Abstract
Purpose
To investigate the effects of acute experimental knee joint pain on maximum force generation and rate of force development (RFD) of the quadriceps muscle during isometric and dynamic muscle activations.
Methods
The right knee of 20 healthy people was injected with hypertonic saline to create an acute pain experience. Measurements of maximum knee extensor torque during isometric, concentric, and eccentric contractions were undertaken using a Biodex dynamometer. The RFD was also examined during the isometric contractions. Quadriceps muscle activity was obtained using electromyography (EMG). The outcome measures were obtained at baseline, during pain, and after knee pain had resolved.
Results
Maximum joint torque and peak EMG were significantly reduced during pain, but there were no differences across the three types of contraction. The maximum RFD and rate of EMG rise were also reduced during pain, primarily at 50–100 ms post-contraction onset. The RFD and EMG rise were largely unaffected at later time periods following contraction onset (150–200 ms).
Conclusions
Acute joint pain has a similar impact on isometric and isokinetic contractions despite differences in neural control strategies. Joint pain also impairs rapid muscle activation and the RFD. These findings are important for people with musculoskeletal pain as it likely contributes to impairments in joint function in these populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is extensive literature demonstrating that pain reduces the strength of muscle contraction (for reviews see Graven-Nielsen and Arendt-Nielsen 2009, and Hodges and Tucker 2011). This is evident in both chronic pain conditions and in experimental pain models, where the influence of pain can be examined more specifically without the confounding effects of behavioral adaptations to chronic conditions or changes in the integrity of the musculoskeletal system. There can be marked clinical implications of reduced muscle strength, particularly in the lower limb. At the knee, the joint most commonly affected by osteoarthritis, quadriceps weakness has been associated with impaired stability (Felson et al. 2007), reduced function (Liikavainio et al. 2008), and falls (Moreland et al. 2003).
The majority of studies have investigated the effects of chronic and experimental pain during maximal concentric and isometric muscle activation, despite the fact that there are notable differences in force generation capacity, fatigue properties, and neural control of eccentric muscle activations (Enoka 1996). At the knee specifically, eccentric activation is critical for weight acceptance while walking downstairs or downhill, transitioning from standing to sitting, or landing from jumps or falls. The few studies that have investigated the effect of pain on eccentric muscle activation have reported findings that challenge the notion of generalized impairment in muscle strength with pain. Three studies have reported a relative loss of concentric compared to eccentric strength in trunk muscles of people with low back pain (Dvir and Keating 2003; Marshall et al. 2010; Shirado et al. 1995). No studies have examined the impact of experimental pain on the relative strength of eccentric, concentric and isometric muscle activations. This would enable any differences in the effect of pain on the different types of activation and a clearer picture of how pain may influence function to be determined.
An additional relevant characteristic of muscle contraction is the rate of force development (RFD). In comparison to maximal strength, the RFD is more strongly related to function (Maffiuletti et al. 2010; Tillin et al. 2013) and is more sensitive to changes in the integrity of the neuromuscular system (Angelozzi et al. 2012; Crameri et al. 2007; Jenkins et al. 2014; Penailillo et al. 2015). Maximal RFD is dependent on muscle size and strength as well as neural drive, with the early component (0–75 ms) primarily determined by neural drive to the muscle and the later component more dependent on structural contractile properties of the muscle (Andersen and Aagaard 2006; Andersen et al. 2010; Folland et al. 2014). Several studies have shown that maximal RFD is reduced in chronic musculoskeletal pain conditions (Andersen et al. 2008; Chourasia et al. 2012; Gapeyeva et al. 2007; Vahtrik et al. 2012; Winters et al. 2014). Given the aforementioned issues when chronic pain is present, it would be more ideal to examine the effects of pain on the RFD in a controlled acute pain paradigm to enable a clearer delineation of the effect of pain on the ability to rapidly develop muscle force.
The aim of this study was to investigate the effects of acute experimental knee joint pain on maximum force generation of the quadriceps muscle during isometric and dynamic muscle activations. We hypothesized that acute pain would differentially impact the maximum force developed during the different types of muscle activation. A further goal was to determine the effects of acute pain on the RFD during isometric muscle activation. We hypothesized that acute pain would impair the early component of RFD more than the later component and that pain-induced changes in early RFD would be related to impaired muscle activation, whereas the pain-induced changes in the later component of RFD would be more strongly related to changes in peak torque.
