknowledge that there may be limitations with our data set and readers should consider this in their interpretation.
We aimed to collect sweat samples from all participants but we only managed to collect enough sweat from 60% of our participants. The measurement technique or the sample location is not appropriate for individuals with very low sweat rates, as is often the case for older adults and those with low aerobic fitness. Our results may be confounded by this and more data from this cohort is required to confirm the relation between advancing age and aerobic fitness on sweat NaCl concentration.
Conclusion
Whilst there is evidence that age affects some aspects of sudomotor function, our data indicate that the maximum ion reabsorption rate of eccrine sweat glands is not affected by age, spanning 50–84 years. Although aldosterone concentration declined moderately with age, this does not appear to modulate the eccrine sweat glands' ion reabsorption rate. Maintaining cardiorespiratory fitness can attenuate the age-related decline in some sudomotor parameters but the sweat glands' maximum ion reabsorption rate was not associated with VO2max in older adults (> 50 years).
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- ASG:
-
Activated sweat glands
- Bpm:
-
Beats per minute
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- ENaC:
-
Epithelial sodium channels
- H+ :
-
Hydrogen
- GSC:
-
Galvanic skin conductance
- K+ :
-
Potassium
- MAP:
-
Mean arterial pressure
- Na+ :
-
Sodium
- NaCl:
-
Sodium chloride
- RPM:
-
Revolutions per minute
- SGO:
-
Sweat gland output
- SR:
-
Sweat rate
- Tb:
-
Body temperature
- Tor:
-
Sublingual temperature
- Tsk:
-
Skin temperature
- VO2max :
-
Maximal oxygen uptake
- WBSR:
-
Whole body sweat rate
References
Abdel-Rahman TA, Collins KJ, Cowen T, Rustin M (1992) Immunohistochemical, morphological and functional changes in the peripheral sudomotor neuro-effector system in elderly people. J Auton Nerv Syst 37:187–197. https://doi.org/10.1016/0165-1838(92)90040-N
Amano T, Gerrett N, Inoue Y et al (2016) Determination of the maximum rate of eccrine sweat glands’ ion reabsorption using the galvanic skin conductance to local sweat rate relation. Eur J Appl Physiol 116:281–290
Amano T, Hirose M, Konishi K et al (2017) Maximum rate of sweat ions reabsorption during exercise with regional differences, sex, and exercise training. Eur J Appl Physiol 117:1317–1327. https://doi.org/10.1007/s00421-017-3619-8
Baccini M, Biggeri A, Accetta G et al (2008) Heat effects on mortality in 15 European cities. Epidemiology 19:711–719. https://doi.org/10.1097/EDE.0b013e318176bfcd
Baker LB, Nuccio RP, Reimel AJ et al (2020) Cross-validation of equations to predict whole-body sweat sodium concentration from regional measures during exercise. Physiol Rep 8:e14524. https://doi.org/10.14814/phy2.14524
Buono MJ, Ball KD, Kolkhorst FW (2007) Sodium ion concentration vs. sweat rate relation in humans. J Appl Physiol 103:990–994. https://doi.org/10.1152/japplphysiol.00015.2007
Buono MJ, Claros R, Deboer T, Wong J (2008) Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate. J Appl Physiol 105:1044–1048. https://doi.org/10.1152/japplphysiol.90503.2008
Chaseling GK, Crandall CG, Gagnon D (2020) Skin blood flow measurements during heat stress: technical and analytical considerations. Am J Physiol - Regul Integr Comp Physiol 318:R57–R69
Cheuvront SN, Bearden SE, Kenefick RW et al (2009) A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol 107:69–75. https://doi.org/10.1152/japplphysiol.00250.2009
Coull NA, West AM, Hodder SG et al (2020) Body mapping of regional sweat distribution in young and older males. Eur J Appl Physiol. https://doi.org/10.1007/s00421-020-04503-5
D’Souza AW, Notley SR, Kenny GP (2020) The relation between age and sex on whole-body heat loss during exercise-heat stress. Med Sci Sports Exerc 52:2242–2249. https://doi.org/10.1249/MSS.0000000000002373
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Nutrition 5:303
Gerrett N, Amano T, Inoue Y et al (2018a) The effects of exercise and passive heating on the sweat glands ion reabsorption rates. Physiol Rep 6:e13619. https://doi.org/10.14814/phy2.13619
Gerrett N, Griggs K, Redortier B et al (2018b) Sweat from gland to skin surface: production, transport, and skin absorption. J Appl Physiol 125:459–469. https://doi.org/10.1152/japplphysiol.00872.2017
Gerrett N, Amano T, Inoue Y, Kondo N (2020) The sweat glands maximum ion reabsorption rates following heat acclimation in healthy older adults. Exp Physiol. https://doi.org/10.1113/EP088486
Gisolfi CV, Wenger CB (1984) Temperature regulation during exercise: old concepts, new ideas. Exerc Sport Sci Rev 12:339–372
Golding LA (2000) YMCA fitness testing and assessment manual. Published for the YMCA of the USA by Human Kinetics
Greenhaff PL (1989) Cardiovascular fitness and thermoregulation during prolonged exercise in man. Br J Sp Med. https://doi.org/10.1136/bjsm.23.2.109
Hall SK, Stableforth DE, Green A (1990) Sweat sodium and chloride concentrations-essential criteria for the diagnosis of cystic fibrosis in adults. Ann Clin Biochem an Int J Biochem Lab Med 27:318–320. https://doi.org/10.1177/000456329002700406
Hamouti N, Del CJ, Ortega JF, Mora-Rodriguez R (2011) Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans. Eur J Appl Physiol 111:2873–2881. https://doi.org/10.1007/s00421-011-1911-6
