Abstract
Purpose
The aim of the present study was to examine the frequency effects (20 Hz and 100 Hz) on neuromuscular fatigue using stimulation parameters favoring an indirect motor unit recruitment through the afferent pathway.
Methods
Nineteen subjects were divided into two groups: 20 Hz (n = 10) and 100 Hz (n = 9). The electrical stimulation session consisted of 25 stimulation trains (20 s ON/20 s OFF, pulse width: 1 ms) applied over the tibial nerve and delivered at an intensity evoking 10% maximal voluntary isometric contraction (MVIC). Before and after these protocols, MVIC was assessed, while neural changes were evaluated by the level of activation (VAL) and muscle changes were evaluated by the twitch associated with the maximal M-wave (Pt). For all stimulation trains, the real and the theoretical values of the torque-time integral (TTIr and TTIth, respectively) were calculated. The TTIr/TTIth ratio of the first train was calculated to evaluate the presence of extra torque.
Results
The main results showed a similar decrease in MVIC torque after both protocols accompanied by neural and muscle changes, as evidenced by the decrease in VAL and Pt. TTIr values across the 20-Hz trains remained constant, whereas they significantly decreased during the 100-Hz stimulation trains. The relative MVIC decrease was negatively correlated with TTIr/TTIth.
Conclusion
Results give evidence of an identical neuromuscular fatigue development between protocols, while lower stimulation frequency permitted preservation of a given torque level during the stimulation trains. The negative correlation between this fatigue development and TTIr/TTIth suggests that extra torque production induces greater voluntary torque losses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular electrical stimulation (NMES) consists of evoking contractions by applying an electrical current over the muscle or motor nerve via surface electrodes and is mainly used in rehabilitation and training programs (Maffiuletti 2010). Although NMES is an efficient stimulus to induce torque increases (Hortobágyi and Maffiuletti 2011), its use is limited by the development of neuromuscular fatigue. Indeed, due to the non-physiological motor units’ (MU) recruitment as compared to voluntary contractions (Gregory and Bickel 2005; Jubeau et al. 2007), metabolic stress is sharply increased under NMES (Vanderthommen et al. 2003), leading to marked and early fatigue development (Theurel et al. 2007). This important drawback limits the wide acceptance and use of NMES in the clinical context.
To counteract this problem, numerous studies have focused on determining the stimulation protocol that would minimize muscle fatigue and maximize muscle performance, by varying stimulation parameters such as stimulation frequency and pulse duration (Binder-Macleod and Scott 2001; Kesar and Binder-Macleod 2006; Scott et al. 2007; Kesar et al. 2008). A new stimulation paradigm has been proposed using wide-pulse durations (1 ms) combined with high frequencies (> 80 Hz) and delivered at low stimulation intensities (evoking 5–10% MVIC), called wide-pulse high frequency (WPHF) stimulation (Collins et al. 2001, 2002; Collins 2007). Indeed, wide-pulse durations combined with low current intensities favor the recruitment of sensory axons (Veale et al. 1973; Kiernan et al. 2004) and minimize the antidromic collision in solicited motor axons, permitting in that way action potentials traveling from the spinal cord to reach muscles (Dean et al. 2007; Bergquist et al. 2011a). These stimulation conditions induce an indirect MU recruitment by the afferent volley (Bergquist et al. 2011b; Dean et al. 2014) and give rise to higher torques from what would be expected from the direct recruitment of motor axons alone (i.e., extra torque, ET). The reflex activation of spinal motoneurons and their contribution to the torque generation, induced independently of the stimulation frequency (Vitry et al. 2019), likely proceed to a more physiological MU recruitment [following the Henneman’s size principle (Henneman et al. 1965)]. Consequently, increasing the indirect MU recruitment during NMES has been shown to induce less torque losses during acute NMES application (1 stimulation train of 30 s) as compared with the recruitment of motor axons alone, implying that these stimulation modalities improve fatigue resistance of electrically evoked contractions (Lagerquist et al. 2009).