Methods
Participants
Twenty healthy people (11 female, age 18–49 years) participated in the study. Participants were excluded if they reported a history of significant trauma to the lower limbs and/or trunk with any current musculoskeletal or neural symptoms, any diagnosed neurological or spinal disorders, use of pain medications on the day or day prior to testing, pain > 2/10 anywhere in the body on the day of testing, or a score > 30 on the Pain Catastrophizing Scale (Osman et al. 2000; Sullivan et al. 2009). All participants gave written informed consent and the study received ethical approval from the local institution.
Isokinetic dynamometer
Knee extensor torque was measured using isokinetic dynamometry (Biodex system 3, Biodex Medical Systems, New York). Participants were positioned in the dynamometer with adjustable straps placed around their shoulders, waist, and right thigh to restrain movement. The center of the right knee joint was aligned with the axis of rotation of the dynamometer and the shank restrained by a resistance cuff. For isometric contractions, the knee joint was set to 65° (0° = full extension). For concentric and eccentric contractions, the velocity was 30°/s and the range of movement was from 0 to 100° (Henriksen et al. 2011). To enable the effects of gravity to be corrected, prior to testing the weight of the limb was obtained by asking participants to relax their leg with the limb held stationary. Joint position and torque data from the dynamometer were sampled at 2 kHz (LabChart v7.2.1, AD Instruments Pty Ltd, NSW).
Electromyography
Muscle activity from the vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) muscles was recorded using surface electromyography (EMG). Standard skin preparation was implemented prior to positioning the electrodes. Electrodes were placed according to SENIAM guidelines, with a ground positioned over the anterior tibia. EMG data were amplified (AMT-8, Bortec Biomedical, Canada), filtered (10–1000 Hz), and sampled at 2 kHz (LabChart v7.2.1, AD Instruments Pty Ltd, NSW).
Experimental pain
With the participant positioned in the dynamometer, an injection of 1.0 ml of 5.8% hypertonic saline was administered using a 27-G needle. The injection was given into the anterior medial aspect of the right infrapatellar fat pad at a 45° angle, with a needle insertion depth of 1 cm. A 0–10 numerical rating scale was used to rate pain intensity at 30 s following needle withdrawal, and then every 90 s until pain resolved (approximately 15 min).
Procedure
Participants attended a practice session that involved familiarization with the equipment and tasks. At least 1 week later, they attended a data collection session. Maximum voluntary contractions (MVCs) were recorded during isometric, eccentric and concentric muscle activation. For each type of muscle activation, five MVCs were performed. Participants were instructed to perform all MVCs as “hard and fast as possible” and were verbally encouraged throughout. Each MVC was sustained for 3 s, with a 3–5-s rest between each of the 5 MVCs. After the 5 MVCs for one type of muscle activation were completed, a 30-s rest was given before completing MVCs in the next type of muscle activation. To control for order effects, the order of muscle activation types was randomized among participants using a counterbalanced design.
MVCs were collected at three time points: baseline, during experimental knee pain (pain), and after the experimental knee pain had resolved (post-pain). A 5-min rest was given after completion of the baseline MVCs before experimental knee pain was induced. The MVCs during knee pain were initiated once a numerical pain rating of ≥ 3/10 was reached. When the numerical pain rating returned to 0, participants waited a further 2 min before completing the post-pain MVCs.
Data processing
Peak torque was determined as the maximum torque value during any of the 5 MVCs in each type of muscle activation (isometric, concentric, eccentric). RFD was analyzed in the isometric muscle activations only. RFD data were obtained from the trial that showed the highest peak slope of the torque–time curve and was determined as the average slope of the curve over time intervals of 0–50, 50–100, 100–150, and 150–200 ms (Tillin et al. 2010), relative to the onset of contraction. The onset of contraction was defined as the time point at which torque exceeded 7.5 Nm (Aagaard et al. 2002).
EMG data were filtered using a moving root mean square (rms) filter with a time constant of 50 ms (Aagaard et al. 2002). For all types of muscle activation, the peak EMG amplitude was determined from the trial in which peak torque was identified. For the isometric muscle activation trials, the rate of EMG rise was analyzed in the trial that was used to obtain RFD data. Integrated EMG was determined in time intervals of 0–50, 50–100, 100–150, and 150–200 ms relative to onset of EMG, with the onset of EMG defined as 70 ms prior to the onset of contraction (Aagaard et al. 2002). For all EMG analyses, data from the three muscles were averaged to provide a single measure of quadriceps muscle activation (Ruiter et al. 2004).