Harvey BJ, Higgins M (2000) Nongenomic effects of aldosterone on Ca2+ in M-1 cortical collecting duct cells. Kidney Int 57:1395–1403. https://doi.org/10.1046/j.1523-1755.2000.00981.x
Hegarty JM, Harvey BJ (1999) Aldosterone rapidly accelerates a Na+ -H+ exchange dependent pH recovery after acid loading in cultured human eccrine sweat gland epithelial cells. J Physiol 517P:20P
Ichinose-Kuwahara T, Inoue Y, Iseki Y et al (2010) Sex differences in the effects of physical training on sweat gland responses during a graded exercise. Exp Physiol 95:1026–1032. https://doi.org/10.1113/expphysiol.2010.053710
Inoue Y (1996) Longitudinal effects of age on heat-activated sweat gland density and output in healthy active older men. Eur J Appl Physiol Occup Physiol 74:72–77. https://doi.org/10.1007/BF00376497
Inoue Y, Shibasaki M (1996) Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Appl Physiol 74:78–84
Inoue Y, Nakao M, Araki T, Murakami H (1991) Regional differences in the sweating responses of older and younger men. J Appl Physiol 71:2453–2459. https://doi.org/10.1152/jappl.1991.71.6.2453
Inoue Y, Nakao M, Okudaira S et al (1995) Seasonal variation in sweating responses of older and younger men. Eur J Appl Physiol Occup Physiol 70:6–12. https://doi.org/10.1007/BF00601802
Inoue Y, Havenith G, Kenney WL et al (1999) Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol 42:210–216. https://doi.org/10.1007/s004840050107
Inoue Y, Kuwahara T, Araki T (2004) Maturation- and aging-related changes in heat loss effector function. J Physiol Anthropol Appl Human Sci 23:289–294
Kaminsky LA, Arena R, Myers J (2015) Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing data from the fitness registry and the importance of exercise national database. Mayo Clin Proc 90:1515–1523. https://doi.org/10.1016/j.mayocp.2015.07.026
Kenney WL, Fowler SR (1988) Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol 65:1082–1086
Larose J, Boulay P, Sigal RJ et al (2013) Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS ONE 8:e83148. https://doi.org/10.1371/journal.pone.0083148
Machado-Moreira CA, Edkins E, Iabushita AS, et al (2009) Sweat gland recruitment following thermal and psychological stimuli. In: Castellini JW (ed) 13th International Conference of Environmental Ergonomics. pp 478–481
Nanba K, Vaidya A, Rainey WE (2018) Aging and adrenal aldosterone production. Hypertension 71:218–223. https://doi.org/10.1161/HYPERTENSIONAHA.117.10391
Notley SR, Meade RD, Kenny GP (2020) Effect of aerobic fitness on the relation between age and whole-body heat exchange during exercise-heat stress: a retrospective analysis. Exp Physiol 105:1550–1560. https://doi.org/10.1113/EP088783
Patterson MJ, Galloway SD, Nimmo MA (2000) Variations in regional sweat composition in normal human males. Exp Physiol 85:869–875
Quinton PM (2007) Cystic fibrosis: lessons from the sweat gland. Physiology (bethesda) 22:212–225. https://doi.org/10.1152/physiol.00041.2006
Ravanelli N, Gagnon D, Imbeault P, Jay O (2020) A retrospective analysis to determine if exercise training-induced thermoregulatory adaptations are mediated by increased fitness or heat acclimation. Exp Physiol. https://doi.org/10.1113/EP088385
Reddy M, Quinton P (2003) Functional interaction of CFTR and ENaC in sweat glands. Pflügers Arch - Eur J Physiol 445:499–503. https://doi.org/10.1007/s00424-002-0959-x
Sato K, Sato F (1983) Individual variations in structure and function of human eccrine sweat gland. Am J Physiol Regul Integr Comp Physiol 245:R203-208
Smith CJ, Alexander LM, Kenney WL (2013) Nonuniform, age-related decrements in regional sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol 305:R877–R885. https://doi.org/10.1152/ajpregu.00290.2013
Vilches JJ, Ceballos D, Verdú E, Navarro X (2002) Changes in mouse sudomotor function and sweat gland innervation with ageing. Auton Neurosci Basic Clin 95:80–87. https://doi.org/10.1016/S1566-0702(01)00359-9
Funding
This study was supported by a Grant-in-Aid for Scientific Research (16H04851 and 17H0253) from the Japan Society for the Promotion of Science from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Contributions
N.G. and N.K. conceptualized the research question and all authors contributed to the research design. N.G. and N.K. performed experiments; N.G. analysed data; and all authors interpreted the results of the experiments. N.G. prepared the figures and drafted the manuscript; all authors edited and revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The Human Subjects Committee of the Graduate School of Human Development and Environment at Kobe University (Japan) approved the study (report no. 259), which conforms to the standards set out by the latest version of the Declaration of Helsinki (except for registration in a database).
Informed consent
Prior to the study, participants were informed about the procedures and provided verbal and written consent.
Additional information
Communicated by George Havenith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gerrett, N., Amano, T., Inoue, Y. et al. Eccrine sweat glands’ maximum ion reabsorption rates during passive heating in older adults (50–84 years). Eur J Appl Physiol 121, 3145–3159 (2021). https://doi.org/10.1007/s00421-021-04766-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04766-6