Contrary to the acute WPHF application, greater evoked torque decreases during repeated WPHF stimulation trains (20 trains) have been reported as compared with protocols using low-frequency, short-pulse duration and higher stimulation intensity (Neyroud et al. 2014). The fact that, despite a lower total muscle work induced during WPHF stimulation trains, similar decreases on subjects’ maximal torque generation capacity were observed after the two protocols, suggests that this new stimulation paradigm induces a greater level of muscle fatigue (Neyroud et al. 2014). This has been explained by the greater metabolic cost per activated MU associated with the higher stimulation frequency (Gorgey et al. 2009). However, there is evidence that the greater torque declines observed during stimulation trains can be also ascribed to changes in axonal excitability threshold of the solicited MU (Bostock and Bergmans 1994; Kiernan et al. 1996), which seem greater under high stimulation frequency (Papaiordanidou et al. 2014). Indeed, repetitive activation of motor axons can induce increases in their excitability threshold, as a result of axonal hyperpolarization (Kiernan et al. 1996, 2004). An important feature to highlight is that these results have been obtained using stimulation over the muscle belly, a stimulation location that has been shown to proceed to MU activation through the efferent pathway (i.e., direct recruitment) (Bergquist et al. 2011a), thereby limiting partially the development of ET. NMES application over the motor nerve maximizes the indirect MU recruitment through the afferent pathway as compared with stimulation over the muscle belly (Bergquist et al. 2011a), which seems a prerequisite for ET development (Vitry et al. 2019). It is, therefore, still controversial whether electrically evoked contractions permitting ET development are less fatiguing, in terms of impact on maximal voluntary torque-generating capacity, and whether they allow torque preservation during repetitive contractions.
The aim of the present study was to investigate the frequency effects on the extent and origin of neuromuscular fatigue of the triceps surae. To maximize the indirect MU recruitment and hence permit ET development, low stimulation intensity associated with wide-pulse duration and applied over the motor nerve was used, while two stimulation frequencies were tested (20 and 100 Hz). It was hypothesized that low stimulation frequency that minimizes activity-dependent increases in axonal excitability threshold and limits the metabolic cost per activated MU would induce less torque decreases during and after NMES as compared to high stimulation frequency.
Methods
Subjects
Nineteen healthy young adults (22.73 ± 4.89 years, 177.05 ± 8.45 cm, 70.63 ± 9.35 kg) with no history of neurological or muscular disorders gave written informed consent to participate in the present study. The experimental design was approved by the scientific committee of the Faculty of Sport Sciences, University of Burgundy in Dijon and was in accordance with the principles of the Declaration of Helsinki for human experimentation.
Experimental design
Subjects were randomly assigned into two groups: the 20-Hz group (8 men and 2 women, 22.3 ± 3.2 years, 179.1 ± 8.6 cm, 73.0 ± 9.3 kg) and the 100-Hz group (7 men and 2 women, 23.2 ± 6.6 years, 174.8 ± 8.1 cm, 68.0 ± 9.1 kg). Participants visited the laboratory for one experimental session. After a warm-up, including 8–10 submaximal contractions of the plantar flexors, the maximal M-wave (MMAX) stimulation intensity was determined. Subsequently, two MVICs with superimposed twitch at supramaximal intensity (120% MMAX) were performed to assess subjects’ maximal torque-generating capacity and voluntary activation level (VAL). The stimulation intensity evoking 10% of maximal voluntary isometric contraction (MVIC) was then identified and 25 stimulation trains of 20 s were delivered at 20 or 100 Hz according to the experimental group. Immediately after these fatiguing protocols, one MVIC with superimposed twitch at supramaximal intensity was performed. After each MVIC (pre- and post-measurements), a stimulation at MMAX was evoked at rest to record the potentiated twitch.