For all torque and EMG outcome measures, change scores were also determined to measure the impact of pain. Change scores were determined by subtracting the baseline measure from the measure obtained during pain, which was then expressed as a percentage of the baseline value.
Statistical analysis
To compare the effect of pain on maximum force generation across the three forms muscle activation, peak torque and EMG peak amplitude were analyzed using a two-way repeated measures ANOVA with factors of muscle activation type (isometric, concentric, eccentric) and time (baseline, pain, post-pain). To investigate the effect of pain on RFD, torque slope and integrated EMG from the isometric trials were analyzed using a one-way ANOVA with the factor of time (baseline, pain, post-pain). Separate ANOVAs were completed for each time interval (0–50, 50–100, 100–150, 150–200 ms). A Huynh–Feldt correction was used to adjust P values when Epsilon < 1. Significant main effects were further investigated using paired T tests.
All statistical analyses were performed using SPSS (v22, IBM Corp., Armonk, NY). Statistical significance was set at P < 0.05. Data are presented as mean±standard deviation unless otherwise indicated.
Results
Experimental pain duration
One participant did not complete data collection due to an adverse reaction to the injection of hypertonic saline. Pain ratings following injection of hypertonic saline were not recorded in one participant. The average time from injection of hypertonic saline until the numerical pain rating returned to 0 was 18 ± 4 min. The average peak pain rating was 5.8 ± 1.9 out of 10.
Maximum force generation: peak torque
Figure 1a shows peak torque values during the three types of muscle activation. There was a main effect of activation type (F2,36 = 77.7; P < 0.001) and time (F2,36 = 5.9; P = 0.008), but the interaction was not significant (F4,72 = 0.5; P = 0.8). Further analysis indicated that peak torque was significantly greater at baseline than during pain (P = 0.01) and at the post-pain period (P = 0.008). The difference between pain and post-pain was not significant (P = 0.3). Across all participants, peak torque during pain decreased by 9.7 ± 16.9, 5.8 ± 15.9, and 7.4 ± 15.4% during isometric, eccentric, and concentric muscle activation, respectively. Figure 2 shows the average torque profiles at baseline and during pain for the CON and ECC trials. In both ECC and CON contractions, torque is primarily inhibited in the midrange from 40 to 65°, which encompasses the point of peak torque.
As expected, analysis of the main effect of activation type revealed that peak torque was significantly greater during eccentric activation compared to concentric (P < 0.001) and isometric (P < 0.001) activation, and was greater during isometric compared to concentric activation (P < 0.001).
Maximum force generation: peak EMG
Figure 1b shows peak EMG data during the three types of muscle activation. Analysis of peak EMG revealed a main effect of time (F2,36 = 4.1; P = 0.03) but not activation type (F2,36 = 1.1; P = 0.3). The interaction also was not significant (F4,72 = 1.0; P = 0.4). Peak EMG was reduced during pain compared to baseline (P = 0.01), but was not different at post-pain compared to baseline (P = 0.3). In the isometric condition, the change in peak torque during pain was strongly correlated with the change in EMG peak during pain (Pearson r = 0.75; P < 0.001).
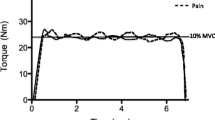
RFD: torque
Figure 3a shows the group torque slope data over the four time periods analyzed, while Fig. 3b shows the average baseline and pain torque profile across participants. At baseline, there was a strong positive correlation between peak torque and peak torque slope (Pearson’s r = 0.79; P < 0.001).
a Rate of force development group results showing the torque slope before, during, and after pain for isometric contractions. Data for the peak slope are shown as well as the four discrete time periods. *P < 0.05. Bars are 1 standard error. b Group averaged torque profile showing the initial 450 ms following contraction onset at baseline and during pain
The main effect of time was significant for peak torque slope (F2,36 = 5.0; P = 0.02) and the slope measured over 50–100 ms (F2,36 = 6.8; P = 0.003). For both of these variables, the torque slope during pain was less than baseline (both P < 0.005). While peak torque slope increased post-pain and was no different to baseline (P = 0.6), torque slope over 50–100 ms was still reduced from baseline at post-pain (P = 0.03). The main effect of time was not significant for torque slope over 0–50 ms (F2,36 = 2.5; P = 0.1), 100–150 ms (F2,36 = 0.8; P = 0.4), or 150–200 ms (F2,36 = 2.7; P = 0.08).
To test the hypothesis that the change in peak torque with pain would be more strongly related to the change in the later components of RFD, we correlated the change scores between these variables. There were moderate-strong correlations between the change in peak torque during pain and the change in torque slope across all time periods, with the strongest relationship evident in the 150–200-ms time period (0–50 ms: r = 0.62, P = 0.005; 50–100 ms: r = 0.55, P = 0.014; 100–150 ms: r = 0.57, P = 0.013; 150–200 ms: r = 0.70; P = 0.001).
RFD: rate of EMG rise
The rate of EMG rise was measured using EMG area. Figure 4 shows the group results over the four time periods analyzed, while Fig. 5 shows the average EMG profiles for the three muscles. There was a main effect of time in the time periods of 50–100 ms (F2,36 = 5.8; P = 0.009) and 100–150 ms (F2,36 = 5.0; P = 0.01). At both of these time periods, EMG area was significantly lower than baseline during pain (both P < 0.015) and at post-pain (both P < 0.05). The main effect of time was not significant for EMG area over 0–50 ms (F2,36 = 3.3; P = 0.053) or 150–200 ms (F2,36 = 1.3; P = 0.3).
To test the hypothesis that the early component of RFD would be more affected by changes in EMG during pain than the later component, we correlated the change in EMG area at each time period with the changes in torque slope over the equivalent time period. The correlation between these variables was significant at all time periods (all P < 0.03) but was stronger at 0–50 ms (r = 0.71), 50–100 ms (r = 0.67), and 100–150 ms (r = 0.67) than the final time period of 150–200 ms (r = 0.52).
Discussion
The first aim of this study was to investigate the effect of experimental joint pain on maximal knee extension torque during isometric and isokinetic activation, and to determine the impact on maximal RFD. We found that peak torque and EMG were equally impaired during isometric, concentric, and eccentric muscle activation in the presence of joint pain. We also provide novel evidence that the RFD is reduced in the presence of joint pain, and that this is accompanied by a reduction in the rate of EMG rise. Notably, the impact of pain on the RFD appeared to differ across the time periods examined. Thus, our findings indicate that not only does knee joint pain reduce the ability to maximally activate the quadriceps muscle, it also impairs the maximal rate of activation.
The reduction in isometric and concentric MVC was a similar magnitude to comparable studies of acute experimental pain on knee extensor torque (Henriksen et al. 2011; Salomoni et al. 2016), although it was modest in comparison to others (Asaki et al. 2018; Graven‐Nielsen et al. 2002; Park and Hopkins 2013). Supporting Salomoni et al. (2016), the effects of pain were quite variable among participants, with only 10 participants (50%) showing greater than 5% reduction in peak torque in the isometric condition. Despite previous research indicating an altered eccentric/concentric strength ratio in people with chronic pain (Dvir and Keating 2003; Marshall et al. 2010; Shirado et al. 1995), we found no evidence that eccentric muscle activation is impacted any differently by acute pain. Compared to concentric activation, eccentric muscle actions are served by different neural control strategies (Enoka 1996; McHugh et al. 2002), have greater resistance to fatigue (Enoka 1996), and are capable of generating more force (Lindstedt et al. 2001). Thus, there is certainly potential for nociceptive input to have a differing effect on eccentric actions. It is possible that the effect of acute pain on eccentric and concentric actions is different from that of chronic pain, where fear of movement (Karayannis et al. 2013; Nederhand et al. 2006) or altered patterns of activation (Arendt-Nielsen et al. 1996; Hodges and Richardson 1996) may be influential.
The exact mechanisms of joint torque inhibition during pain are unknown; however, it is widely postulated that it is due to central adaptations (Henriksen et al. 2009, 2011; Salomoni et al. 2016; Slater et al. 2003). Supporting this view, the strong correlation between changes in peak EMG and peak torque suggest the impaired force was related to reduced neural drive. Based on findings showing a relationship between voluntary activation and force impairment, Salomoni et al. (2016) speculated that this was at least in part due to impaired descending voluntary drive. In the upper limb, studies using transcranial magnetic stimulation have provided good evidence that acute pain is associated with reduced corticomotor excitability (Burns et al. 2016), but there is evidence for the opposite in the quadriceps muscle (Rice et al. 2015). Thus, impaired activation of the quadriceps cannot be solely explained by changes in corticomotor excitability.