Experimental procedures
Nerve stimulation
The posterior tibial nerve was electrically stimulated using a self-adhesive electrode with a 30-mm diameter (Durastick Plus, DJO Global, Vista, CA, USA) placed in the popliteal fossa. The optimal stimulation site (highest muscle response with the lowest stimulation intensity) was initially identified with a hand-held cathode ball electrode. Once this site was determined, the stimulation electrode was tightly fixed in this location using straps. The anode (5 × 10 cm, Compex SA, Ecublens, Switzerland) was placed over the patella. Monophasic rectangular pulses (1 ms pulse duration) were delivered using a Digitimer stimulator (DS7AH, Digitimer, Hertfordshire, UK), triggered by a commercially available software (Tida, Heka Elektronik, Lambrecht/Pfalz, Germany). Recruitment curve was built at rest to determine soleus (SOL) MMAX intensity. Stimulation intensity was increased using 5-mA increments from M-wave threshold until MMAX (four stimulations at each intensity). The intensity where SOL MMAX plateaued was then increased by at least 20% (supramaximal intensity). Stimulation trains were also delivered through tibial nerve stimulation in the popliteal fossa. The fatiguing protocol consisted of 25 stimulation trains of 20-s duration delivered either at 20 Hz or at 100 Hz with a pulse duration of 1 ms. A 20-s rest period was applied between trains. Intensity was set to evoke an initial torque level corresponding to 10% MVIC and was determined in the initial phase of the experimental procedures with a train lasting 2 s delivered at the corresponding frequency. The stimulation intensity varied between 5.5 and 23 mA (mean 11.25 ± 5.6 mA) for the 20-Hz group and between 3.5 and 22 mA (mean 11.9 ± 6 mA) for the 100-Hz group.
Data acquisition
Torque recording
Voluntary and evoked torques developed by the plantar flexors were recorded using an isokinetic dynamometer (Biodex Medical Systems Inc., Shirley, NY, USA). Subjects were seated on the chair of the dynamometer with the right foot strapped to the pedal at the ankle level. The dynamometer's axis of rotation was aligned with the anatomical plantar- and dorsi-flexion axis of the ankle joint. The ankle angle was set at 0° (90° between the leg and the sole of the foot) and the knee joint was at 110° (180° full extension). This configuration was kept constant during the entire session (pre- and post-measurements, fatiguing protocol).
EMG recording
Electromyographic activity (EMG) recordings were performed to determine MMAX amplitude of the SOL muscle. After shaving and dry cleaning the skin with alcohol to get low impedance (< 5 kΩ), EMG signals were obtained using two silver-chloride circular (7 mm recording diameter) surface electrodes (Contrôle Graphique Medical, Brie-Compte-Robert, France) placed with an inter-electrode center-to-center distance of 2 cm. Electrodes were placed 2 cm below the insertion of the two gastrocnemii over the Achilles’ tendon. The reference electrode was placed in a central position between the stimulation and recording sites between upper-gastrocnemii insertions. EMG signals were amplified (gain = 1000), filtered (5 kHz) and collected with a sampling frequency of 10 kHz. Torque and EMG data were recorded with Biopac Software (Biopac MP150, Biopac System Inc, USA).
Data analysis
Torque analysis
Maximal voluntary torque was considered as the highest torque value attained during MVIC (for pre the mean value of the two MVICs was considered). The amplitude of the potentiated twitches evoked by supramaximal intensity (Pt) was also analyzed offline (for pre the mean value of the two twitches was considered). It was defined as the maximum value of torque production to a twitch evoked by supramaximal single stimulation (Maffiuletti et al. 2002; Lattier et al. 2004; Papaiordanidou et al. 2010). The VAL was estimated according to the modified version of VAL formula proposed by Strojnik and Komi (1998).
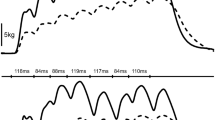
For each stimulation train, the real torque-time integral value (TTIr) was quantified. It corresponded to the integral of the entire 20-s period. The sum of the TTIr of the 25 stimulation trains was calculated to determine the total TTI developed by each subject during the session (∑TTI). The TTIr mean values of each stimulation train were also calculated to observe their evolution across the session. Because during stimulation trains the phenomenon of ET can be observed (a progressively increasing torque output that arises in addition to what would be expected from the direct response to motor axon stimulation) (Collins et al. 2001, 2002), the theoretical value of torque-time integral (TTIth) was also estimated for each first stimulation train. This corresponded to the integral of the first 2 s of the torque plateau multiplied by 10 (Fig. 1b, left panel), while when there was no clear identified plateau (Fig. 1b, right panel), 1 s before and 1 s after the instant when torque reached 10% MVIC were considered to calculate TTIth. This parameter expresses the TTI that would be theoretically developed during this first train without the presence of ET, fatigue or changes in axonal excitability threshold of motor axons (Vitry et al. 2019). The ratio between these two values of the first train was calculated for each subject to evaluate the presence or not of ET (ET corresponds to TTIr/TTIth > 1) (Vitry et al. 2019).