The maximum EMG recovered to baseline levels following the cessation of pain but the maximum torque did not. Other studies have also shown a sustained impairment in maximum isometric or concentric peak torque following the resolution of acute pain (Ervilha et al. 2004; Henriksen et al. 2007, 2009, 2011; Salomoni et al. 2016; Shakespeare et al. 1985; Slater et al. 2003) and ongoing suppression of corticomotor excitability (Burns et al. 2016). Together, these findings suggest that the impact of nociception on motor output persists beyond the period of subjective pain experience.
This is the first study to show that the rate of force development and EMG rise is impaired in the presence of acute experimental pain. Aagaard and colleagues (2002) were among the first to delineate different temporal components of RFD and speculate different contributing factors. Subsequent cross-sectional studies have used various techniques (e.g., twitch force comparisons, muscle or fiber types, comparison to clinical populations) to more clearly identify the limiting factors across the early and late components (reviewed in Maffiuletti et al. 2016). There is now good evidence that maximal motor unit discharge rate is critical for initial force development (< 75 ms) (Del Vecchio et al. 2019), while the later component is more strongly influenced by the contractile properties of muscle and the peak MVC force itself. It is known that acute nociceptive activation can reduce the firing rate of motor units recruited during submaximal tasks (Farina et al. 2004, 2005, 2008; Sohn et al. 2000; Tucker et al. 2009). There is also evidence that muscle contractile properties are not impacted by experimentally induced acute muscle pain (Farina et al. 2004; Graven‐Nielsen et al. 2002). Therefore, it was hypothesized that acute pain would preferentially impair the earlier components of the RFD, and that this would be associated with impaired EMG rise. Our hypotheses are somewhat supported by the finding that peak torque slope and slope from 50 to 100 ms were the only periods to be significantly inhibited during pain. EMG area was also significantly inhibited from 50 to 100 and 100–150 ms, with a trend for inhibition over 0–50 ms (P = 0.053). The strength of the correlations between the changes in torque slope and EMG area during pain also decreased from the early to late components, while the relationship between change in torque with pain and change in RFD was strongest for the late component.
Two studies have used a delayed onset muscle soreness (DOMS) model to induce quadriceps muscle pain and examine the impact on RFD (Molina and Denadai 2012; Vila-Chã et al. 2012). In the presence of DOMS-induced pain, both studies reported a reduction in MVC and extensor torque slope, while Vila-Cha et al. (2012) also reported reduced quadriceps EMG activity. In DOMS, the muscular nociceptive pain has been related to inflammation following tissue damage (MacIntyre et al. 1995). While acknowledging this muscle damage and the potential impact on force development, the reduced EMG activity during ballistic activation indicates there is also neural contribution to impaired RFD. Similar to our findings, Molina and Denadai (2012) reported that the change in peak torque correlated with the change in RFD, at least at 24 h following DOMS inducement. The benefit of our acute experimental pain model was that we manipulated MVC capacity in a single session where the effects of electrode placement, body position, and changes in muscle properties were eliminated. Our study findings, therefore, more clearly show that acute joint pain impairs the maximal RFD, and that it primarily affects the early component.
Clinical relevance
Given the documented relationship between RFD in the quadriceps and function (Maffiuletti et al. 2010; Tillin et al. 2013), our finding of impaired RFD during acute pain is important and relevant for people with long-term pain conditions, such as osteoarthritis. Indeed, other studies have provided evidence that the RFD is lower in people with knee osteoarthritis (Gapeyeva et al. 2007; Vahtrik et al. 2012; Winters et al. 2014) as well as in chronic upper limb musculoskeletal pain conditions (Andersen et al. 2008; Chourasia et al. 2012). In these clinical populations, the RFD has been correlated with both pain (Andersen et al. 2008; Chourasia et al. 2012) and function (Andersen et al. 2008; Chourasia et al. 2012; Winters et al. 2014) measures. Along with our findings, the correlations with pain evident support an impact of pain on RFD in these populations, although Anderson et al. (2008) also acknowledge psychological effects associated with fear of movement that are likely to be influential in people with long-term pain. The finding that quadriceps RFD remains impaired following total knee joint replacement (Chourasia et al. 2012; Winters et al. 2014) highlights the importance of addressing pain control and targeting rapid torque generation through explosive/power training in clinical populations.
Limitations
There were several limitations to the current study. First, maintaining the desired pain intensity for a period long enough to complete the tasks involved is difficult with a single injection of hypertonic saline, and three participants reached a pain rating of 0/10 before they had completed all the pain condition measures. We did not include a control (isotonic saline) injection in the study due to the potential risk to the participants. Therefore, the changes in MVC and RFD during pain may have been due to fatigue; however, this is unlikely given the rest periods provided and the return to baseline of some measures in the post-pain period. We also did not record EMG activity from the hamstring muscles and are, therefore, unable to determine any changes in co-activation during pain. However, a reduction in antagonist activation during pain has been shown in other studies (Ciubotariu et al. 2007; Salomoni et al. 2016). Finally, there was a large age range of the participants in the study and there is evidence that speed-related force development properties are reduced with ageing, which may have influenced the variability of our findings (Klass et al. 2008).
Abbreviations
- ANOVA:
-
Analysis of variance
- EMG:
-
Electromyography
- MVC:
-
Maximum voluntary contraction
- RFD:
-
Rate of force development
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326. https://doi.org/10.1152/japplphysiol.00283.2002
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52. https://doi.org/10.1007/s00421-005-0070-z
Andersen LL, Holtermann A, Jorgensen MB, Sjogaard G (2008) Rapid muscle activation and force capacity in conditions of chronic musculoskeletal pain. Clin Biomech 23:1237–1242. https://doi.org/10.1016/j.clinbiomech.2008.08.002
Andersen LL, Andersen JL, Zebis MK, Aagaard P (2010) Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports 20:e162–169. https://doi.org/10.1111/j.1600-0838.2009.00933.x
Angelozzi M, Madama M, Corsica C, Calvisi V, Properzi G, McCaw ST, Cacchio A (2012) Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 42:772–780. https://doi.org/10.2519/jospt.2012.3780
Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P (1996) The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain 64:231–240. https://doi.org/10.1016/0304-3959(95)00115-8
Asaki T, Wang K, Luo Y, Arendt-Nielsen T, Graven-Nielsen T, Arendt-Nielsen L (2018) Acid-induced experimental knee pain and hyperalgesia in healthy humans. Exp Brain Res 236:587–598. https://doi.org/10.1007/s00221-017-5155-5
Burns E, Chipchase LS, Schabrun SM (2016) Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur J Pain 20:1203–1213. https://doi.org/10.1002/ejp.859
Chourasia AO, Buhr KA, Rabago DP, Kijowski R, Irwin CB, Sesto ME (2012) Effect of lateral epicondylosis on grip force development. J Hand Ther 25:27–36. https://doi.org/10.1016/j.jht.2011.09.003
Ciubotariu A, Arendt-Nielsen L, Graven-Nielsen T (2007) Localized muscle pain causes prolonged recovery after fatiguing isometric contractions. Exp Brain Res 181:147–158. https://doi.org/10.1007/s00221-007-0913-4
Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583:365–380. https://doi.org/10.1113/jphysiol.2007.128827
Del Vecchio A, Negro F, Holobar A, Casolo A, Folland JP, Felici F, Farina D (2019) You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol 597(9):2445–2456. https://doi.org/10.1113/JP277396
Dvir Z, Keating JL (2003) Trunk extension effort in patients with chronic low back dysfunction. Spine 28:685–692. https://doi.org/10.1097/01.brs.0000051917.04731.a4
Enoka RM (1996) Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol 81:2339–2346. https://doi.org/10.1152/jappl.1996.81.6.2339
Ervilha UF, Arendt-Nielsen L, Duarte M, Graven-Nielsen T (2004) Effect of load level and muscle pain intensity on the motor control of elbow-flexion movements. Eur J Appl Physiol 92:168–175. https://doi.org/10.1007/s00421-004-1083-8
Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T (2004) Effect of experimental muscle pain on motor unit firing rate and conduction velocity. J Neurophysiol 91:1250–1259. https://doi.org/10.1152/jn.00620.2003
Farina D, Arendt-Nielsen L, Graven-Nielsen T (2005) Experimental muscle pain reduces initial motor unit discharge rates during sustained submaximal contractions. J Appl Physiol 98:999–1005. https://doi.org/10.1152/japplphysiol.01059.2004