Torque-time integral (TTI) evolution during the sessions and typical traces of the method of TTIth calculation. a TTIr remained constant during the 20-Hz session, while it significantly decreased from the 8th stimulation train for the 100-Hz group. Dotted lines represent the average TTIth of the first train for the 20-Hz group (black line) and for the 100-Hz group (grey line). Although during the first stimulation train there was development of extra torque for both protocols (TTIr value > TTIth value), this held true for the entire session only for the 20-Hz group (excepted for trains 14–18). Despite the important TTIr decrease for the 100-Hz group, there was only a tendency for ∑TTI during the 100-Hz being lower than the one developed during the 20-Hz protocol (P = 0.07). b Torque traces of two subjects during a 20-s stimulation train at 20 Hz. The dark hatched area corresponds to the first 2 s in the torque plateau used to estimate TTIth (left panel). This value was then multiplied by 10. When there was no clear identified plateau (right panel), 1 s before and 1 s after the instant when torque reached 10% MVIC were considered to calculate TTIth. TTIr corresponds to the integral of the entire 20-s period. Extra torque corresponds to TTIr/TTIth > 1. TTIr, real Torque-time integral; TTIth, theoretical torque-time integral. *P < 0.05, significant difference from the first train. #P < 0.05, significant difference from TTIth
EMG analysis
SOL EMG activity associated with the twitches elicited at supramaximal intensity was recorded and averaged for the analysis. The peak-to-peak amplitude of MMAX was calculated.
Statistical analysis
Normal distribution (Shapiro–Wilk test, P > 0.05) and homogeneity of variances (Levene’s test, P > 0.05) for all variables were checked to ensure that parametric ANOVA could be used. A two-way ANOVA [frequency (20 Hz vs 100 Hz) × time (pre vs post)] was performed to assess differences on mechanical (MVIC, VAL, Pt) and EMG data (MMAX), while a two-way ANOVA [frequency (20 Hz vs 100 Hz) × stimulation trains (train 1–train 25)] was performed to compare the evolution of TTIr values across the session. To compare TTIr and TTIth values a two-way ANOVA [frequency (20 Hz vs 100 Hz) × stimulation trains (TTIth of the first train + 25 TTIr values)] was performed. When a significant main or interaction effect was observed, the HSD Tukey post hoc test was performed. A t test analysis was used to compare the ∑TTI developed by each group as well as to assess differences between pre-values of both groups for MVIC, VAL and Pt. Correlations between selected variables (TTIr/TTIth ratio of the first train, relative decrease of MVIC, ∑TTI) were tested with Pearson coefficient. A significance level of P < 0.05 was accepted for all analyses. Effect sizes were calculated and are reported as partial eta squared (\(\eta_{{\text{p}}}^{2}\)) with small, moderate and large effects considered for \(\eta_{{\text{p}}}^{2}\) ≥ 0.01, ≥ 0.07 and ≥ 0.14, respectively, and Cohen’s d values are reported with small, moderate and large effects considered for d ≥ 0.2, ≥ 0.5 and ≥ 0.8, respectively. All data are expressed as mean ± standard deviation in text and as mean ± standard error in figures. All statistical tests were performed using Statistica software (Statsoft, version 7.1, Tulsa, OK, USA), except the Cohen’s effect sizes that were calculated with G * Power software (version 3.1.9, Universität Düsseldorf, Germany).
Results
There were no differences in pre-values of the two groups for MVIC, VAL and Pt (P > 0.05). Plantar flexors MVIC showed a significant pre/post-effect (F1,17 = 17.57, P < 0.001, \(\eta_{{\text{p}}}^{2}\) = 0.51), indicating a similar loss in maximal voluntary torque production after the 25 stimulation trains for both protocols. Indeed, MVIC pre-value was 109.97 ± 36.96 Nm and post-value was 104.35 ± 39.55 Nm.
Although t test analysis did not reveal any significant difference in ∑TTI values between the two groups (P = 0.07), there was a strong tendency for ∑TTI of 100 Hz being smaller compared with the ∑TTI of 20 Hz (3850 ± 1523 Nm.s and 6577 ± 4052 Nm s for the 100- and 20-Hz group respectively).
The two-way ANOVA showed an interaction effect frequency × stimulations trains (F25,425 = 1.75, P < 0.05, \(\eta_{{\text{p}}}^{2}\) = 0.09) for the evolution of TTIr values across the session. Post hoc analysis revealed that during the 100-Hz protocol, TTIr significantly decreased from the 8th stimulation train (P < 0.05) and remained constant thereafter except for the 16th, the 24th and the 25th stimulation train (P > 0.05), whereas during the 20-Hz session TTIr remained constant throughout the session (P > 0.05, Fig. 1). This same analysis showed that each TTIr was not significantly different from TTIth for the 20-Hz, whereas TTIr of the 8th, 11th and 21th trains was significantly different from TTIth for the 100-Hz.
Interestingly, the TTIr/TTIth ratio of the first stimulation train was negatively correlated with the relative decrease of MVIC (r = − 0.86; P < 0.001) for both protocols, showing that subjects having the higher TTIr/TTIth presented the greater MVIC decrease after the 25 stimulation trains (Fig. 2). On the contrary, no significant correlation was found between the relative MVIC decrease and ∑TTI (r = 0.12; P = 0.62).
Correlation between TTIr/TTIth and the relative MVIC decrease (ΔMVIC). There was a negative correlation between TTIr/TTIth and ΔMVIC (r = − 0.86; P < 0.001), indicating that subjects having the higher TTIr/TTIth, presented the greater MVIC decrease after the stimulation protocols. The dashed horizontal line at 1 represents equal TTIr and TTIth values. All points that are above this line have a TTIr/TTIth > 1, indicating the presence of extra torque. TTIr, real torque-time integral; TTIth, theoretical torque-time integral; MVIC, maximal voluntary isometric contraction
Statistical analysis revealed a significant pre/post-effect for VAL (F1,17 = 6.05, P < 0.05, \(\eta_{{\text{p}}}^{2}\) = 0.26) and Pt (F1,17 = 49.78, P < 0.001, \(\eta_{{\text{p}}}^{2}\) = 0.74). VAL significantly decreased after the 25 stimulation trains for both protocols (87.68 ± 5.20% vs. 85.71 ± 7.11% for pre -and post-values, respectively). These results were also accompanied by a significant decrease of Pt for both frequencies (16.43 ± 3.48 Nm vs. 14.2 ± 3.43 Nm for pre- and post-values, respectively). MMAX remained unchanged after the 25 stimulation trains for both protocols (P > 0.05, \(\eta_{{\text{p}}}^{2}\) = 0.03).
Discussion
The present study investigated the frequency effects on neuromuscular fatigue of the triceps surae after an NMES session applied over the motor nerve and delivered at low stimulation intensity. It was hypothesized that low stimulation frequency would induce less torque decreases during and after NMES as compared with the higher stimulation frequency. The main results showed a similar significant decrease in MVIC torque after both protocols accompanied by neural and muscle changes, as evidenced by the decrease in VAL and Pt. These results were obtained despite a strong tendency for ∑TTI values being smaller during the 100-Hz protocol. TTIr values across the 20-Hz trains remained constant, whereas they significantly decreased during the 100-Hz stimulation trains. Finally, the relative MVIC torque decrease was negatively correlated with the TTIr/TTIth ratio of the first stimulation train (subjects with the initial higher TTIr/TTIth ratio were the ones who lost the most).