Farina D, Arendt-Nielsen L, Roatta S, Graven-Nielsen T (2008) The pain-induced decrease in low-threshold motor unit discharge rate is not associated with the amount of increase in spike-triggered average torque. Clin Neurophysiol 119:43–51. https://doi.org/10.1016/j.clinph.2007.10.003
Felson DT et al (2007) Knee buckling: Prevalence, risk factors, and associated limitations in function. Ann Intern Med 147:534–540
Folland JP, Buckthorpe MW, Hannah R (2014) Human capacity for explosive force production: neural and contractile determinants. Scand J Med Sci Sports 24:894–906. https://doi.org/10.1111/sms.12131
Gapeyeva H, Buht N, Peterson K, Ereline J, Haviko T, Pääsuke M (2007) Quadriceps femoris muscle voluntary isometric force production and relaxation characteristics before and 6 months after unilateral total knee arthroplasty in women. Knee Surg Sports Traumatol Arthrosc 15:202–211. https://doi.org/10.1007/s00167-006-0166-y
Graven-Nielsen T, Arendt-Nielsen L (2009) Impact of clinical and experimental pain on muscle strength and activity. Curr Rheumatol Rep 10:475. https://doi.org/10.1007/s11926-008-0078-6
Graven-Nielsen T, Lund H, Arendt-Nielsen L, Danneskiold-Samsøe B, Bliddal H (2002) Inhibition of maximal voluntary contraction force by experimental muscle pain: a centrally mediated mechanism. Muscle Nerve 26:708–712. https://doi.org/10.1002/mus.10225
Henriksen M, Alkjaer T, Lund H, Simonsen EB, Graven-Nielsen T, Danneskiold-Samsoe B, Bliddal H (2007) Experimental quadriceps muscle pain impairs knee joint control during walking. J Appl Physiol 103:132–139. https://doi.org/10.1152/japplphysiol.01105.2006
Henriksen M, Alkjaer T, Simonsen EB, Bliddal H (2009) Experimental muscle pain during a forward lunge-the effects on knee joint dynamics and electromyographic activity. Br J Sports Med 43:503–507. https://doi.org/10.1136/bjsm.2008.050393
Henriksen M, Rosager S, Aaboe J, Graven-Nielsen T, Bliddal H (2011) Experimental knee pain reduces muscle strength. J Pain 12:460–467. https://doi.org/10.1016/j.jpain.2010.10.004
Hodges PW, Richardson CA (1996) Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine 21:2640–2650
Hodges PW, Tucker K (2011) Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152:S90–S98. https://doi.org/10.1016/j.pain.2010.10.020
Jenkins ND et al (2014) The rate of torque development: a unique, non-invasive indicator of eccentric-induced muscle damage? Int J Sports Med 35:1190–1195. https://doi.org/10.1055/s-0034-1375696
Karayannis NV, Smeets RJEM, van den Hoorn W, Hodges PW (2013) Fear of movement is related to trunk stiffness in low back pain. PLoS ONE 8:e67779. https://doi.org/10.1371/journal.pone.0067779
Klass M, Baudry S (1985) Duchateau J (2008) Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104(3):739–746. https://doi.org/10.1152/japplphysiol.00550.2007
Liikavainio T, Lyytinen T, Tyrvainen E, Sipila S, Arokoski JP (2008) Physical function and properties of quadriceps femoris muscle in men with knee osteoarthritis. Arch Phys Med Rehabil 89:2185–2194
Lindstedt SL, LaStayo PC, Reich TE (2001) When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol Sci 16:256–261
MacIntyre DL, Reid WD, McKenzie DC (1995) Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med 20:24–40. https://doi.org/10.2165/00007256-199520010-00003
Maffiuletti NA, Bizzini M, Widler K, Munzinger U (2010) Asymmetry in quadriceps rate of force development as a functional outcome measure in TKA. Clin Orthop 468:191–198. https://doi.org/10.1007/s11999-009-0978-4
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J (2016) Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116:1091–1116. https://doi.org/10.1007/s00421-016-3346-6
Marshall PW, Mannion J, Murphy BA (2010) The eccentric, concentric strength relationship of the hamstring muscles in chronic low back pain. J Electromyogr Kinesiol 20:39–45. https://doi.org/10.1016/j.jelekin.2009.04.005
McHugh MP, Tyler TF, Greenberg SC, Gleim GW (2002) Differences in activation patterns between eccentric and concentric quadriceps contractions. J Sports Sci 20:83–91. https://doi.org/10.1080/026404102317200792
Molina R, Denadai BS (2012) Dissociated time course recovery between rate of force development and peak torque after eccentric exercise. Clin Physiol Funct Imaging 32:179–184. https://doi.org/10.1111/j.1475-097X.2011.01074.x
Moreland JD et al (2003) Progressive resistance strengthening exercises after stroke: a single-blind randomized controlled trial. Arch Phys Med Rehabil 84:1433–1440