Results of the present study showed a decrease in MVIC torque observed after an NMES session applied over the motor nerve at a low intensity (10% MVIC) for both stimulation frequencies tested (20 and 100 Hz). Indeed, the mean decrease was of a magnitude of 6 ± 6% (4.8 ± 7.3% for the 20-Hz and 7.3 ± 6.3% for the 100-Hz) and was accompanied by a significant decline in VAL as well as in Pt amplitude. These torque losses are of the same magnitude with those reported by previous studies having applied low-intensity NMES over the muscle (Neyroud et al. 2014), but lower than those observed by studies having used maximal tolerated intensity, evoking 40–55% MVIC. Indeed, torque losses of a magnitude of 10% have been observed after fatiguing NMES protocols using either high (Boerio et al. 2005) or low stimulation frequencies (Papaiordanidou et al. 2010), but short pulse duration (400–450 µs). These differences can be explained in part by the greater torque levels adopted in previous protocols, suggesting that since only a small fraction of MU participating in MVIC torque production was activated during the NMES session in the present study, the magnitude of the observed fatigue was less. Concerning the origin of the observed MVIC decrease, results indicate that it could be attributed to both neural and muscle mechanisms for both protocols. The decrease in VAL indicates a reduction in neural drive reaching the working muscles and is an evidence of decreased MU recruitment and/or firing rate (Kent-Braun 1999), alterations that take place within the central nervous system at the supraspinal and/or spinal levels. Concomitant to this neural fatigue, the muscle origin of electrically induced fatigue, as evidenced by the diminished Pt amplitude after both protocols (Fitts 2008), was also observed. This is consistent with repeated activation of the same muscle fibers and can be explained by altered muscle contractile properties (Allen and Westerblad 2001), while it has widely been reported in the literature (Boerio et al. 2005; Zory et al. 2005; Neyroud et al. 2014).
These results (same extend and origin of neuromuscular fatigue after the 20- and 100-Hz protocols) were obtained despite a strong tendency for ∑TTI during the 100-Hz protocol being smaller than the one developed during the 20-Hz session (P = 0.07). Indeed, the evolution of TTIr values across the 20-Hz trains was constant, whereas TTIr sharply decreased between the first and second 100-Hz train, despite this decrease becoming significant from the 8th stimulation train. Moreover, TTIr values of the 100-Hz trains were lower from TTIth values (except the first train). These observations indicate that under high stimulation frequency, torque decreases during repeated stimulation trains are greater than those obtained during low stimulation frequency. The presence of ET under these conditions, observed only for the first train, is a transitory physiological phenomenon, not sufficient to maintain a given torque level due to the progressive appearance of other physiological phenomena related to high stimulation frequency, such as higher metabolic cost per activated MU (Gorgey et al. 2009) and/or decreased number of activated MU caused by activity-dependent changes in axonal excitability threshold (Bostock and Bergmans 1994; Kiernan et al. 1996; Vagg et al. 1998). Indeed, the repetitive activation of MU at high frequency may have induced on one hand a greater fatigue of the solicited MU and on the other hand a reduction in the number of solicited MU which have an excitability threshold close to the pulse amplitude. This would have contributed to the greater evoked torque decrease during the high-frequency stimulation trains (Matkowski et al. 2015). Although the latter phenomenon may have occurred during both protocols, its contribution to torque losses seems greater during high stimulation frequencies (Papaiordanidou et al. 2014). In fact, during the 20-Hz protocol TTIr values were not different from TTIth and, as shown in Fig. 1, their mean values were even higher from TTIth for the majority of the stimulation trains. It can, therefore, be concluded that under low stimulation frequency ET development can persist throughout the NMES session, avoiding important torque decreases due to MU fatigue or to changes in the excitability threshold of motor axons.
Despite the importance of ET development in the maintenance of a given torque level during repeated stimulation trains, the present results give evidence of greater MVIC torque losses after the two stimulation protocols for subjects who initially developed greater ET. MVIC torque losses for both protocols were negatively correlated with the TTIr/TTIth ratio of the first train, indicating that subjects producing more ET in the beginning of the protocol were the ones who lost the most and this independently of the stimulation frequency. Although stimulation parameters were adjusted to evoke an initial torque level corresponding to 10% MVIC, development of ET, as evidenced by a TTIr/TTIth > 1 (Vitry et al. 2019), occurred for some of the subjects and these subjects presented the greater MVIC declines at post-conditions. The presence of ET has been commonly ascribed to additional synaptic recruitment of motor neurons and/or to activation of persistent inward currents in spinal neurons (Collins et al. 2001; Lagerquist et al. 2009). Independently of the exact physiological mechanism, the electrically evoked afferent volley during NMES permitting the development of ET in the initial phase of the stimulation protocols resulted in greater voluntary torque declines after the protocols. This could not be explained by a greater ∑TTI, since the total muscle work did not show any significant correlation with the MVIC losses.
To conclude, to study the frequency effects of electrically induced neuromuscular fatigue, it appears important to clearly distinguish phenomena occurring during repetitive electrical stimulation from their impact on maximal force generating capacity after the repeated trains. Results of the present study give evidence of an identical neuromuscular fatigue development between protocols using high or low stimulation frequency, in terms of amount and origin (neural vs. muscle mechanisms). The negative correlation between this fatigue development (MVIC torque decrease) and the TTIr/TTIth ratio of the first train suggests that ET production induces greater torque losses. On the other hand, increased metabolic stress of the solicited MU and changes in axonal excitability threshold occurring during high stimulation frequency preclude torque maintenance during repeated stimulation trains. This can be avoided under lower stimulation frequency when ET development permits preservation of a given torque level.
Abbreviations
- ANOVA:
-
Analysis of variance
- EMG:
-
Electromyographic activity
- ET:
-
Extra torque
- M MAX :
-
Maximal M-wave
- MU:
-
Motor unit
- MVIC:
-
Maximal voluntary isometric contraction
- NMES:
-
Neuromuscular electrical stimulation
- Pt:
-
Peak torque amplitude of the potentiated twitch associated to MMAX
- SOL:
-
Soleus
- TTI:
-
Torque-time integral
- TTIr:
-
Real torque-time integral
- TTIth:
-
Theoretical torque-time integral
- VAL:
-
Voluntary activation level
- WPHF:
-
Wide-pulse high frequency
References
Allen DG, Westerblad H (2001) Role of phosphate and calcium stores in muscle fatigue. J Physiol 536:657–665
Bergquist AJ, Clair JM, Collins DF (2011a) Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J Appl Physiol 110:627–637
Bergquist AJ, Clair JM, Lagerquist O et al (2011b) Neuromuscular electrical stimulation: implications of the electrically evoked sensory volley. Eur J Appl Physiol 111:2409–2426
Binder-Macleod SA, Scott WB (2001) Comparison of fatigue produced by various electrical stimulation trains. Acta Physiol Scand 172:195–203
Boerio D, Jubeau M, Zory R, Maffiuletti NA (2005) Central and peripheral fatigue after electrostimulation-induced resistance exercise. Med Sci Sports Exerc 37:973–978
Bostock H, Bergmans J (1994) Post-tetanic excitability changes and ectopic discharges in a human motor axon. Brain 117:913–928
Collins DF (2007) Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev 35:102–109
Collins DF, Burke D, Gandevia SC (2001) Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21:4059–4065
Collins DF, Burke D, Gandevia SC (2002) Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol 538:289–301
Dean J, Yates L, Collins DF (2007) Turning on the central contributions to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol 9:102–109
Dean JC, Clair-Auger JM, Lagerquist O, Collins DF (2014) Asynchronous recruitment of low-threshold motor units during repetitive, low-current stimulation of the human tibial nerve. Front Hum Neurosci 8:1–12