Nederhand MJ, Hermens HJ, Ijzerman MJ, Groothuis KG, Turk DC (2006) The effect of fear of movement on muscle activation in posttraumatic neck pain disability. Clin J Pain 22:519–525. https://doi.org/10.1097/01.ajp.0000202979.44163.da
Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L (2000) The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med 23:351–365. https://doi.org/10.1023/a:1005548801037
Park J, Hopkins JT (2013) Induced anterior knee pain immediately reduces involuntary and voluntary quadriceps activation. Clin J Sport Med 23:19–24. https://doi.org/10.1097/JSM.0b013e3182717b7b
Penailillo L, Blazevich A, Numazawa H, Nosaka K (2015) Rate of force development as a measure of muscle damage. Scand J Med Sci Sports 25:417–427. https://doi.org/10.1111/sms.12241
Rice DA, Graven-Nielsen T, Lewis GN, McNair PJ, Dalbeth N (2015) The effects of experimental knee pain on lower limb corticospinal and motor cortex excitability. Arthritis Res Ther 17:204. https://doi.org/10.1186/s13075-015-0724-0
Ruiter CJd, Kooistra RD, Paalman MI, Haan Ad (2004) Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol 97:1693–1701. https://doi.org/10.1152/japplphysiol.00230.2004
Salomoni S, Tucker K, Hug F, McPhee M, Hodges PW (2016) Reduced maximal force during acute anterior knee pain is associated with deficits in voluntary muscle activation. PLoS ONE 11:e0161487. https://doi.org/10.1371/journal.pone.0161487
Shakespeare DT, Stokes M, Sherman KP, Young A (1985) Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clin Physiol 5:137–144
Shirado O, Ito T, Kaneda K, Strax TE (1995) Concentric and eccentric strength of trunk muscles: influence of test postures on strength and characteristics of patients with chronic low-back pain. Arch Phys Med Rehabil 76:604–611
Slater H, Arendt-Nielsen L, Wright A, Graven-Nielsen T (2003) Experimental deep tissue pain in wrist extensors - a model of lateral epicondylalgia. Eur J Pain 7:277–288. https://doi.org/10.1016/s1090-3801(02)00141-6
Sohn MK, Graven-Nielsen T, Arendt-Nielsen L, Svensson P (2000) Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve 23:1219–1226. https://doi.org/10.1002/1097-4598(200008)23:8%3c1219:aid-mus10%3e3.0.co;2-a
Sullivan M, Tanzer M, Stanish W, Fallaha M, Keefe FJ, Simmonds MA, Dunbar M (2009) Psychological determinants of problematic outcomes following Total Knee Arthroplasty. Pain 143:123–129. https://doi.org/10.1016/j.pain.2009.02.011
Tillin NA, Jimenez-Reyes P, Pain MT, Folland JP (2010) Neuromuscular performance of explosive power athletes versus untrained individuals. Med Sci Sports Exerc 42:781–790. https://doi.org/10.1249/MSS.0b013e3181be9c7e
Tillin NA, Pain MTG, Folland J (2013) Explosive force production during isometric squats correlates with athletic performance in rugby union players. J Sports Sci 31:66–76. https://doi.org/10.1080/02640414.2012.720704
Tucker K, Butler J, Graven-Nielsen T, Riek S, Hodges P (2009) Motor unit recruitment strategies are altered during deep-tissue pain. J Neurosci 29:10820–10826. https://doi.org/10.1523/jneurosci.5211-08.2009
Vahtrik D et al (2012) Quadriceps femoris muscle function prior and after total knee arthroplasty in women with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20:2017–2025. https://doi.org/10.1007/s00167-011-1808-2
Vila-Chã C, Hassanlouei H, Farina D, Falla D (2012) Eccentric exercise and delayed onset muscle soreness of the quadriceps induce adjustments in agonist-antagonist activity, which are dependent on the motor task. Exp Brain Res 216:385–395. https://doi.org/10.1007/s00221-011-2942-2
Winters JD, Christiansen CL, Stevens-Lapsley JE (2014) Preliminary investigation of rate of torque development deficits following total knee arthroplasty. Knee 21:382–386. https://doi.org/10.1016/j.knee.2013.10.003
Author information
Authors and Affiliations
Contributions
DR, PM, and JM conceived and designed the research. LF and DR collected the data. LF and GL analyzed the data. GL and LF wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rice, D.A., Mannion, J., Lewis, G.N. et al. Experimental knee pain impairs joint torque and rate of force development in isometric and isokinetic muscle activation. Eur J Appl Physiol 119, 2065–2073 (2019). https://doi.org/10.1007/s00421-019-04195-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04195-6