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104:551–558
Gorgey AS, Black CD, Elder CP, Dudley GA (2009) Effects of electrical stimulation parameters on fatigue in skeletal muscle. J Orthop Sport Phys Ther 39:684–692
Gregory CM, Bickel CS (2005) Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85:358–364
Henneman E, Somjen G, Carpenter DO (1965) Functional significance of cell size in spinal motoneurons. J Neurophysiol 28:560–580
Hortobágyi T, Maffiuletti NA (2011) Neural adaptations to electrical stimulation strength training. Eur J Appl Physiol 111:2439–2449
Jubeau M, Gondin J, Martin A et al (2007) Random motor unit activation by electrostimulation. Int J Sports Med 28:901–904
Kent-Braun JA (1999) Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol 80:57–63
Kesar T, Binder-Macleod S (2006) Effect of frequency and pulse duration on human muscle fatigue during repetitive electrical stimulation. Exp Physiol 91:967–976
Kesar T, Chou LW, Binder-Macleod SA (2008) Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol 18:662–671
Kiernan M, Mogyoros I, Burke D (1996) Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain 119:1099–1105
Kiernan MC, Lin CS-Y, Burke D (2004) Differences in activity-dependent hyperpolarization in human sensory and motor axons. J Physiol 558:341–349
Lagerquist O, Walsh LD, Blouin J-S et al (2009) Effect of a peripheral nerve block on torque produced by repetitive electrical stimulation. J Appl Physiol 107:161–167
Lattier G, Millet G, Martin A, Martin V (2004) Fatigue and recovery after high-intensity exercise. Part I: neuromuscular fatigue. Int J Sport Med 25:450–456
Maffiuletti NA (2010) Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol 110:223–234
Maffiuletti NA, Pensini M, Martin A (2002) Activation of human plantar flexor muscles increases after electromyostimulation training. J Appl Physiol 92:1383–1392
Matkowski B, Lepers R, Martin A (2015) Torque decrease during submaximal evoked contractions of the quadriceps muscle is linked not only to muscle fatigue. J Appl Physiol 118:1136–1144
Neyroud D, Dodd D, Gondin J et al (2014) Wide-pulse-high-frequency neuromuscular stimulation of triceps surae induces greater muscle fatigue compared with conventional stimulation. J Appl Physiol 116:1281–1289
Papaiordanidou M, Guiraud D, Varray A (2010) Does central fatigue exist under low-frequency stimulation of a low fatigue-resistant muscle? Eur J Appl Physiol 110:815–825
Papaiordanidou M, Stevenot JD, Mustacchi V et al (2014) Electrically induced torque decrease reflects more than muscle fatigue. Muscle Nerve 50:604–607
Scott WB, Lee SCK, Johnston TE et al (2007) Effect of electrical stimulation pattern on the force responses of paralyzed human quadriceps muscles. Muscle Nerve 35:471–478
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol 84:344–350
Theurel J, Lepers R, Pardon L, Maffiuletti NA (2007) Differences in cardiorespiratory and neuromuscular responses between voluntary and stimulated contractions of the quadriceps femoris muscle. Respir Physiol Neurobiol 157:341–347
Vagg R, Mogyoros I, Kiernan MC, Burke D (1998) Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol 507:919–925
Vanderthommen M, Duteil S, Wary C et al (2003) A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-NMRS in humans. J Appl Physiol 94:1012–1024
Veale JL, Mark RF, Rees S (1973) Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry 36:75–86
Vitry F, Martin A, Deley G, Papaiordanidou M (2019) Effect of reflexive activation of motor units on torque development during electrically-evoked contractions of the triceps surae muscle. J Appl Physiol 126:386–392
Zory R, Boërio D, Jubeau M, Maffiuletti NA (2005) Central and peripheral fatigue of the knee extensor muscles induced by electromyostimulation. Int J Sports Med 26:847–853
Author information
Authors and Affiliations
Contributions
FV, AM and MP conceived and designed research; FV conducted experiments; FV analyzed data; FV, AM and MP interpreted results of experiments; FV prepared figures; FV and MP drafted manuscript; FV, AM and MP edited and revised manuscript; FV, AM and MP approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vitry, F., Martin, A. & Papaiordanidou, M. Impact of stimulation frequency on neuromuscular fatigue. Eur J Appl Physiol 119, 2609–2616 (2019). https://doi.org/10.1007/s00421-019-04239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04239-x